Abstract
The etiology and pathologic findings of bovine respiratory disease (BRD) in adult dairy cows (n = 35) from a commercial dairy herd in Southern Brazil were investigated. Pulmonary samples were examined for histopathologic patterns and specific features within these patterns, while immunohistochemical (IHC) assays were designed to detect the intralesional antigens of viral infectious disease agents and Mycoplasma bovis. Pneumonia was diagnosed in 91.4% (32/35) of these cases; neither pneumonia nor any of the infectious disease pathogens evaluated occurred in three cows. The presence of multiple respiratory pathogens in 75% (24/32) of these cases indicated the complex origin of pneumonia in cattle. Interstitial pneumonia, necrosuppurative bronchopneumonia and suppurative bronchopneumonia were the principal patterns of pulmonary disease identified by histopathology. The most frequent pathogens identified by IHC were bovine viral diarrhea virus (BVDV; n = 18), M. bovis (n = 16) and bovine alphaherpesvirus type 1 (BoHV‐1; n = 14), followed by bovine respiratory syncytial virus (BRSV; n = 11) and bovine parainfluenza virus type 3 (BPIV‐3; n = 5). Obliterative bronchiolitis and peribronchial lymphocytic cuffings were the characteristic histopathologic features associated with M. bovis. Necrohemorrhagic bronchitis with bronchial angiogenesis was associated with BoHV‐1. Necrotizing bronchitis and bronchiolitis were associated with BVDV, BoHV‐1 and BRSV. Ballooning degeneration of the bronchial and bronchiolar epithelia was associated with BRSV and BoHV‐1. This is the first report from Brazil that correlated the histopathologic findings of BRD with the associated infectious disease agents by immunohistochemistry. M. bovis was frequently detected in the tissues of cows with fatal pulmonary disease during this study and may be a possible primary disease pathogen associated with the development of BRD in dairy cows. Additionally, the histopathologic features identified within patterns of pulmonary disease during this investigation may be an efficient diagnostic tool to associate histopathologic findings with specific agents of BRD in dairy cows.
Keywords: BoHV‐1, bovine pulmonary mycoplasmosis, bovine respiratory syncytial virus, bovine viral diarrhea virus, diagnostic immunohistochemistry, interstitial pneumonia, respiratory pathogens
1. INTRODUCTION
The bovine respiratory disease (BRD) is a multifactorial and multi‐aetiological disease associated with several infectious disease agents (Fulton et al., 2009; Panciera & Confer, 2010). Data relative to the occurrence of BRD in Brazil are scarce and incipient. Most of these investigations used polymerase chain reaction (PCR) and identified infectious agents of BRD such as Histophilus somni (Headley, Alfieri, Oliveira, Beuttemmuller, & Alfieri, 2014; Headley et al., 2018), bovine alphaherpesvirus type 1, BoHV‐1 (Suarez Heinlein et al., 1993), bovine respiratory syncytial virus, BRSV (Arns et al., 2003; Headley et al., 2017), bovine viral diarrhea virus, BVDV (Cortez et al., 2006; Flores, Ridpath, Weiblen, Vogel, & Gil, 2002; Otonel et al., 2014; Silveira et al., 2017), bovine coronavirus, BCoV (Headley et al., 2018), Pasteurella multocida (Baptista et al., 2017; Headley et al., 2018), Mannheimia haemolytica (Baptista et al., 2017; Headley et al., 2018) and Mycoplasma bovis (Tortorelli et al., 2017). Furthermore, studies done in Brazil using serology identified seropositivity to infectious disease agents including BoHV‐1 (Barbosa, Brito, & Alfaia, 2005; Fernandes, Pimenta, Pituco, Brasil, & Azevedo, 2016), bovine parainfluenza virus type 3, BPIV‐3 (Gonçalves et al., 2003), BRSV (Driemeier et al., 1997), and BVDV (Flores et al., 2005; Wageck Canal, Strasser, Hertig, Masuda, & Peterhans, 1998) and M. bovis (Pretto et al., 2001). Additionally, there is the isolation of M. bovis (Pretto et al., 2001), while few studies from Brazil have investigated only BRSV by immunohistochemistry, IHC (Brasil et al., 2013; Peixoto et al., 2000).
The detection of antigen‐coding sequences in tissues by PCR in the absence of histopathologic findings does not necessarily indicate that the identified agent is associated with a specific lesion or disease (Maes et al., 2013). Disease due to infectious agents associated with BRD is confirmed by the simultaneous presence of pathogens within the affected tissues (Fulton & Confer, 2012). The IHC assay is a sensitive diagnostic technique that can be used to identify the intralesional presence of specific protein of infectious disease agents associated with histopathologic lesions in formalin‐fixed paraffin embedded (FFPE) tissues sections (Fulton & Confer, 2012; Maes et al., 2013), and the results obtained are strong evidence of an associated disease process within the affected tissues (Fulton & Confer, 2012).
The disease pathogens associated with BRD have been evaluated extensively mainly in North America (Fulton, 2009; Fulton et al., 2009; Panciera & Confer, 2010; Wolfger, Timsit, White, & Orsel, 2015) and Australia (Cusack, McMeniman, & Lean, 2003; Hay, Morton, Mahony, Clements, & Barnes, 2016). However, only a few studies have used histopathologic diagnosis with related IHC assays to confirm the participation of infectious disease agents associated with BRD (Gershwin et al., 2015; Haines, Martin, Clark, Jim, & Janzen, 2001; Haines et al., 2004; Rodríguez, Bryson, Ball, & Forster, 1996). This study describes the histopathologic patterns with associated histologic features and the IHC findings associated with M. bovis and four viral agents of BRD in a commercial dairy herd from Southern Brazil.
2. MATERIAL AND METHODS
2.1. Animals, clinical history and study location
This study investigated the occurrence of infectious disease agents of BRD and the pathologic findings in Holstein cows (n = 35) from a commercial dairy establishment in Eastern Central Paraná, Southern Brazil. This establishment consisted of 1,500 Holstein dairy cows with milk production of 45,000 L/day and an average of 29.2 L/cow/day. Due to the purchase of heifer and cows from different neighbouring herds, as well as from farms from neighbouring cities, this farm is considered as an open dairy cattle herd.
Between January and September 2017, there were reports of recurrent respiratory distress of the affected dairy cows that demonstrated clinical signs of inappetence, reluctance to walk and pulmonary distress (dyspnea, extended head and neck, and audible noise when breathing) associated with BRD, and eventually died spontaneously. Respiratory diseases were predominant in recently calved cows that demonstrated low morbidity (10%; 150/1,500) and mortality (2.3%; 35/1,500). Autopsies were performed by on‐site veterinarians during this seven‐month period as mortality occurred; pulmonary samples were collected and submitted for laboratory diagnosis after autopsy. The clinical course of the respiratory disease and possible antibiotic therapies are not known.
2.2. Histopathologic examination
Refrigerated pulmonary sections were submitted for pathologic diagnostics; tissues sections were fixed by immersion in 10% buffered formalin solution for 24 hr and then routinely processed for histopathologic evaluation with the haematoxylin and eosin (H&E) stain. Histopathologic patterns were classified (TESO, SAH) and recorded according to the presence/absence of bronchopneumonia (suppurative, necrosuppurative, fibrinous or fibrinosuppurative) and interstitial pneumonia. Additionally, the occurrence of specific histopathologic features associated with these patterns of pulmonary disease such as obliterative bronchiolitis, syncytial formation, necrotizing bronchitis/bronchiolitis/alveolitis, necrohemorrhagic bronchiolitis, abscesses and intralesional bacterial accumulations was identified and recorded. Furthermore, whenever necessary new histologic slides were evaluated with the Brown–Brenn modified staining technique to detect Gram‐positive or Gram‐negative bacteria, the Giemsa stain was used to detect intralesional accumulations of Mycoplasma spp.
2.3. Immunohistochemical identification of infectious agents associated with BRD
Immunohistochemistry assays were performed on lung sections to investigative the presence of five pathogens associated with BRD: BoHV‐1, BRSV, BVDV, BPIV‐3 and M. bovis. Selected FFPE tissue sections from the lung of each cow were prepared on silanized slides with Poly‐l‐lysine 0,1% (Sigma‐Aldrich, St. Louis, MO, USA), deparaffinized, hydrated in alcohol baths and subjected to antigen retrieval. The dilutions of the monoclonal antibodies used during this investigation are shown in Table 1. Antigen retrieval (Table 1) was achieved by using citrate buffer (pH 6.0) or Tris‐EDTA buffer with 0.05% Tween (pH 9.0). Both solutions were utilized with the pressure cooker system (Electrolux Pressure Cooker PCC10, São Paulo, Brazil) for 5 min. Endogenous peroxidase was blocked with distilled water and hydrogen peroxide (6%) for 30 min in a dark chamber.
Table 1.
List of antibodies, dilutions, method of antigen retrieval and source manufactures of the immunohistochemical assays
| Antibody (clone) | Antigen retrieval | Dilution | Source |
|---|---|---|---|
| BoHV‐1 (MAb gC‐gIII) | Citrate buffer (pH 6.0) | 1:700 | VRMD (Pullman, WA, USA) |
| BPIV‐3 | EDTA buffer (pH 9.0) | 1:40 | Gently ceded by Dr. Eduardo F. Flores, UFSM |
| BRSV (15c7) | Citrate buffer (pH 6.0) | 1:300 | Gently ceded by Dr. Eduardo F. Flores, UFSM |
| BVDV (15c5) | Citrate buffer (pH 6.0) | 1:1,500 | Gently ceded by Dr. Eduardo F. Flores, UFSM |
| Mycoplasma bovis | Citrate buffer (pH 6.0) | 1:10 | Gently ceded by Dr. Lucienne Pretto‐Giordano, UEL |
Abbreviations: BoHV‐1: bovine alphaherpesvirus type 1; BPIV‐3: bovine parainfluenza virus type 3; BRSV: bovine respiratory syncytial virus; BVDV: bovine viral diarrhea virus.
The primary incubation was achieved with the monoclonal antibodies shown in Table 1 during 24 hr at 4°C. Incubation with the secondary antibody SuperPicture™ Polymer Detection kit (Invitrogen Corporation, Camarillo, CA, USA) was done in a humid chamber for 30 min at 25°C, after which the chromogen 3,3′‐diaminobenzidine (DAB, Invitrogen® Life Technologies, Frederick, MD, USA) was added for 4 min. The slides were counterstained with Harris’ haematoxylin, dehydrated in successive alcohol baths to xylol and then assembled with commercial resins and coverslips. Positive controls consisted of pulmonary sections from other cases known to be infected with BRSV (Headley et al., 2018), BoHV‐1 (Oliveira, Lorenzetti, Alfieri, & Lisbôa, 2015), BVDV (Lunardi, Headley, Lisboa, Amude, & Alfieri, 2008) and M. bovis (Pretto et al., 2001); positive controls for BPIV‐3 were obtained from tissue culture maintained within our laboratory. Negative control consisted of using the same tissue, with substitution of the primary antibody by its diluent. Positive and negative controls were included in each IHC assay.
3. RESULTS
3.1. Histopathologic patterns and features associated with BRD
An overview of the principal histopathologic patterns of pulmonary disease observed in adult dairy cows during this study and their associated histologic features are given in Table 2. Pneumonia was diagnosed in 91.4% (32/35) of the affected cows, and at least one infectious disease agent was identified in each animal by IHC (Table 3); however, agents associated with BRD were not identified in 8.6% (3/35) of cows without pneumonia. Interstitial pneumonia (46.8%; 15/32) was the most predominant pattern of pulmonary disease observed, followed by necrosuppurative bronchopneumonia (28.1%; 9/32; Figure 1a) with peribronchial lymphocytic cuffings (21.9%; 7/32), and suppurative bronchopneumonia (18.7%; 6/32). Additionally, two cows without histopathologic evidence of pneumonia had necrotizing bronchitis. Accumulations of intralesional, Giemsa‐stained, coccoid bacteria were associated with necrosuppurative and suppurative bronchopneumonia; Gram‐positive or Gram‐negative bacteria were not detected by the modified Brown–Brenn stain.
Table 2.
Association of patterns of pulmonary disease with specific histologic features observed in dairy cows with bovine respiratory disease
| Pulmonary patterns | Agent | Associated features |
|---|---|---|
| Interstitial pneumonia (n = 15) |
BVDV BoHV‐1 BRSV BPIV‐3 |
Necrotizing bronchitis Necrotizing bronchiolitis Necrohemorrhagic bronchitis with angiogenesis Syncytial formation Ballooning degeneration of bronchial/bronchiolar epithelium Hyperplasia of type II pneumocytes |
| Necrosuppurative bronchopneumonia (n = 9) | M. bovis | Accumulations of intralesional Giemsa‐stained coccoid bacteria |
| Peribronchial lymphocytic cuffings | ||
| Suppurative bronchopneumonia (n = 6) | M. bovis | Accumulations of intralesional Giemsa‐stained coccoid bacteria |
| Peribronchial lymphocytic cuffings | ||
| Without pneumonia (n = 2) | BRSV | Necrotizing bronchitis |
Abbreviations: BoHV‐1: bovine alphaherpesvirus type 1; BPIV‐3: bovine parainfluenza virus type 3; BRSV: bovine respiratory syncytial virus; BVDV: bovine viral diarrhea virus; and M. bovis: Mycoplasma bovis.
Table 3.
Principal histopathologic diagnosis/features and immunohistochemical findings of bovine respiratory disease in 32 dairy cows
| Histopathologic diagnosis/features | Detection of pathogens in the lungs (number/total) |
|---|---|
| Obliterative bronchiolitis | M. bovis (10/10) |
| Accumulations of intralesional Giemsa‐stained coccoid bacteria | M. bovis (9/9) |
| Necrosuppurative bronchopneumonia | M. bovis (9/9) |
| Peribronchial lymphocytic cuffings | M. bovis (8/8) |
| Suppurative bronchopneumonia | M. bovis (6/6) |
| Interstitial pneumonia | BVDV (10/15) |
| BoHV‐1 (9/15) | |
| BRSV (4/15) | |
| BPIV‐3 (1/15) | |
| Necrotizing bronchitis | BVDV (4/6) |
| BRSV (4/6) | |
| BoHV‐1 (2/6) | |
| Necrotizing bronchiolitis | BVDV (4/4) |
| BRSV (2/4) | |
| Necrohemorrhagic bronchitis with bronchial angiogenesis | BoHV‐1 (2/2) |
| Syncytial formation | BRSV (4/4) |
| Ballooning degeneration of bronchial epithelium | BoHV‐1 (2/3) |
| BRSV (1/3) | |
| Hyperplasia of type II pneumocytes | BRSV (2/2) |
| Ballooning degeneration of bronchial epithelium of bronchiolar epithelium | BoHV‐1 (1/1) |
Abbreviations: BoHV‐1: bovine alphaherpesvirus type 1; BPIV‐3: bovine parainfluenza virus type 3; BRSV: bovine respiratory syncytial virus; BVDV: bovine viral diarrhea virus; M. bovis: Mycoplasma bovis.
Figure 1.
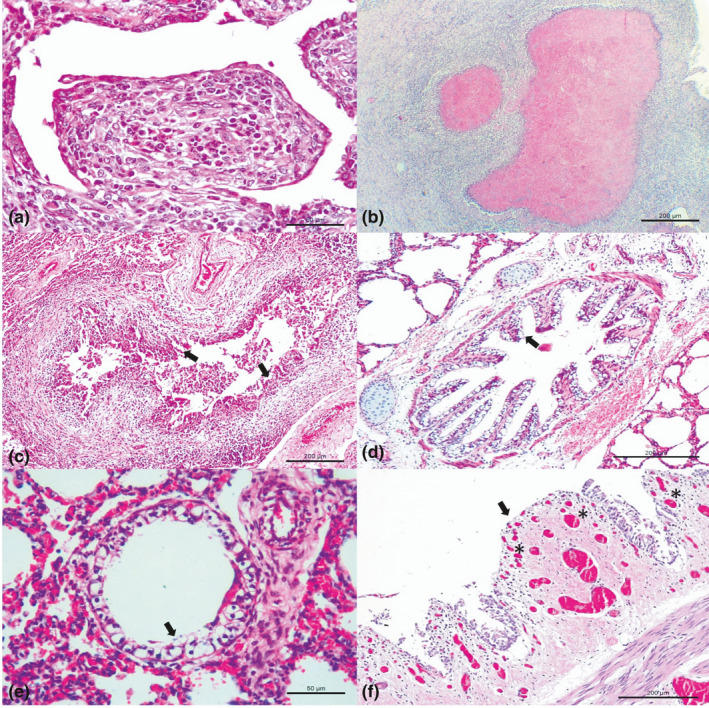
Histopathologic features observed in dairy cattle with bovine respiratory disease. (a) Observe obliterative bronchiolitis and (b) necrosuppurative bronchopneumonia with large areas of necrosis filled with hypereosinophilic (pink–red) granular debris associated with M. bovis. (c) Observe BVDV associated with necrotizing bronchiolitis (arrows) while (d) ballooning degeneration of bronchial (arrow) (e) and bronchiolar epithelium (arrow) (f) and focal area of necrohemorrhagic bronchitis with angiogenesis (asterisk) at the lamina propria (arrow) with detachment of the bronchial epithelium within the lumen were seen with BoHV‐1. M. bovis‐associated lesions at A and B; BVDV associated lesion at C; and BoHV‐1‐associated lesions at D‐F. Hematoxylin and eosin stain. A, E 50 μm; B‐D, F 200 μm [Colour figure can be viewed at wileyonlinelibrary.com]
The histopathologic features associated with the patterns of pneumonia are summarized in Table 3; these lesions included obliterative bronchiolitis (28.6%; 10/35; Figure 1b), necrotizing bronchitis (18.7%; 6/32) and bronchiolitis (12.5%; 4/32; Figure 1c), syncytial formation (12.5%; 4/32), ballooning degeneration of the bronchial (12.5%; 4/32; Figure 1d), and bronchiolar (3.1%; 1/32; Figure 1e) epithelia, hyperplasia of type II pneumocytes (6.2%; 2/32) and necrohemorrhagic bronchitis with angiogenesis at the lamina propria of the bronchus (6.2%; 2/32; Figure 1f).
3.2. Immunohistochemical identification of viral agents associated with BRD
Tissues from 32 positive cows contained antigens of at least one of the infectious disease pathogens evaluated. The most frequent infectious disease agents identified were BVDV (56.2%; 18/32), M. bovis (50%; 16/32) and BoHV‐1 (43.7%; 14/32), followed by BRSV (34.4%; 11/32) and BPIV‐3 (15.6%; 5/32). Viral infections were identified in 50% (16/32) of the cases without any association with M. bovis, while the other cases (50%; 16/32) were associated with intralesional accumulations of M. bovis. In six cases (18.7%; 6/32), there was the IHC detection of M. bovis in simultaneous disease without the presence of BVDV.
During this investigation, singular and mixed infections were identified (Table 4). However, dual (31.3%; 10/32) and triple (18.7%; 6/32) associations were the most frequent forms at this outbreak, followed by quadruple (6.3%; 2/32) and quintuple (3.1%; 1/32, cow #34) simultaneous association of infectious disease agents associated with BRD. Singular infections were caused by BVDV (15.6%; 5/32), M. bovis (12.5%; 4/32), BRSV (9.4%; 3/32) and BoHV‐1 (3.1%; 1/32); singular infections were not associated with BPIV‐3.
Table 4.
Distribution of the infectious disease agents observed in adult dairy cows with bovine respiratory disease
| Positive immunoreactivity for single and/or combined infections disease pathogens | Number of cows (n = 35) | Agea |
|---|---|---|
| BVDV, BoHV‐1, BRSV, BPIV‐3, M. bovis | 1 | 4 |
| BVDV, BoHV‐1, BRSV, M. bovis | 1 | 5 |
| BRSV, BoHV‐1, BPIV‐3, M. bovis | 1 | 5 |
| BVDV, BRSV, BPIV‐3 | 2 | NI |
| BVDV, BoHV‐1, M. bovis | 3 | 4, 4, 5 |
| BRSV, BoHV‐1, M. bovis | 1 | NI |
| BVDV, BoHV‐1 | 4 | 2, NI |
| BVDV, BPIV‐3 | 1 | 2 |
| BVDV, M. bovis | 1 | 2 |
| BRSV, M. bovis | 2 | 2, 5 |
| BoHV‐1, M. bovis | 2 | 2, NI |
| BVDV | 5 | 2, 2, NI, NI, 4 |
| M. bovis | 4 | 2, 3, NI, NI |
| BRSV | 3 | NI, 2, 2 |
| BoHV‐1 | 1 | 3 |
| Negative to all | 3 | 2, 2, 2 |
NI: not informed.
Abbreviations: BoHV‐1: bovine alphaherpesvirus type 1; BPIV‐3: bovine parainfluenza virus type 3; BRSV: bovine respiratory syncytial virus; BVDV: bovine viral diarrhea virus; M. bovis: Mycoplasma bovis.
Estimated age based on number of calving seasons.
3.3. Correlation between the immunohistochemical identification of BRD pathogens and histopathologic patterns
All antibodies demonstrated cytoplasmic immunolabelling within epithelial cells of bronchi and/or bronchioles of the infected cows. When the histopathologic patterns were correlated with the immunohistochemical identification (Table 5), all cows with obliterative bronchiolitis (100%; 10/10; Figure 2a), necrosuppurative bronchopneumonia with intralesional, Giemsa‐positive, bacterial accumulations within the foci of necrosis (100%; 9/9; Figure 2b) and peribronchial lymphocytic cuffings (100%; 7/7; Figure 2c,d) demonstrated positive immunoreactivity for M. bovis.
Table 5.
Association of principal histopathologic patterns/features and positive immunoreactivity with infectious disease agents of bovine respiratory disease in dairy cows
| Histopathologic patterns/features | Positive immunoreactivity | ||||
|---|---|---|---|---|---|
| M. bovis | BVDV | BoHV‐1 | BRSV | BPIV‐3 | |
| Necrossupurative and suppurative bronchopneumonia | X | ||||
| Obliterative bronchiolitis | X | ||||
| Peribronchial lymphocytic cuffings | X | ||||
| Bronchial angiogenesis | X | ||||
| Ballooning degeneration of bronchial, bronchiolar and alveolar epithelium | X | X | |||
| Necrotizing bronchitis and bronchiolitis | X | X | X | ||
| Necrohemorrhagic bronchitis | X | X | |||
| Hyaline cartilage of the bronchus | X | X | X | X | |
| Mixed peribronchial glands | X | X | X | ||
| Syncytial formation | X | ||||
| Interstitial pneumonia | X | X | X | X | |
Abbreviations: BoHV‐1, bovine alphaherpesvirus type 1; BPIV‐3, bovine parainfluenza virus type 3; BRSV, bovine respiratory syncytial virus; BVDV, bovine viral diarrhea virus; M. bovis, Mycoplasma bovis.
Figure 2.
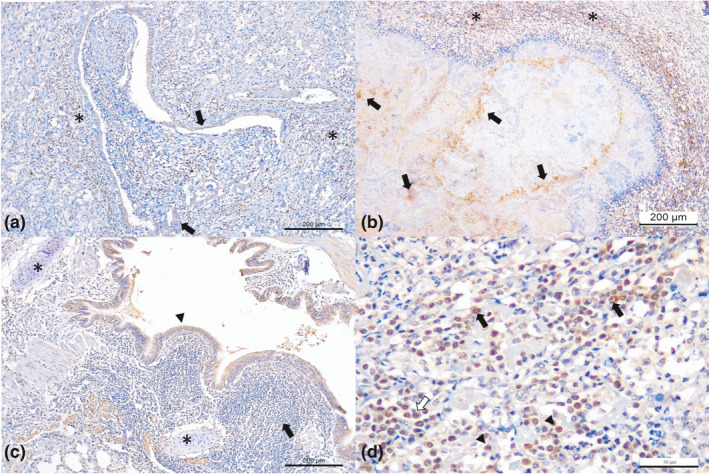
Immunohistochemical findings observed in dairy cattle with bovine respiratory disease associated with Mycoplasma bovis. (a) There is positive intracytoplasmic immunoreactivity to antigens of M. bovis in obliterative bronchiolitis; observe immunoreactivity within epithelial cells of the bronchiole (arrows) and peripheral macrophage (asterisk). (b) There is necrosuppurative bronchopneumonia with intralesional immunolabelling of bacteria within foci of necrosis (arrows), (c) bronchiolar epithelium (arrow head), at the bronchial hyaline cartilage (asterisk) and peribronchial lymphocytic cuffings (arrow). (d) Closer view of peribronquial lymphocytic cuffing demonstrating positive immunoreactivity for M. bovis with macrophages (black arrows), lymphocytes (arrow head) and plasma cells (red arrow). Immunoperoxidase counterstained with haematoxylin. Bar, A–C 200 μm; D 50 μm [Colour figure can be viewed at wileyonlinelibrary.com]
Interstitial pneumonia was associated with all viral disease pathogens evaluated. Positive immunostaining of the vascular endothelia was related with BoHV‐1 (64.3%; 9/14; Figure 3a) and BVDV (11.1%; 2/18; Figure 3b), while hydropic degeneration of vascular endothelia was associated only with positive immunolabelling for BVDV (Figure 3b). Additionally, necrotizing bronchitis/bronchiolitis was associated with BVDV (44.4%; 8/18; Figure 3c), BRSV (54.5%; 6/11; Figure 3d) and BoHV‐1 (15.4%; 2/13). Positive immunoreactivity to chondrocytes of hyaline cartilage of the bronchi was associated with BoHV‐1 (28.6%; 4/14), BRSV (21.4%; 3/14; Figure 3d), BVDV (11.1%; 2/18; Figure 3e) and M. bovis (12.5%; 2/16; Figure 2c). It must be highlighted that one BVDV‐positive cow (#34) was carrying a fetus in the final third semester of gestation when it died, and there was positive immunoreactivity for BVDV at the hyaline cartilage of the bronchus (Figure S1) of this fetus. Syncytial formation (100%; 4/4) and hyperplasia of type II pneumocytes (18.2%; 2/11) demonstrated positive immunoreactivity only for antigens of BRSV.
Figure 3.
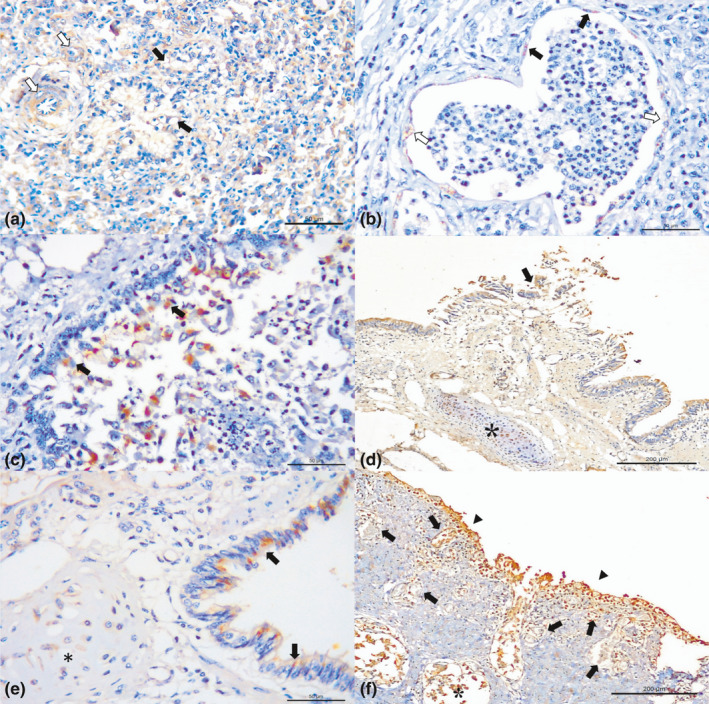
Immunohistochemical findings observed in dairy cattle with bovine respiratory disease. (a) There is positive immunoreactivity to antigens of BoHV‐1 ballooning degeneration of the bronchiole (black arrows) and endothelial staining in alveolar capillaries (white arrows). (b) There is positive immunoreactivity for BVDV at the endothelium of alveolar venule (black arrows), within degenerated endothelial cells (white arrows), and (c) necrotizing bronchiolitis (arrow). (d) Observe positive immunoreactivity for antigens of BRSV at the bronchial epithelium, within detached bronchial epithelial cells (arrow), in bronchial hyaline cartilage (asterisk). (e) Observe positive immunolabelling for antigens of BVDV at the bronchial epithelium and within chondrocytes of the bronchial hyaline cartilage (asterisk). (f) There is positive immunoreactivity associated with BoHV‐1 at the bronchial epithelium of a cow with diffused ulcerative bronchitis and epithelial necrotizing bronchitis (arrows heads), at the newly formed capillaries (arrows) and mixed peribronchial glands (asterisks). Immunoperoxidase counterstained with haematoxylin. Bar, A–C, E 50 μm; D, F 200 μm [Colour figure can be viewed at wileyonlinelibrary.com]
In cases of necrohemorrhagic bronchitis (14.3%; 2/14), an unusual lesion was identified, which was histologically characterized as angiogenesis of the capillaries within the lamina propria of the bronchus and trachea (Figure 3f) that demonstrated positive immunolabelling at the epithelia and endothelial cells for BoHV‐1. Ballooning degeneration of the bronchial and bronchiolar epithelium was associated with positive immunoreactivity for BoHV‐1 (14.3%; 2/14; Figure 3a) and BRSV (1/11; Figure 4a); mixed bronchial glands demonstrated positive immunoreactivity for BVDV (9.1%; 9/18; Figure 4b), BoVH‐1 (21.4%; 3/14) and BRSV (1/11; Figure 4c). Mild interstitial pneumonia was associated with positive immunolabelling for BPIV‐3 at alveolar macrophages (100%; 5/5) and the detached bronchiolar epithelia (60%; 3/5; Figure 4d).
Figure 4.
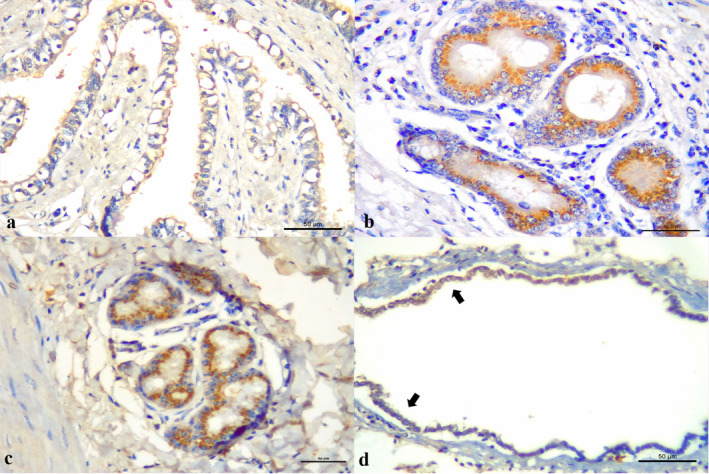
Immunohistochemical findings observed in dairy cows with bovine respiratory disease. (a) There is positive immunoreactivity to antigens of BoHV‐1 within ballooning degenerated bronchial epithelial cells. Observe positive immunoreactivity associated with (b) BVDV and (c) BRSV at the mixed peribronchial glands. (d) There is positive immunolabelling of mildly detached bronchiole epithelium associated with BPIV‐3. Immunoperoxidase counterstained with hematoxylin. Bar, A–D 50 μm [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
The findings of this study have demonstrated the multifactorial nature of BRD during which 59.4% (19/32) of the dairy cows submitted by veterinary clinicians for diagnostic were concomitantly infected by two or more infectious disease agents. Although viral infectious disease agents were the predominant causes (87.5%; 28/32) of BRD, fatal pneumonia associated with M. bovis occurred in 12.5% (4/32) of these cases.
Primary pulmonary infections due to BVDV were demonstrated experimentally (Fulton et al., 2016; Gershwin et al., 2015; Rodríguez et al., 1996) and observed in field outbreaks of BRD (Fulton et al., 2000, 2004; Szeredi, Janosi, & Palfi, 2010). The role of BVDV in the development of the mixed infections herein described, especially with respect to those of bovine pneumonic mycoplasmosis, must be highlighted. Several studies have suggested that synergism between BVDV and M. bovis (Bürgi, Josi, Bürki, Schweizer, & Pilo, 2018; Haines et al., 2001, 2004; Shahriar, Clark, Janzen, West, & Wobeser, 2002) and BVDV and BRSV (Liu, Lehmkuhl, & Kaeberle, 1999) may affect the severity of BRD due to the increased virulence of pathogens caused by synergy in coinfections (Ridpath, 2010). This aspect is relevant not only for the interpretation of experimental data, but because BVDV may interact with M. bovis in field conditions (Bürgi et al., 2018). Additionally, some strains of BVDV are immunosuppressive in cattle, due to the loss of alveolar macrophage functionality (Bürgi et al., 2018; Welsh, Adair, & Foster, 1995) and severe and extensive apoptosis of lymphocytes (Chase, 2013). Consequently, immunosuppression due to BVDV associated with BoHV‐1, BRSV and M. bovis may have resulted in an increased severity of disease in this dairy herd, due to incapacity of the innate immune system to control these infections.
In our study, several chondrocytes of the hyaline cartilage of the bronchus demonstrated positive immunoreactivity for BRSV, BoHV‐1 and BVDV, with diffused immunoreactivity to BVDV occurring within the chondrocytes of two cows. Similar positive immunoreactivity to BVDV was described in 60% (6/10) of persistently infected (PI) calves, where it was proposed that the presence of BVDV antigen in respiratory cartilage is an indication that this viral disease pathogen predisposes cows to secondary bacterial infection (Confer, Fulton, Step, Johnson, & Ridpath, 2005). In the cases herein described, we were unable to confirm these cows as being PI animals since two biological samples were not available for testing. However, there was positive immunoreactivity to BVDV (using Mab 15c5) within the chondrocytes of the hyaline cartilage of the bronchus of the fetus of one of these cows. Collectively, these findings may suggest that the fetus of the gravid cow and probably the cow itself were PI animals; moreover, positive immunoreactivity to BVDV within the pulmonary cartilage of these two cows can correlate with the occurrence of simultaneous infections in these animals (Confer et al., 2005). Additionally, it must be highlighted that the MAb used during this study to identify intralesional antigens of BVDV has elevated specificity and sensitivity for the diagnosis of this infectious disease pathogen (Haines, Clark, & Dubovi, 1992).
The results of this study suggest that M. bovis‐associated pneumonias might be important causes of BRD associated mortality in dairy cattle from Brazil. Until the present moment, the authors are unaware of studies done in Brazil that confirmed BRD due to infections by M. bovis using pulmonary tissues derived from adult dairy cattle with fatal pulmonary disease. Two studies have previously identified the presence of M. bovis associated with BRD in Brazil. The first identified M. bovis‐associated pulmonary disease in 26.1% (12/46) of calves due to a combination of bacterial culture and direct immunofluorescence using lung sections (Pretto et al., 2001). While the other amplified nucleic acids of M. bovis from 5.6% (1/18) of nasal swabs from dairy cows (Tortorelli et al., 2017). The marked difference between these and the current study was the confirmation of disease (Fulton & Confer, 2012), in our investigation due to the intralesional detection of antigens of M. bovis by IHC and intralesional accumulations of bacteria by the Giemsa stain, while the frequency of disease was more elevated. Additionally, during this investigation IHC assays were done from pulmonary tissues with gross evidence of disease, which may have also contributed towards the elevated number of positive cases, considering the elevated sensitivity of this diagnostic technique to identify intralesional antigens of infectious disease pathogens (Fulton & Confer, 2012). Since positive Giemsa staining was observed in most pulmonary sections that contained intralesional antigens of M. bovis by IHC, we postulate that Giemsa staining may be an adequate but simple method to characterize infections associated with this pathogen.
Necrosuppurative bronchopneumonia and peribronchial lymphocytic cuffings are the hallmarks of chronic pulmonary mycoplasmosis in cattle (Nicholas & Ayling, 2003) and calves (Hermeyer et al., 2012; Khodakaram‐Tafti & López, 2004), and were the main findings observed in the affected dairy cows during this study. These findings were previously described (Caswell & Archambault, 2007; Gagea et al., 2006; Haines et al., 2004) and demonstrate the ability of M. bovis to invade the pulmonary parenchyma, as was observed in experimental (Gershwin et al., 2015; Hermeyer et al., 2012; Rodríguez et al., 1996; Thomas, Howard, Stott, & Parsons, 1986) and spontaneous (Gagea et al., 2006) cases of pulmonary disease in cattle. Furthermore, M. bovis may induce cytotoxicity and induction of apoptosis of the alveolar macrophage (Bürgi et al., 2018), and neutrophils, inhibit the production of nitric oxide in cows (Jimbo et al., 2017) and infects a wide range of epithelial and immune cells (Bürki, Frey, & Pilo, 2015). These mechanisms then favour the dissemination of M. bovis and would have facilitated simultaneous infections in this herd with BVDV, BoHV‐1 and BRSV.
The silent immunomodulatory and immunosuppressive effects of M. bovis predisposes the respiratory tract of calves to other bacterial infections (Margineda et al., 2017; Nicholas, 2011; Poumarat et al., 2001). These effects have been demonstrated experimentally (Poumarat et al., 2001; Rodríguez et al., 1996; Vanden Bush & Rosenbusch, 2003) and were described in spontaneous cases (Haines et al., 2004; Rodríguez et al., 1996; Yİlmaz et al., 2016), and there are reports of pneumonic diseases in which M. bovis was the only pathogen identified (Gershwin et al., 2015; Nicholas, 2011). These findings may suggest that M. bovis can act as a primary disease pathogen and produce BRD. However, the possibility of M. bovis being a primary disease agent is still controversial, since this agent may colonize and perpetuate pulmonary lesions that were initiated by other bacteria, such as M. haemolytica or others virus (Caswell, Bateman, Cai, & Castillo‐Alcala, 2010). Nevertheless, the immunosuppressive nature of BVDV‐associated infections cannot be overlooked as contributing towards the development of BRD in these cows, since in most cases of combined infections associated with M. bovis, intralesional antigens of BVDV were detected.
As far as the authors are aware, this manuscript may represent the first description of M. bovis‐related BRD in dairy cows. This pathogen was frequently associated with several disease syndromes in dairy calves (Mahmood et al., 2017) and feedlot cattle (Caswell & Archambault, 2007; Haines et al., 2001), including descriptions of M. bovis‐related pneumonia (Gagea et al., 2006; Mahmood et al., 2017), but we did not locate reports of M. bovis‐induced BRD in adult dairy cows on searching major databases. Furthermore, M. bovis was not identified in recent studies done by our group to identify this pathogen associated with BRD in feedlot cattle from different geographical regions of Brazil (Baptista et al., 2017; Headley et al., 2014, 2018). Additionally, there are only two reports (Pretto et al., 2001; Tortorelli et al., 2017) of M. bovis‐associated pulmonary disease in cattle from this country. Although the exact reasons for the low detection rate of M. bovis‐associated BRD in Brazil are unknown, we believe that the reduced identification of this agent may be related to the diagnostic strategy used and the type of cattle evaluated, that is beef against dairy. All previous cases of M. bovis‐associated BRD were diagnosed in dairy cows (Pretto et al., 2001; Tortorelli et al., 2017), while this bacterial pathogen was not identified in studies done with beef cattle (Headley et al., 2014, 2017, 2018). It must be highlighted that nasal swabs were tested by PCR in the beef cattle surveys done by our group which resulted in negative results; however, these negative results could have been associated with the sporadic elimination of M. bovis so that PCR testing would not be an efficient method to detect this pathogen (Nicholas, 2011). This sporadic shedding of M. bovis may also explain the low results (5.6%; 1/18) obtained in a study done by another group from Brazil that used the same molecular testing strategy in dairy cattle (Tortorelli et al., 2017), as compared with the elevated results (26.1%; 12/46) obtained by bacterial culture and immunofluorescence assay using pulmonary tissue with characteristic lesions (Pretto et al., 2001). Since pulmonary mycoplasmosis is a chronic disease (Caswell & Archambault, 2007), dairy cows that are maintained for longer durations at a herd would be more prone to develop this infection as compared with beef cattle which are younger on entering feedlots and are maintained on feed for approximately 120–150 days. Moreover, the higher detection rate in dairy cattle as opposed to beef cattle may be because the dairy cows, herein described, could have probably demonstrated extra‐pulmonary manifestations of mycoplasmosis, such as mastitis and reproductive diseases, with concomitant pulmonary disease. Lastly, pulmonary mycoplasmosis of cattle may probably be underdiagnosed in Brazil, considering that isolation can be difficult in severe chronic cases (Nicholas & Ayling, 2003), the sporadic shedding of M. bovis (Nicholas, 2011) in affected cows, and the molecular testing frequently used to identify this pathogen in association with BRD. Consequently, this pathogen should be suspected in dairy cows with clinical manifestations of respiratory distress and be included in the differential diagnosis of infectious disease agents associated with BRD in adult dairy cattle from Brazil. Moreover, pulmonary tissue rather than nasal swabs may be more efficient to diagnose M. bovis in dairy cattle.
Necrohemorrhagic bronchitis with bronchial angiogenesis was exclusively associated with infections induced by BoHV‐1 in this study. We have not seen this lesion previously described in the development of pulmonary disease. It was demonstrated that BoHV‐1 infects bronchial epithelial cells, induced increase in neutrophil adhesion and activation, and the infection elicits a rapid secreting proinflammatory cytokines such as interleukin 1 (IL‐1), IL‐8 and tumour necrosis factor (TNF‐α), causing the influx of neutrophils, degranulation and tissue necrosis (Rivera‐Rivas, Kisiela, & Czuprynski, 2009). Although the pathogenesis of angiogenesis observed at the lamina propria of the bronchus with ulcerative or necrosis induced by BoHV‐1 is not fully elucidated, these cytokines may be associated with the development of this lesion due to inflammatory reaction, and necrosis; in addition, IL‐8 has angiogenic and repair qualities (Koch et al., 1992). Consequently, further studies are needed to understand the pathophysiology and the importance of this unique lesion.
Few studies have demonstrated synergism of BRSV with BVDV (Liu et al., 1999), H. somni (Agnes et al., 2013; Headley et al., 2017) and M. bovis (Thomas et al., 1986). Experimentally it was demonstrated that this synergism induced alveolar cell retraction and increased degradation of collagen by TNF‐α (Agnes et al., 2013). Both mechanisms may facilitate pulmonary damage resulting in subsequent pneumonia and the dissemination of the infectious pathogen (Agnes et al., 2013). Additionally, BRSV infects type I and type II pneumocytes (Bryson, McConnell, McAliskey, & McNulty, 1991), with consequent apoptosis to these cells (Viuff et al., 2002), and produces necrosis of alveoli and small airways (Bryson et al., 1991), due to alveolar neutrophilic exudation (Agnes et al., 2013), resulting in suppurative alveolitis/bronchiolitis and necrotizing bronchiolitis/bronchitis (Andrews & Kennedy, 1997; Brasil et al., 2013; Gershwin et al., 2015; Peixoto et al., 2000). Moreover, during this investigation, hyperplasia of type II pneumocytes was observed in association only with positive immunoreactivity to BRSV; similar findings were previously associated with chronic epithelial injury in the proximal alveolar region (Barry, Miller, & Crapo, 1985) and identified in beef cattle with chronic pulmonary disease (Driemeier et al., 1997). The low number of cases with syncytial formation (4/11) in the present study may be due to the fact that the manifestations were chronic (Driemeier et al., 1997). These histopathologic findings were observed in cattle infected by BRSV during this investigation and may have contributed to the increased severity of pulmonary lesions observed in the dairy cows herein described.
5. CONCLUSIONS
This is the first report from Brazil that demonstrated and correlated the histopathologic findings of bovine respiratory disease by immunohistochemistry. Necrosuppurative bronchopneumonia, obliterative bronchiolitis and peribronchial lymphocytic cuffings were the characteristic histopathologic features associated with mycoplasma pneumonia. Necrotizing bronchitis and bronchiolitis were associated with BVDV, BoHV‐1 and BRSV. Necrohemorrhagic bronchitis with bronchial angiogenesis was associated with infection by BoHV‐1. M. bovis was commonly detected in the tissues of fatal pulmonary disease during this outbreak and may be a possible primary disease pathogen associated with the development of bovine respiratory disease. Moreover, the histopathologic features observed within the patterns of pulmonary disease may be an excellent diagnostic tool to identify some infectious disease agents of BRD in dairy cattle.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Supporting information
ACKNOWLEDGEMENTS
The authors are grateful for the monoclonal antibody against BVDV used during this investigation, gently provided by Dr. Ruben O. Donis, University of Nebraska, Lincoln, USA. The authors thank the National Institutes of Science and Technology ‐ Dairy Production Chain (INCT‐Leite; Brazil), the Brazilian National Counsel of Scientific and Technological Development (CNPq; Brazil), and the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES; Brazil) for financial support. Oliveira, TES, Pelaquim, IF, Flores, EF, Alfieri, AA, Saut, JPE, and Headley, SA are recipients of CNPq Fellowships. Massi, RP and Valdiviezo, MJJ are recipients of CAPES Fellowships.
Oliveira TES, Pelaquim IF, Flores EF, et al. Mycoplasma bovis and viral agents associated with the development of bovine respiratory disease in adult dairy cows. Transbound Emerg Dis.2020;67:82–93. 10.1111/tbed.13223;
Part of the PhD thesis of the first author (TESO).
REFERENCES
- Agnes, J. T. , Zekarias, B. , Shao, M. , Anderson, M. L. , Gershwin, L. J. , & Corbeil, L. B. (2013). Bovine respiratory syncytial virus and Histophilus somni interaction at the alveolar barrier. Infection and Immunity, 81, 2592–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews, G. A. , & Kennedy, G. A. (1997). Respiratory diagnostic pathology. Veterinary Clinics of North America: Food Animal Practice, 13, 515–547. [DOI] [PubMed] [Google Scholar]
- Arns, C. W. , Campalans, J. , Costa, S. C. B. , Domingues, H. G. , D'Arce, R. C. F. , & Almeida, R. S. (2003). Characterization of bovine respiratory syncytial virus isolated in Brazil. Brazilian Journal of Medical and Biological Research, 36, 213–218. [DOI] [PubMed] [Google Scholar]
- Baptista, A. L. , Rezende, A. L. , Fonseca, P. A. , Massi, R. P. , Nogueira, G. M. , Magalhães, L. Q. , … Saut, J. P. E. (2017). Bovine respiratory disease complex associated mortality and morbidity rates in feedlot cattle from southeastern Brazil. The Journal of Infection in Developing Countries, 11, 791–799. [DOI] [PubMed] [Google Scholar]
- Barbosa, A. C. V. C. , Brito, W. M. E. D. , & Alfaia, B. T. (2005). Seroprevalence and risk factors to the infectious by bovine herpesvirus type 1 (BHV‐1) in Goiás State, Brazil (in Portuguese). Ciência Rural, 35, 1368–1373. [Google Scholar]
- Barry, B. E. , Miller, F. J. , & Crapo, J. D. (1985). Effects of inhalation of 0.12 and 0.25 parts per million ozone on the proximal alveolar region of juvenile and adult rats. Laboratory Investigation, 53, 692–704. [PubMed] [Google Scholar]
- Brasil, N. D. A. , Hinnah, F. L. , Fiss, L. , Sallis, E. S. V. , Grecco, F. B. , Ladeira, S. R. L. , … Schild, A. L. (2013). Respiratory diseases in calves in southern Rio Grande do Sul: Study of 33 outbreaks (in Portuguese). Pesquisa Veterinária Brasileira, 33, 745–751. [Google Scholar]
- Bryson, D. G. , McConnell, S. , McAliskey, M. , & McNulty, M. S. (1991). Ultrastructural features of alveolar lesions in induced respiratory syncytial virus pneumonia of calves. Veterinary Pathology, 28, 286–292. [DOI] [PubMed] [Google Scholar]
- Bürgi, N. , Josi, C. , Bürki, S. , Schweizer, M. , & Pilo, P. (2018). Mycoplasma bovis co‐infection with bovine viral diarrhea virus in bovine macrophages. Veterinary Research, 49, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürki, S. , Frey, J. , & Pilo, P. (2015). Virulence, persistence and dissemination of Mycoplasma bovis . Veterinary Microbiology, 179, 15–22. [DOI] [PubMed] [Google Scholar]
- Caswell, J. L. , & Archambault, M. (2007). Mycoplasma bovis pneumonia in cattle. Animal Health Research Reviews, 8, 161–186. [DOI] [PubMed] [Google Scholar]
- Caswell, J. L. , Bateman, K. G. , Cai, H. Y. , & Castillo‐Alcala, F. (2010). Mycoplasma bovis in respiratory disease of feedlot cattle. Veterinary Clinics of North America: Food Animal Practice, 26, 365–379. [DOI] [PubMed] [Google Scholar]
- Chase, C. C. (2013). The impact of BVDV infection on adaptive immunity. Biologicals: Journal of the International Association of Biological Standardization, 41, 52–60. [DOI] [PubMed] [Google Scholar]
- Confer, A. W. , Fulton, R. W. , Step, D. L. , Johnson, B. J. , & Ridpath, J. F. (2005). Viral antigen distribution in the respiratory tract of cattle persistently infected with bovine viral diarrhea virus subtype 2a. Veterinary Pathology, 42, 192–199. [DOI] [PubMed] [Google Scholar]
- Cortez, A. , Heinemann, M. B. , Castro, A. M. M. G. , Soares, R. M. , Pinto, A. M. V. , Alfieri, A. A. , … Richtzenhain, L. J. (2006). Genetic characterization of Brazilian bovine viral diarrhea virus Genetic characterization of Brazilian bovine viral diarrhea virus isolates by partial nucleotide sequencing of the 5′‐UTR region. Pesquisa Veterinária Brasileira, 26, 211–216. [Google Scholar]
- Cusack, P. M. , McMeniman, N. , & Lean, I. J. (2003). The medicine and epidemiology of bovine respiratory disease in feedlots. Australian Veterinary Journal, 81, 480–487. [DOI] [PubMed] [Google Scholar]
- Driemeier, D. , Gomes, M. J. P. , Moojen, V. , Arns, C. W. , Vogg, G. , Kessler, L. , & Costa, U. M. (1997). Clinic‐pathological aspects in the natural infection of Bovine Respiratory Syncytial Virus (BRVS) in extensive management of cattle in Rio Grande do Sul, Brazil (in Portuguese). Pesquisa Veterinária Brasileira, 17, 77–81. [Google Scholar]
- Fernandes, L. G. , Pimenta, C. L. R. M. , Pituco, E. M. , Brasil, A. W. L. , & Azevedo, S. S. (2016). Risk factors associated with BoHV‐1 and BVDV seropositivity in buffaloes (Bubalus bubalis) from the State of Paraiba, Northeastern Brazil. Semina: Ciências Agrárias, 37, 1929–1936. [Google Scholar]
- Flores, E. F. , Ridpath, J. F. , Weiblen, R. , Vogel, F. S. , & Gil, L. H. (2002). Phylogenetic analysis of Brazilian bovine viral diarrhea virus type 2 (BVDV‐2) isolates: Evidence for a subgenotype within BVDV‐2. Virus Research, 87, 51–60. [DOI] [PubMed] [Google Scholar]
- Flores, E. F. , Weiblen, R. , Flores‐Vogel, F. S. , Roehe, P. M. , Alfieri, A. A. , & Pituco, E. M. (2005). Bovine viral diarrhea virus (BVDV) infection in Brazil: History, current situation and perspectives (in Portuguese). Pesquisa Veterinária Brasileira, 25, 125–134. [Google Scholar]
- Fulton, R. W. (2009). Bovine respiratory disease research (1983‐2009). Animal Health Research Reviews, 10, 131–139. [DOI] [PubMed] [Google Scholar]
- Fulton, R. W. , Blood, K. S. , Panciera, R. J. , Payton, M. E. , Ridpath, J. F. , Confer, A. W. , … Reck, A. (2009). Lung pathology and infectious agents in fatal feedlot pneumonias and relationship with mortality, disease onset, and treatments. Journal of Veterinary Diagnostic Investigation, 21, 464–477. [DOI] [PubMed] [Google Scholar]
- Fulton, R. W. , Briggs, R. E. , Payton, M. E. , Confer, A. W. , Saliki, J. T. , Ridpath, J. F. , … Duff, G. C. (2004). Maternally derived humoral immunity to bovine viral diarrhea virus (BVDV) 1a, BVDV1b, BVDV2, bovine herpesvirus‐1, parainfluenza‐3 virus bovine respiratory syncytial virus, Mannheimia haemolytica and Pasteurella multocida in beef calves, antibody decline by half‐life studies and effect on response to vaccination. Vaccine, 22, 643–649. [DOI] [PubMed] [Google Scholar]
- Fulton, R. W. , & Confer, A. W. (2012). Laboratory test descriptions for bovine respiratory disease diagnosis and their strengths and weaknesses: Gold standards for diagnosis, do they exist? Canadian Journal of Veterinary Research, 53, 754–761. [PMC free article] [PubMed] [Google Scholar]
- Fulton, R. W. , d'Offay, J. M. , Landis, C. , Miles, D. G. , Smith, R. A. , Saliki, J. T. , … Payton, M. E. (2016). Detection and characterization of viruses as field and vaccine strains in feedlot cattle with bovine respiratory disease. Vaccine, 34, 3478–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton, R. W. , Purdy, C. W. , Confer, A. W. , Saliki, J. T. , Loan, R. W. , Briggs, R. E. , & Burge, L. J. (2000). Bovine viral diarrhea viral infections in feeder calves with respiratory disease: Interactions with Pasteurella spp., parainfluenza‐3 virus, and bovine respiratory syncytial virus. Canadian Journal of Veterinary Research, 64, 151–159. [PMC free article] [PubMed] [Google Scholar]
- Gagea, M. I. , Bateman, K. G. , Shanahan, R. A. , van Dreumel, T. , McEwen, B. J. , Carman, S. , … Caswell, J. L. (2006). Naturally occurring Mycoplasma bovis‐associated pneumonia and polyarthritis in feedlot beef calves. Journal of Veterinary Diagnostic Investigation, 18, 29–40. [DOI] [PubMed] [Google Scholar]
- Gershwin, L. J. , Van Eenennaam, A. L. , Anderson, M. L. , McEligot, H. A. , Shao, M. X. , Toaff‐Rosenstein, R. , … Womack, J. (2015). Single pathogen challenge with agents of the bovine respiratory disease complex. PLoS ONE, 10, e0142479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves, D. A. , Spilki, F. R. , Chiminazzo, C. , Oliveira, M. A. , Franco, A. C. , & Roehe, P. M. (2003). Isolation of bovine Parainfluenza virus type 3 in Rio Grande do Sul, Brazil (in Portuguese). Ciência Rural, 33, 953–956. [Google Scholar]
- Haines, D. M. , Clark, E. G. , & Dubovi, E. J. (1992). Monoclonal antibody‐based immunohistochemical detection of bovine viral diarrhea virus in formalin‐fixed, paraffin‐embedded tissues. Veterinary Pathology, 29, 27–32. [DOI] [PubMed] [Google Scholar]
- Haines, D. M. , Martin, K. M. , Clark, E. G. , Jim, G. K. , & Janzen, E. D. (2001). The immunohistochemical detection of Mycoplasma bovis and bovine viral diarrhea virus in tissues of feedlot cattle with chronic, unresponsive respiratory disease and/or arthritis. Canadian Journal of Veterinary Research, 42, 857–860. [PMC free article] [PubMed] [Google Scholar]
- Haines, D. M. , Moline, K. M. , Sargent, R. A. , Campbell, J. R. , Myers, D. J. , & Doig, P. A. (2004). Immunohistochemical study of Hemophilus somnus, Mycoplasma bovis, Mannheimia hemolytica, and bovine viral diarrhea virus in death losses due to myocarditis in feedlot cattle. Canadian Journal of Veterinary Research, 45, 231–234. [PMC free article] [PubMed] [Google Scholar]
- Hay, K. E. , Morton, J. M. , Mahony, T. J. , Clements, A. C. , & Barnes, T. S. (2016). Associations between animal characteristic and environmental risk factors and bovine respiratory disease in Australian feedlot cattle. Preventive Veterinary Medicine, 125, 66–74. [DOI] [PubMed] [Google Scholar]
- Headley, S. A. , Alfieri, A. F. , Oliveira, V. H. , Beuttemmuller, E. A. , & Alfieri, A. A. (2014). Histophilus somni is a potential threat to beef cattle feedlots in Brazil. Veterinary Record, 175, 249. [DOI] [PubMed] [Google Scholar]
- Headley, S. A. , Balbo, L. C. , Alfieri, A. F. , Baptista, A. L. , Saut, J. P. E. , & Alfieri, A. A. (2017). Bovine respiratory disease associated with Histophilus somni and bovine respiratory syncytial virus in a beef cattle feedlot from Southeastern Brazil. Semina: Ciêncas Agrárias, 38, 283–294. [Google Scholar]
- Headley, S. A. , Okano, W. , Balbo, L. C. , Marcasso, R. A. , Oliveira, T. E. , Alfieri, A. F. , … Alfieri, A. A. (2018). Molecular survey of infectious agents associated with bovine respiratory disease in a beef cattle feedlot in southern Brazil. Journal of Veterinary Diagnostic Investigation, 30, 249–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermeyer, K. , Buchenau, I. , Thomasmeyer, A. , Baum, B. , Spergser, J. , Rosengarten, R. , & Hewicker‐Trautwein, M. (2012). Chronic pneumonia in calves after experimental infection with Mycoplasma bovis strain 1067: Characterization of lung pathology, persistence of variable surface protein antigens and local immune response. Acta Veterinaria Scandinavica, 54, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimbo, S. , Suleman, M. , Maina, T. , Prysliak, T. , Mulongo, M. , & Perez‐Casal, J. (2017). Effect of Mycoplasma bovis on bovine neutrophils. Veterinary Immunology and Immunopathology, 188, 27–33. [DOI] [PubMed] [Google Scholar]
- Khodakaram‐Tafti, A. , & López, A. (2004). Immunohistopathological findings in the lungs of calves naturally infected with Mycoplasma bovis . Journal of Veterinary Medicine A, Physiology, Pathology, Clinical Medicine, 51, 10–14. [DOI] [PubMed] [Google Scholar]
- Koch, A. E. , Polverini, P. J. , Kunkel, S. L. , Harlow, L. A. , DiPietro, L. A. , Elner, V. M. , … Strieter, R. M. (1992). Interleukin‐8 as a macrophage‐derived mediator of angiogenesis. Science, 258, 1798–1801. [DOI] [PubMed] [Google Scholar]
- Liu, L. , Lehmkuhl, H. D. , & Kaeberle, M. L. (1999). Synergistic effects of bovine respiratory syncytial virus and non‐cytopathic bovine viral diarrhea virus infection on selected bovine alveolar macrophage functions. Canadian Journal of Veterinary Research, 63, 41–48. [PMC free article] [PubMed] [Google Scholar]
- Lunardi, M. , Headley, S. A. , Lisboa, J. A. , Amude, A. M. , & Alfieri, A. A. (2008). Outbreak of acute bovine viral diarrhea in Brazilian beef cattle: Clinicopathological findings and molecular characterization of a wild‐type BVDV strain subtype 1b. Research in Veterinary Science, 85, 599–604. [DOI] [PubMed] [Google Scholar]
- Maes, R. K. , Langohr, I. M. , Wise, A. G. , Smedley, R. C. , Thaiwong, T. , & Kiupel, M. (2013). Beyond H&E: Integration of nucleic acid–based analyses into diagnostic pathology. Veterinary Pathology, 51, 238–256. [DOI] [PubMed] [Google Scholar]
- Mahmood, F. , Khan, A. , Hussain, R. , Khan, I. A. , Abbas, R. Z. , Ali, H. M. , & Younus, M. (2017). Patho‐bacteriological investigation of an outbreak of Mycoplasma bovis infection in calves ‐ emerging stealth assault. Microbial Pathogenesis, 107, 404–408. [DOI] [PubMed] [Google Scholar]
- Margineda, C. A. , Zielinski, G. O. , Jurado, S. , Alejandra, F. , Mozgovoj, M. , Alcaraz, A. C. , & López, A. (2017). Mycoplasma bovis pneumonia in feedlot cattle and dairy calves in Argentina. Brazilian Journal of Veterinary Pathology, 10, 79–86. [Google Scholar]
- Nicholas, R. A. (2011). Bovine mycoplasmosis: Silent and deadly. Veterinary Record, 168, 459–462. [DOI] [PubMed] [Google Scholar]
- Nicholas, R. A. , & Ayling, R. D. (2003). Mycoplasma bovis: Disease, diagnosis, and control. Research in Veterinary Science, 74, 105–112. [DOI] [PubMed] [Google Scholar]
- Oliveira, R. A. M. , Lorenzetti, E. , Alfieri, A. A. , & Lisbôa, J. A. N. (2015). Prevalence of latent infection with BoHV‐1 and BoHV‐5 in beef cattle of Parana, Brazil (in Portuguese). Arquivo Brasileiro de Medicina Veterinária e Zootecnia, 67, 1217–1225. [Google Scholar]
- Otonel, R. A. , Alfieri, A. F. , Dezen, S. , Lunardi, M. , Headley, S. A. , & Alfieri, A. A. (2014). The diversity of BVDV subgenotypes in a vaccinated dairy cattle herd in Brazil. Tropical Animal Health and Production, 46, 87–92. [DOI] [PubMed] [Google Scholar]
- Panciera, R. J. , & Confer, A. W. (2010). Pathogenesis and pathology of bovine pneumonia. Veterinary Clinics of North America: Food Animal Practice, 26, 191–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto, P. V. , Mota, R. A. , Brito, M. F. , Corbellini, L. G. , Driemeier, D. , & Souza, M. I. (2000). Spontaneous BRSV infection in cattle of the state of Alagoas, Brazil Spontaneous BRSV infection in cattle of the state of Alagoas, Brazil (in Portuguese). Pesquisa Veterinária Brasileira, 20, 171–175. [Google Scholar]
- Poumarat, F. , Le Grand, D. , Philippe, S. , Calavas, D. , Schelcher, F. , Cabanie, P. , … Navetat, H. (2001). Efficacy of spectinomycin against Mycoplasma bovis induced pneumonia in conventionally reared calves. Veterinary Microbiology, 80, 23–35. [DOI] [PubMed] [Google Scholar]
- Pretto, L. G. , Müller, E. E. , Freitas, J. C. , Mettifoga, E. , Buzinhani, M. , & Yamaguti, M. (2001). Mycoplasma bovis isolation from calves with pneumonia (in Portuguese). Veterinária Notícias, 7, 69–73. [Google Scholar]
- Ridpath, J. (2010). The contribution of infections with Bovine viral diarrhea viruses to bovine respiratory disease. Veterinary Clinics of North America: Food Animal Practice, 26, 335–348. [DOI] [PubMed] [Google Scholar]
- Rivera‐Rivas, J. J. , Kisiela, D. , & Czuprynski, C. J. (2009). Bovine herpesvirus type 1 infection of bovine bronchial epithelial cells increases neutrophil adhesion and activation. Veterinary Immunology and Immunopathology, 131, 167–176. [DOI] [PubMed] [Google Scholar]
- Rodríguez, F. , Bryson, D. G. , Ball, H. J. , & Forster, F. (1996). Pathological and immunohistochemical studies of natural and experimental Mycoplasma bovis pneumonia in calves. Journal of Comparative Pathology, 115, 151–162. [DOI] [PubMed] [Google Scholar]
- Shahriar, F. M. , Clark, E. G. , Janzen, E. , West, K. , & Wobeser, G. (2002). Coinfection with bovine viral diarrhea virus and Mycoplasma bovis in feedlot cattle with chronic pneumonia. Canadian Journal of Veterinary Research, 43, 863–868. [PMC free article] [PubMed] [Google Scholar]
- Silveira, S. , Weber, M. N. , Mosena, A. C. , da Silva, M. S. , Streck, A. F. , Pescador, C. A. , … Canal, C. W. (2017). Genetic diversity of brazilian bovine pestiviruses detected between 1995 and 2014. Transboundary and Emerging Diseases, 64, 613–623. [DOI] [PubMed] [Google Scholar]
- Suarez Heinlein, A. , Metzler, A. E. , Weiblen, R. , Berrios, P. , Schudel, A. A. , & Rodriguez, M. (1993). Molecular characterization of South American bovine herpesvirus‐1 isolates with monoclonal antibodies and SDS‐PAGE. Journal of Veterinary Medicine B, Infectious Diseases and Veterinary Public Health, 40, 125–130. [DOI] [PubMed] [Google Scholar]
- Szeredi, L. , Janosi, S. , & Palfi, V. (2010). Microbiological and pathological examination of fatal calf pneumonia cases induced by bacterial and viral respiratory pathogens. Acta Veterinaria Hungarica, 58, 341–356. [DOI] [PubMed] [Google Scholar]
- Thomas, L. H. , Howard, C. J. , Stott, E. J. , & Parsons, K. R. (1986). Mycoplasma bovis infection in gnotobiotic calves and combined infection with respiratory syncytial virus. Veterinary Pathology, 23, 571–578. [DOI] [PubMed] [Google Scholar]
- Tortorelli, G. , Carrillo Gaeta, N. , Mendonça Ribeiro, B. L. , Miranda Marques, L. , Timenetsky, J. , & Gregory, L. (2017). Evaluation of Mollicutes microorganisms in respiratory disease of cattle and their relationship to clinical signs. Journal of Veterinary Internal Medicine, 31, 1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Bush, T. J. , & Rosenbusch, R. F. (2003). Characterization of the immune response to Mycoplasma bovis lung infection. Veterinary Immunology and Immunopathology, 94, 23–33. [DOI] [PubMed] [Google Scholar]
- Viuff, B. , Tjornehoj, K. , Larsen, L. E. , Rontved, C. M. , Uttenthal, A. , Ronsholt, L. , & Alexandersen, S. (2002). Replication and clearance of respiratory syncytial virus: Apoptosis is an important pathway of virus clearance after experimental infection with bovine respiratory syncytial virus. The American Journal of Pathology, 161, 2195–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wageck Canal, C. , Strasser, M. , Hertig, C. , Masuda, A. , & Peterhans, E. (1998). Detection of antibodies to bovine viral diarrhoea virus (BVDV) and characterization of genomes of BVDV from Brazil. Veterinary Microbiology, 63, 85–97. [DOI] [PubMed] [Google Scholar]
- Welsh, M. D. , Adair, B. M. , & Foster, J. C. (1995). Effect of BVD virus infection on alveolar macrophage functions. Veterinary Immunology and Immunopathology, 46, 195–210. [DOI] [PubMed] [Google Scholar]
- Wolfger, B. , Timsit, E. , White, B. J. , & Orsel, K. (2015). A systematic review of bovine respiratory disease diagnosis focused on diagnostic confirmation, early detection, and prediction of unfavorable outcomes in feedlot cattle. Veterinary Clinics of North America: Food Animal Practice, 31, 351–365. [DOI] [PubMed] [Google Scholar]
- Yİlmaz, R. , Cangul, I. T. , Onat, K. , Akkoc, A. , Ozyİgİt, M. O. , & Akdesİr, E. (2016). Histopathological, immunohistochemical and bacteriological characterization of Mycoplasma bovis pneumonia in cattle. Pakistan Veterinary Journal, 36, 316–321. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


