To the Editor,
Testing for SARS‐CoV‐2 RNA has become the standard for COVID‐19 diagnosis. 1 , 2 However, a number of false negative results have been reported, 2 resulting in a failure to quarantine infected patients. If unchecked, this could cause a major setback in containing viral transmission. 3
Titers of SARS‐CoV‐2 antibodies can reflect the progress of viral infection. Around 60 convalescent patients (with an onset time of 6‐7 weeks) in a ward in the Wuhan Tongji Hospital were tested for specific antibodies against SARS‐CoV‐2. All patients tested positive for the IgG against the virus, while 13 patients tested negative for immunoglobulin M (IgM), with the immunoglobulin G (IgG) titer being greater than the IgM titer (Table 1 and Figure 1). Meanwhile, the IgM and IgG titers in 10 convalescent patients were tested twice (1 week apart); both titers showed a decrease, with the IgG titer being greater than the IgM titer (Table 2 and Figure 1). In these patients, two consecutive SARS‐CoV‐2 RNA tests were negative and the chest computed tomography findings indicated improvement. Considering this, their antibody titers and consistent clinical manifestations suggested that antibody detection could act as an indicator of the stage of COVID‐19 progression and that the antibodies in convalescent patients are not always maintained at a high level. The immune status fitted both, the clinical and general characteristics of the humoral response. In one report, while 38 patients in the acute phase of the infection tested positive for SARS‐CoV‐2, 31 (81.6%) of them tested negative for IgM and IgG in serological assays, 4 thereby demonstrating that these patients were in the early stages of infection, as both the antibody titers were relatively low (Supplemental table 1). COVID‐19 patients will develop immunity after recovery; however, the persistence, attenuation, and duration of protection of SARS‐CoV‐2 antibodies requires further investigation. 5
Table 1.
Serological data from 60 convalescent patients in the Wuhan Tongji Hospital
| Patients | Onset | Tested on | IgM titer, AU/mL | IgG titer, AU/mL |
|---|---|---|---|---|
| 1 | 13/1/2020 | 9/3/2020 | 29.26 (+) | 231.67 (+) |
| 2 | 22/1/2020 | 3/3/2020 | 33.71 (+) | 198.77 (+) |
| 3 | 15/1/2020 | 9/3/2020 | 17.17 (+) | 464.69 (+) |
| 4 | 24/1/2020 | 9/3/2020 | 63.54 (+) | 178.23 (+) |
| 5 | 22/1/2020 | 9/3/2020 | 15.36 (+) | 369.07 (+) |
| 6 | 12/1/2020 | 8/3/2020 | 50.01 (+) | 203.07 (+) |
| 7 | 30/1/2020 | 9/3/2020 | 58.26 (+) | 203.00 (+) |
| 8 | 17/1/2020 | 9/3/2020 | 41.21 (+) | 405.85 (+) |
| 9 | 5/2/2020 | 9/3/2020 | 12.41 (+) | 175.03 (+) |
| 10 | 4/2/2020 | 10/3/2020 | 13.47 (+) | 31.60 (+) |
| 11 | 15/1/2020 | 12/3/2020 | 20.90 (+) | 162.72 (+) |
| 12 | 10/2/2020 | 12/3/2020 | 18.32 (+) | 192.90 (+) |
| 13 | 18/1/2020 | 9/3/2020 | 99.85 (+) | 220.03 (+) |
| 14 | 27/1/2020 | 10/3/2020 | 21.88 (+) | 139.36 (+) |
| 15 | 17/1/2020 | 9/3/2020 | 76.84 (+) | 177.61 (+) |
| 16 | 1/2/2020 | 9/3/2020 | 123.06 (+) | 161.16 (+) |
| 17 | 26/1/2020 | 8/3/2020 | 16.45 (+) | 194.57 (+) |
| 18 | 27/1/2020 | 8/3/2020 | 115.23 (+) | 194.80 (+) |
| 19 | 20/1/2020 | 9/3/2020 | 36.49 (+) | 96.75 (+) |
| 20 | 23/1/2020 | 9/3/2020 | 27.08 (+) | 136.96 (+) |
| 21 | 18/1/2020 | 9/3/2020 | 176.27 (+) | 369.4 (+) |
| 22 | 28/1/2020 | 9/3/2020 | 82.71 (+) | 177.69 (+) |
| 23 | 3/2/2020 | 9/3/2020 | 65.15 (+) | 241.57 (+) |
| 24 | 24/1/2020 | 9/3/2020 | 47.84 (+) | 200.31 (+) |
| 25 | 26/1/2020 | 8/3/2020 | 63.52 (+) | 165.49 (+) |
| 26 | 21/1/2020 | 8/3/2020 | 164.18 (+) | 346.98 (+) |
| 27 | 20/1/2020 | 9/3/2020 | 168.04 (+) | 171.85 (+) |
| 28 | 22/1/2020 | 8/3/2020 | 99.28 (+) | 137.58 (+) |
| 29 | 26/1/2020 | 8/3/2020 | 66.79 (+) | 188.75 (+) |
| 30 | 3/2/2020 | 8/3/2020 | 27.13 (+) | 208.82 (+) |
| 31 | 2/2/2020 | 6/3/2020 | 55.03 (+) | 186.47 (+) |
| 32 | 3/2/2020 | 5/3/2020 | 164.96 (+) | 180.42 (+) |
| 33 | 27/1/2020 | 6/3/2020 | 84.12 (+) | 162.14 (+) |
| 34 | 26/1/2020 | 9/3/2020 | 287.54 (+) | 299.75 (+) |
| 35 | 22/1/2020 | 9/3/2020 | 346.62 (+) | 962.01 (+) |
| 36 | 28/1/2020 | 9/3/2020 | 163.58 (+) | 733.95 (+) |
| 37 | 20/1/2020 | 8/3/2020 | 150.93 (+) | 158.48 (+) |
| 38 | 20/1/2020 | 8/3/2020 | 114.81 (+) | 338.99 (+) |
| 39 | 27/1/2020 | 9/3/2020 | 176.29 (+) | 206.03 (+) |
| 40 | 23/1/2020 | 9/3/2020 | 147.93 (+) | 965.24 (+) |
| 41 | 16/1/2020 | 6/3/2020 | 45.35 (+) | 134.35 (+) |
| 42 | 20/1/2020 | 8/3/2020 | 78.34 (+) | 112.56 (+) |
| 43 | 24/1/2020 | 6/3/2020 | 307.14 (+) | 420.72 (+) |
| 44 | 27/1/2020 | 8/3/2020 | 181.75 (+) | 1077.09 (+) |
| 45 | 26/1/2020 | 6/3/2020 | 41.68 (+) | 233.06 (+) |
| 46 | 26/1/2020 | 8/3/2020 | 20.1 (+) | 162.79 (+) |
| 47 | 25/1/2020 | 6/3/2020 | 155.64 (+) | 279.45 (+) |
| 48 | 24/1/2020 | 3/2/2020 | 8.82 (‐) | 170.22 (+) |
| 49 | 1/2/2020 | 14/3/2020 | 8.91 (‐) | 73.35 (+) |
| 50 | 30/1/2020 | 8/3/2020 | 7.33 (‐) | 185.74 (+) |
| 51 | 27/1/2020 | 9/3/2020 | 2.59 (‐) | 133.14 (+) |
| 52 | 30/1/2020 | 8/3/2020 | 5.65 (‐) | 121.43 (+) |
| 53 | 30/1/2020 | 9/3/2020 | 5.53 (‐) | 20.2 (+) |
| 54 | 23/1/2020 | 14/3/2020 | 9.15 (‐) | 191.61 (+) |
| 55 | 12/1/2020 | 12/3/2020 | 3.65 (‐) | 15.16 (+) |
| 56 | 26/1/2020 | 10/3/2020 | 4.12 (‐) | 25.61 (+) |
| 57 | 24/1/2020 | 7/3/2020 | 3.4 (‐) | 213.32 (+) |
| 58 | 20/1/2020 | 11/3/2020 | 3.16 (‐) | 183.32 (+) |
| 59 | 23/1/2020 | 4/3/2020 | 5.01 (‐) | 208.08 (+) |
| 60 | 20/1/2020 | 8/3/2020 | 6.75 (‐) | 150.80 (+) |
Note: (+) positive result; (‐) negative result; titer ≤10 AU/mL indicates a negative result according to the manufacturer's instructions.
Abbreviations: AU, arbitrary unit; IgG, immunoglobulin G; IgM, immunoglobulin M.
Figure 1.
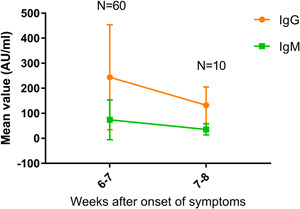
IgM and IgG antibodies level to SARS‐CoV‐2 from the onset of symptoms. IgG, immunoglobulin G; IgM, immunoglobulin M
Table 2.
Serological data from 10 convalescent patients who were tested twice (1 week apart)
| Patients | Onset | 1st Test | 2nd Test | IgM titer (1st test), AU/mL | IgM titer (2nd test), AU/mL | IgG titer (1st test), AU/mL | IgG titer (2nd test), AU/mL |
|---|---|---|---|---|---|---|---|
| 38 | 20/1/2020 | 8/3/2020 | 15/3/2020 | 114.81 (+) | 36.14 (+) | 338.99 (+) | 210.33 (+) |
| 39 | 27/1/2020 | 9/3/2020 | 16/3/2020 | 176.29 (+) | 50.21 (+) | 206.03 (+) | 88.74 (+) |
| 40 | 23/1/2020 | 9/3/2020 | 16/3/2020 | 147.93 (+) | 40.35 (+) | 965.24 (+) | 201.34 (+) |
| 41 | 16/1/2020 | 6/3/2020 | 13/3/2020 | 45.35 (+) | 13.54 (+) | 134.35 (+) | 50.33 (+) |
| 42 | 20/1/2020 | 8/3/2020 | 15/3/2020 | 78.34 (+) | 21.36 (+) | 112.56 (+) | 47.21 (+) |
| 43 | 24/1/2020 | 6/3/2020 | 14/3/2020 | 307.14 (+) | 80.79 (+) | 420.72 (+) | 97.06 (+) |
| 44 | 27/1/2020 | 8/3/2020 | 15/3/2020 | 181.75 (+) | 47.03 (+) | 1077.09 (+) | 242.25 (+) |
| 45 | 26/1/2020 | 6/3/2020 | 15/3/2020 | 41.68 (+) | 12.36 (+) | 233.06 (+) | 95.15 (+) |
| 46 | 26/1/2020 | 8/3/2020 | 15/3/2020 | 20.1 (+) | 9.28 (‐) | 162.79 (+) | 88.68 (+) |
| 47 | 25/1/2020 | 6/3/2020 | 12/3/2020 | 155.64 (+) | 48.49 (+) | 279.45 (+) | 200.58 (+) |
Note: (+) positive result; (‐) negative result; titer ≤10 AU/mL indicates a negative result according to the manufacturer's instructions.
Abbreviations: AU, arbitrary unit; IgG, immunoglobulin G; IgM, immunoglobulin M.
Presently, data regarding the COVID‐19 spectrum are mainly focused on clinical infection with respiratory symptoms. The proportion of subclinical infections and atypical patients remains unknown. Antibody detection will help in the profiling of the COVID‐19 spectrum. Epidemiological surveys of serum antibody levels in the population would help in fully understanding how many people have ever been infected. This information will allow the determination of the proportion of different types of infected individuals and a profiling of the complete disease spectrum of COVID‐19.
High virus volumes and transmission have been reported in the asymptomatic phase. 6 By combining the results of RNA and antibody testing, we can further identify the contribution of different types of infected people (especially the atypical ones and those with subclinical infection) in the spread of the virus and the disease. This will provide a key scientific basis for the discovery and management of infectious sources.
Detection of IgM and IgG against SARS‐CoV‐2 is a fast and simple screening method. As an effective supplement to RNA testing, antibody detection is of epidemiological significance and is an important means to understand the occurrence, development, prognosis, and outcome of COVID‐19. More medical research on the expression levels of antibodies against SARS‐CoV‐2 and on the prognosis of COVID‐19 is required.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
ZD and FZG drafted the manuscript. FXZ, BY, and TBW cared for the patients and collected the data.
Supporting information
Supporting information
ACKNOWLEDGMENTS
This work was supported by the Beijing Natural Science Foundation (7204321). We thank Dr. Bo Yang for collecting and providing serological data.
Funding Information Beijing Natural Science Foundation, Grant/Award Number: 7204321
Zhe Du and Fengxue Zhu contributed equally to this work.
REFERENCES
- 1. The Chinese novel coronavirus pneumonia diagnosis and treatment plan (trial version seven). http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. (Accessed March 20, 2020).
- 2. Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J Med Virol. 2020. 10.1002/jmv.25727. [published online ahead of print February 27, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guoo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID‐19). Clin Infect Dis. 2020. 10.1093/cid/ciaa310. [published online ahead of print March 21, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cassaniti I, Novazzi F, Giardina F, et al. Performance of VivaDiagTM COVID‐19 IgM/IgG rapid test is inadequate for diagnosis of COVID‐19 in acute patients referring to emergency room department. J Med Virol. 2020. 10.1002/jmv.25800. [published online ahead of print March 30, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bao L, Deng W, Gao H, et al. Reinfection could not occur in SARS‐CoV‐2 infected rhesus macaques. BioRxiv. 2020. 10.1101/2020.03.13.990226 [DOI] [Google Scholar]
- 6. Zouu L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


