To the Editor,
I read with great interest the paper published ahead‐of‐print in the Journal of Medical Virology by Liu et al 1 on comparisons of the spike sequences between severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and SARS‐CoV, bat SARS‐like CoV, and other coronaviruses, supporting the fact that snakes, pangolins, also turtles may act as potential intermediate hosts that transmit SARS‐CoV‐2 to humans. As we do believe that the evolutionary analysis and finding on the possible virus reservoirs are helpful in understanding the diffusion of the novel 2019 coronavirus (SARS‐CoV‐2) causing the coronavirus disease (COVID‐19), with this letter I want to discuss the implications that descend from the coincidence of the virus binding site with the membrane angiotensin‐converting enzyme 2 (ACE2) receptor. 2
As it was previously stated, for the establishment of SARS coronavirus infections, the endocytosis of virus particles into host cells is needed. Surprisingly, the ACE2 has been identified as a functional receptor, 3 as ACE2–Spike interaction is the mechanisms that lead through a process of internalization with ACE2, to the endocytosis 4 (Figure 1). This mechanism is shared with all SARS coronavirus family that, now, includes the SARS‐CoV‐2 causing the severe COVID‐19.
Figure 1.
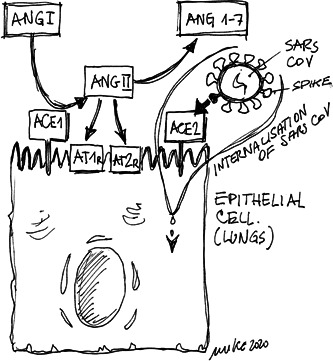
Internalisation of SARS‐CoV into an epithelial cell by using ACE2 as functional receptor. ACE1, angiotensin‐converting‐enzyme 1; ACE2, angiotensin‐converting‐enzyme 2; ANG I, angiotensin I; ANG II, angiotensin II; ANG 1–7, angiotensin from 1 to 7; AT1r, angiotensin receptor 1; AT2r, angiotensin receptor 2
According to the cardiologists, ACE, which belongs to the renin–angiotensin–aldosterone system (RAAS), is involved, at multiple levels, in blood pressure control and, for example, myocardial fibrotic response to damage 5 ; since the discovery of the first ACE inhibitor (ACEi), remarkably found in the peptides from the venom of the snake Bothrops jararaca, 6 it has become a main component for the treatment of high blood pressure and heart failure. 7 Furthermore, it should be underlined that ACE2 is a homolog of ACE that was identified in the 2000s 8 ; indeed, we do have (at least) two ACE receptors, AT1 and AT2. We do have much more information on ACE1 effects, whereas details about ACE2 is only assumed; cardiovascular therapies are focused on ACE1, although ACE2 is supposed to be a promising target. We know also that ACE2 is abundantly present in the epithelia of the human lungs, which might explain the possible routes of the COVID‐19 providing evidence to the recently observed severe case of pneumonia in China with a high mortality rate, and in the intestine, suggesting other possible routes of transmission. 9
Thus, the questions that I want to point out and discuss with virologists are as follows:
-
1.
How is common to share biomolecular targets between viruses and active mediators of the cardiovascular system? Is it a “coincidence” due to the spread over species and ancient phylogeny of renin–angiotensin system? I mean, do viruses use as a binding site a functional receptor that is, usually, involved in the control of vascular tone, arterial blood pressure, and fibrotic response to damage? Is this functional? Or, instead, if this is not common, it is a possible evolution of viruses, eventually, driven by experiments and/or worldwide use of treatment targeting RAAS receptors?
-
2.
This fact raises questions on safety when using ACEi and angiotensin receptor blockers (ARBs) in patients affected by COVID‐19, as these therapies, which are widely used for the treatment of high blood pressure and heart failure, having as main target ACE1, can cause the upregulation of ACE2, with the consequence of open access routes to the COVID‐19. On the contrary, it is not possible to exclude that less‐selective ACEi/ARBs might be also helpful by blocking, even partially, ACE2. In this regard, it might be useful to obtain epidemiological data on patients affected by COVID‐19 that are assuming ACEi/ARB.
REFERENCES
- 1. Liu Z, Xiao X, Wei X, et al. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS‐CoV‐2. J Med Virol. 2020. 10.1002/jmv.25726 [published online ahead of print February 26, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li W, Moore MJ, Vasilieva N, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang H, Yang P, Liu K, et al. SARS coronavirus entry into host cells through a novel clathrin‐ and caveolae‐independent endocytic pathway. Cell Res. 2008;18:290‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ciulla MM, Paliotti R, Esposito A, et al. Different effects of antihypertensive therapies based on losartan or atenolol on ultrasound and biochemical markers of myocardial fibrosis: results of a randomized trial. Circulation. 2004;110(5):552‐557. 10.1161/01.CIR.0000137118.47943.5C [DOI] [PubMed] [Google Scholar]
- 6. Ferreira SH. Angiotensin converting enzyme: history and relevance. Semin Perinatol. 2000;24(1):7‐10. 10.1016/s0146-0005(00)80046-4 [DOI] [PubMed] [Google Scholar]
- 7. Brown NJ, Vaughan DE. Angiotensin‐converting enzyme inhibitors. Circulation. 1998;97(14):1411‐1420. 10.1161/01.cir.97.14.1411 [DOI] [PubMed] [Google Scholar]
- 8. Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin‐converting enzyme‐related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1‐9. Circ Res. 2000;87:E1‐E9. [DOI] [PubMed] [Google Scholar]
- 9. Gao QY, Chen YX, Fang JY. 2019 Novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020. 10.1111/1751-2980.12851 [published online ahead of print February 25, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]


