Abstract
Primary hepatic tuberculosis is a rare clinical entity with non-specific clinical and imaging features that can mimic other liver diseases, representing a diagnostic challenge. We report a case of a 35-year-old man with metabolic syndrome, type 2 diabetes and high alcohol consumption presenting asymptomatic with abnormal liver tests, hepatosplenomegaly and diffuse hepatic steatosis in ultrasound imaging initially suspected to be alcoholic steatohepatitis but later diagnosed as hepatic tuberculosis in the histological specimen. Anti-tuberculosis therapy was started. This clinical case highlights the diagnostic difficulty of hepatic tuberculosis and the importance of not overlooking liver biopsy and to consider it in the differential diagnosis in patients with obvious hepatic injury factors but with atypical clinical presentation.
Keywords: liver disease, alcoholic liver disease, nonalcoholic steatosis
Background
Tuberculosis remains a worldwide public health problem, especially in developing countries.1 2 Hepatic tuberculosis is a rare clinical entity even in countries endemic for tuberculosis, representing less than 1% of all cases of tuberculosis, and is usually associated with a generalised miliary tuberculosis.3 4 Primary hepatic tuberculosis is extremely rare, poorly described in the literature5 and its diagnosis is challenging due to non-specific symptoms and imaging features, sometimes mimicking hepatic tumours or metastases.6 In a recent case series reported by Ch'ng et al,7 calcified and hypodense nodules with biliary duct dilatation associated with lobar atrophy were the most consistent features of hepatic tuberculosis, especially in the presence of active lung disease, although unless there was a high index of suspicion, the diagnosis was often overlooked.
Case presentation
We report a case of a 35-year-old Caucasian immunocompetent male patient, with a prior medical history of obesity, poorly controlled type 2 diabetes, hypertension, dyslipidaemia and high alcohol consumption (60–80 g/day), who was referred to Hepatology Department for asymptomatic abnormal liver tests. There was no history of smoking or drug use. The biochemical analysis showed persistent elevation of aminotransferases (up to two to three times the upper limit of normal (ULN)) and γ-glutamyltransferase (up to three times the ULN), with normal platelets, albumin and coagulation studies. Abdominal ultrasound revealed hepatosplenomegaly and diffuse hepatic steatosis (figure 1). The remaining studies such as viral and auto-immune serologies and iron status were negative and there was no history of hepatotoxic drugs consumption. An alcoholic steatohepatitis was suspected. Transient elastography (Fibroscan) presented a liver stiffness of 13.5 kPa (IQR/med 15%), suggestive of advanced fibrosis (F3) and considerable steatosis (controlled attenuation parameter: 307 dB/m). After alcohol abstinence, improved glycaemic control and loss of 8.7% of total body weight, 3 months later, Fibroscan was repeated and there was a worsening in liver stiffness values (17.1 kPa; IQR/med 13%) corresponding to advanced fibrosis (F3). Due to worsening of high liver stiffness measure, even after lifestyle changes, and the discrepancy between transitory elastography findings and biochemical, imaging and clinical features without alterations suggestive of portal hypertension, a liver biopsy was performed. Hepatic biopsy revealed epithelioid granulomas with Langhans-type giant cells, surrounded by foci of fibrosis. Ziehl-Neelsen stain for acid-fast bacilli was positive (figure 2). No steatosis, Mallory bodies or other lesions were identified. Extrahepatic involvement of tuberculosis was excluded and anti-tuberculosis treatment with rifampicin, pyrazinamide, ethambutol and isoniazid (with pyridoxine supplementation to prevent isoniazid-induced neuropathy) was started.
Figure 1.
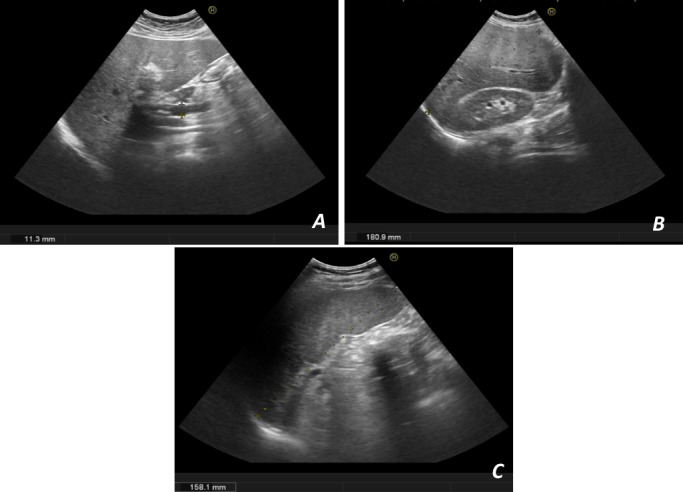
Ultrasonographic images. (A) Diffuse hepatic steatosis, without focal nodular lesions, with normal-sized portal vein; (B) hepatomegaly; (C) splenomegaly.
Figure 2.
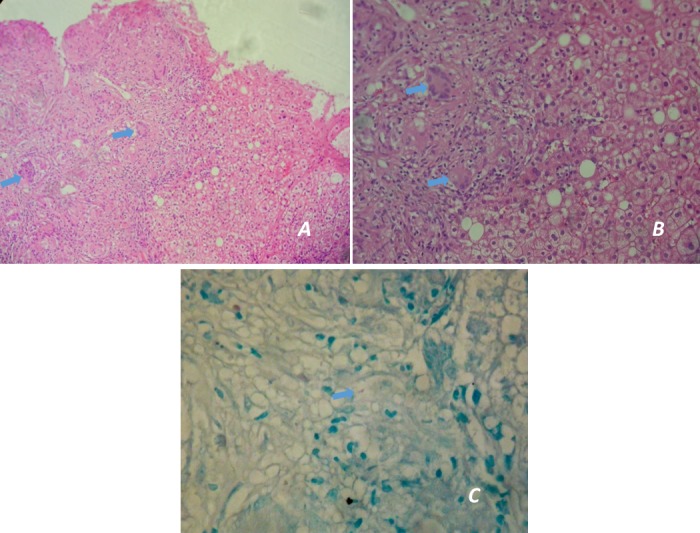
Histological images. (A and B) Granulomatous inflammation on electron microscopy showing epithelioid granulomas with Langhans giant cells (blue arrows) (H&E stain—40× and 100×, respectively); (C) Ziehl-Neelsen staining showing acid-fast bacilli (blue arrow) (ZN stain—400×).
Outcome and follow-up
The patient accomplished anti-tuberculosis treatment during 6 months with clinical response with complete normalisation of clinical parameters and no complications reported. Fibroscan was repeated 1 year after the end of the treatment and the liver stiffness improved (8.7 kPa (F2); IQR/med 6%).
Discussion
Hepatic tuberculosis is a rare entity, mainly in developing countries, and if not timely diagnosed and properly managed, it can culminate in fulminant hepatic failure and death.4 5 8 9 Coexistence of HIV and HBV with tuberculosis infection should not be underestimated and evidence of such co-infections or other immunodeficiency states should be excluded.10 A variety of diseases and conditions favour the development of active tuberculosis such as intravenous drug use, tobacco smoking, malnutrition and severe underweight, diabetes, HIV infection, silicosis, chronic renal failure, immunosuppressive treatment, gastrectomy and jejunoileal bypass.11 In this case, the only recognisable risk factor presented by the patient was diabetes; he was HIV negative without a history of smoking or drug use.
Hepatic tuberculosis can be classified as hepatic involvement in miliary tuberculosis, pulmonary tuberculosis with hepatic compromise, primary hepatic tuberculosis, focal tuberculoma or abscess and tuberculous cholangitis.10 The most common form of hepatic involvement is miliary tuberculosis occurring in 50%–80% of cases due to haematogenous spread via hepatic artery.10 Primary hepatic tuberculosis is rare because low oxygen tension in the liver is unfavourable for the growth of mycobacteria,10 although the rich blood supply and the presence of the reticuloendothelial system facilitate granuloma formation once seeding of the tuberculosis bacillus occurs.12 The proposed mechanism for primary hepatic tuberculosis is that it begins as primary intestinal tuberculosis as a gateway to the tuberculosis bacilli entry into the portal vein and seed the liver parenchyma.12
The granulomas are usually located near the portal tract and there is only mild impairment of hepatic function, so most of these lesions are minimally symptomatic or asymptomatic.8 The most frequently encountered clinical and laboratory findings described in literature are fever, weight loss, abdominal pain, hepatomegaly and elevated alkaline phosphatase level.5 8 In our case report, the patient was asymptomatic, presented with hepatomegaly and elevated aminotransferases and γ-glutamyltransferase.
Hepatic tuberculosis has no characteristic imaging features and can closely mimic primary liver malignancy or metastases.5 8 10 12–16 Ultrasonography and CT scan can show intrahepatic hypodense lesions or linear high-density calcific lesions,16 although these features are not specific. Liver biopsy is the gold standard for its diagnosis and is often required, especially when malignancy needs to be excluded.5 Histological/microbiological confirmation is often necessary to confirm the diagnosis, along with clinical and radiological exclusion of extrahepatic disease. It is essential to keep a high index of suspicion, especially in endemic regions, to avoid unnecessary surgery and start prompt treatment with anti-tuberculosis therapy.12 Although a biopsy is not mandatory for diagnosis of hepatocellular carcinoma or another liver diseases such as non-alcoholic fatty liver disease and alcoholic liver disease, clinical presentation and imaging need to be unequivocal. Any atypical features should prompt question the diagnosis and to consider performing a liver biopsy.
In our case, the clinical context and imaging features of the patient were compatible with alcoholic steatohepatitis, although the worsening of sustained advanced fibrosis in Fibroscan, after lifestyle changes with alcohol withdrawal and, most importantly, the discrepancy between transitory elastography findings and biochemical, imaging and clinical features without alterations suggestive of portal hypertension, lead to doubt the diagnosis, even with recognisable hepatic injury factors, and to perform a liver biopsy. It should be noted that there is the possibility that the values of transient elastography, in an initial phase, were overestimated due to alcohol consumption17 18 and alterations in aminotransferases,19 20 so we expected that after lifestyle changes with alcohol abstinence and weight loss at least there was no aggravation in liver stiffness.
It is described in the literature that hepatic tuberculosis can present as ‘pseudocirrhosis’ as a result of scarring of multiple tubercles or small disseminated diffuse foci during the healing phase but no major hepatic dysfunction results from the cicatrisation process.21
The histopathology result in our case revealed granulomatous inflammation with a positive Ziehl-Neelsen stain. Ziehl-Neelsen stain is positive in only 40% of cases. The PCR for tuberculosis has a sensitivity of 82% and should be considered to confirm the diagnosis in such cases.22 23
Treatment for primary hepatic tuberculosis is anti-tuberculosis therapy. Quadruple therapy (isoniazid, rifampicin, pyrazinamide and ethambutol) is recommended due to the increasing incidence of drug-resistant tuberculosis.5 Although some previous reports5 indicate that at least 1 year of medical therapy is generally required, recent publications1 2 11 advocate that the treatment regimen of choice for virtually all forms of drug-susceptible tuberculosis in adults consists of a 2-month initial phase of isoniazid, rifampin, pyrazinamide and ethambutol followed by 4 months of isoniazid and rifampin. This regimen can cure tuberculosis in more than 90% of patients.11 In fact, although comparative clinical trials of treatment for extrapulmonary tuberculosis are limited, the available evidence suggests the 6-month regimen for patients with pulmonary tuberculosis.1 2 11 However, the drugs and regimen need to be individualised according to the severity and liver function of the patient.12 Anti-tuberculosis treatment is effective and the prognosis is usually good in most of the cases with early diagnosis and treatment.22
Bacteriologic monitoring of patients with extrapulmonary tuberculosis is more difficult and often not feasible. In these cases, the response to treatment must be assessed clinically and radiologically.11 In this report, the patient completed the 6-month regimen with clinical response with hepatic analytical parameters normalisation and no complications were reported. One year after the end of the treatment, there was a substantial improvement of liver stiffness, although without normalisation in a patient who remains with metabolic risk factors. Besides, histology prior to treatment revealed fibrosis surrounding granulomas in hepatic lobules, and minimum fibrosis in portal spaces, so this may also reflect some hepatic fibrosis that still persists.
Learning points.
Primary hepatic tuberculosis is a rare entity that represents a diagnostic challenge.
Primary hepatic tuberculosis presents with a myriad of non-specific clinical and imaging manifestations that can mimic other liver diseases.
Liver biopsy is essential in cases where clinical and imaging features are equivocal, even when there are recognisable etiological factors of liver disease.
Anti-tuberculosis treatment is effective, and the prognosis is usually good in most of cases with early diagnosis and treatment.
Footnotes
Contributors: All authors have contributed to and agreed on the content of the manuscript. MF did the literature research and drafted the manuscript. JM was involved in the patient’s management and revised the manuscript. CM revised the manuscript. JC critically revised the manuscript and approved the final version to be submitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Zumla A, Raviglione M, Hafner R, et al. Tuberculosis. N Engl J Med 2013;368:745–55. 10.1056/NEJMra1200894 [DOI] [PubMed] [Google Scholar]
- 2.Maartens G, Wilkinson RJ, Tuberculosis WRJ. Tuberculosis.. Lancet 2007;370:2030–43. 10.1016/S0140-6736(07)61262-8 [DOI] [PubMed] [Google Scholar]
- 3.Essop AR, Posen JA, Hodkinson JH, et al. Tuberculosis hepatitis: a clinical review of 96 cases. Q J Med 1984;53:465–77. [PubMed] [Google Scholar]
- 4.Sharma SK, Shamim SQ, Bannerjee CK, et al. Disseminated tuberculosis presenting as massive hepatosplenomegaly and hepatic failure. Case report. Am J Gastroenterol 1981;76:153–6. [PubMed] [Google Scholar]
- 5.Mert A, Ozaras R, Tabak F, et al. Localized hepatic tuberculosis. Eur J Intern Med 2003;14:511–2. 10.1016/j.ejim.2003.09.007 [DOI] [PubMed] [Google Scholar]
- 6.Dias LTFF, Rodrigues GC, Barbosa DS, et al. Primary nodular hepatic tuberculosis mimicking hepatic neoplasia in an immunocompetent host. Braz J Infect Dis 2009;13:153–4. 10.1590/S1413-86702009000200016 [DOI] [PubMed] [Google Scholar]
- 7.Ch'ng LS, Amzar H, Ghazali KC, et al. Imaging appearances of hepatic tuberculosis: experience with 12 patients. Clin Radiol 2018;73:321.e11–321.e16. 10.1016/j.crad.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Jain P, Aggarwal G, et al. Primary hepatic tuberculosis: a rare but fatal clinical entity if undiagnosed. Asian Pac J Trop Med 2012;5:498–9. 10.1016/S1995-7645(12)60085-6 [DOI] [PubMed] [Google Scholar]
- 9.Hussain W, Mutimer D, Harrison R, et al. Fulminant hepatic failure caused by tuberculosis. Gut 1995;36:792–4. 10.1136/gut.36.5.792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine C. Primary macronodular hepatic tuberculosis: US and CT appearances. Gastrointest Radiol 1990;15:307–9. 10.1007/BF01888805 [DOI] [PubMed] [Google Scholar]
- 11.Kasper D, Fauci A, Hauser S, et al. Harrison's principles of internal medicine, 19th edition. 19th Edn McGraw-Hill Education, 2015. [Google Scholar]
- 12.Niyogi D, Goel M, Shinde RS, et al. Primary hepatic tuberculosis: a rare occurrence. Ann Hepatobiliary Pancreat Surg 2019;23:80–3. 10.14701/ahbps.2019.23.1.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brookes MJ, Field M, Dawkins DM, et al. Massive primary hepatic tuberculoma mimicking hepatocellular carcinoma in an immunocompetent host. MedGenMed 2006;8:11. [PMC free article] [PubMed] [Google Scholar]
- 14.Singh D, Singh S, Raut SB, et al. Isolated liver tuberculosis: a case report. Pediatr Surg Int 2004;20:727–8. 10.1007/s00383-002-0878-0 [DOI] [PubMed] [Google Scholar]
- 15.Chen H-C, Chao Y-C, Shyu R-Y, et al. Isolated tuberculous liver abscesses with multiple hyperechoic masses on ultrasound: a case report and review of the literature. Liver Int 2003;23:346–50. 10.1034/j.1478-3231.2003.00861.x [DOI] [PubMed] [Google Scholar]
- 16.Wu Z, Wang W-L, Zhu Y, et al. Diagnosis and treatment of hepatic tuberculosis: report of five cases and review of literature. Int J Clin Exp Med 2013;6:845–50. [PMC free article] [PubMed] [Google Scholar]
- 17.Trabut J-B, Thépot V, Nalpas B, et al. Rapid decline of liver stiffness following alcohol withdrawal in heavy drinkers. Alcohol Clin Exp Res 2012;36:1407–11. 10.1111/j.1530-0277.2012.01737.x [DOI] [PubMed] [Google Scholar]
- 18.Bardou-Jacquet E, Legros L, Soro D, et al. Effect of alcohol consumption on liver stiffness measured by transient elastography. World J Gastroenterol 2013;19:516–22. 10.3748/wjg.v19.i4.516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coco B, Oliveri F, Maina AM, et al. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat 2007;14:360–9. 10.1111/j.1365-2893.2006.00811.x [DOI] [PubMed] [Google Scholar]
- 20.Sagir A, Erhardt A, Schmitt M, et al. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 2008;47:592–5. 10.1002/hep.22056 [DOI] [PubMed] [Google Scholar]
- 21.Chaudhary P. Hepatobiliary tuberculosis. Ann Gastroenterol 2014;27:207–11. [PMC free article] [PubMed] [Google Scholar]
- 22.Singh KK, Muralidhar M, Kumar A, et al. Comparison of in house polymerase chain reaction with conventional techniques for the detection of Mycobacterium tuberculosis DNA in granulomatous lymphadenopathy. J Clin Pathol 2000;53:355–61. 10.1136/jcp.53.5.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang W-T, Wang C-C, Chen W-J, et al. The nodular form of hepatic tuberculosis: a review with five additional new cases. J Clin Pathol 2003;56:835–9. 10.1136/jcp.56.11.835 [DOI] [PMC free article] [PubMed] [Google Scholar]


