Abstract
The role of the dopamine D2 receptor (D2R) in regulating appetitive behavior continues to be controversial. Earlier literature suggests that reduced D2R signaling diminishes motivated behavior while more recent theories suggest that reduced D2R, as has been putatively observed in obesity, facilitates compulsive appetitive behavior and promotes overeating. Using a homecage foraging paradigm, we revisit classic neuroleptic pharmacological studies from the 1970s that led to the ‘extinction mimicry’ hypothesis: that dopamine blockade reduces reinforcement leading to an extinction-like reduction in a learned, motivated behavior. We complement this with a selective genetic deletion of D2R in indirect pathway medium spiny neurons (iMSNs). Administration of haloperidol shifts foraging strategy toward less effortful, more thrifty pursuit of food without altering consumption or bodyweight. D2R deletion in iMSNs also reduces effort and energy expended toward food pursuit, but without a compensatory shift in foraging strategy, resulting in loss of body weight, an effect more pronounced under conditions of escalating costs as the knockouts fail to adequately increase effort. The selective knockouts exhibit no change in sucrose preference or sucrose reinforcement. These data suggest that striatal D2R regulates effort in response to costs, mediating cost sensitivity and behavioral thrift. In the context of obesity, these data suggest that reduced D2R is more likely to diminish effort and behavioral energy expenditure rather than increase appetitive motivation and consumption, possibly contributing to reduced physical activity commonly observed in obesity.
Keywords: dopamine D2 receptor, neuroeconomics, striatum, indirect pathway, D2R selective knockout, extinction mimicry, behavioral energy expenditure, neuroleptics
INTRODUCTION
In recent years, there has been increased focus on the role of sedentary behavior in obesity (Chaput et al., 2011; Ladabaum et al., 2014; Shook et al., 2015), with evidence suggesting voluntary activity is reduced in obesity, an effect that can persist even after weight loss (Kravitz et al., 2016). While caloric intake is the prime determinant of body weight in the short-term (Tataranni et al., 2003; Luke et al., 2008; Westerterp and Speakman, 2008; Luke and Cooper, 2013 but see Church et al., 2011), physical activity contributes to long-term maintenance of weight loss, reducing cycles of weight loss and rebound weight gain (Levin and Dunn-Meynell, 2004; MacLean et al., 2009, 2015; DeLany et al., 2014). Moreover, exercise is important for health. Sedentary lifestyles has been associated with increased rates of chronic illness and mortality imposing a significant global economic burden (Healy et al., 2011; Ding et al., 2016). Adaptations in response to increased physical activity suggest that total energy expenditure may be regulated and constrained within limits (Pontzer et al., 2016), perhaps analogous to the notion of a set-point in body weight regulation. How energy expenditure and physical activity are regulated is poorly understood, but such understanding is important in designing interventions aimed at increasing physical activity, both in the treatment of individuals and at a broader population level.
Midbrain dopamine is a key neural substrate mediating addiction (Berke and Hyman, 2000; Di Chiara et al., 2004; Kenny et al., 2013; Pascoli et al., 2015; Volkow et al., 2017). More recently, dopamine has been suggested to play a role in compulsive overeating associated with obesity (Volkow et al., 2011; Blum et al., 2014; Guo et al., 2014; Naef et al., 2015). Widely associated with compulsive reward seeking, DA is also known to regulate voluntary activity (Beeler et al., 2012a). Though both increased caloric intake and reduced activity contribute to positive energy balance and obesity, the potential role of dopamine in the latter has been considerably less examined.
The dopamine D2 receptor (D2R) has been a focus of attention in obesity studies (Volkow et al., 2011; Kenny et al., 2013). Initial reports that D2R was reduced in obesity (Wang et al., 2001) gave rise to the ‘reward deficiency’ hypothesis that suggested animals and people compulsively pursue reward, such as palatable foods, to induce dopamine release to compensate for reduced D2R signaling (Volkow and Wise, 2005; Kenny, 2011; Blum et al., 2014). Subsequent studies have yielded contradictory results (Volkow et al., 2008; de Weijer et al., 2011; Kessler et al., 2014; negative: Dunn et al., 2012; Eisenstein et al., 2013; de Weijer et al., 2014; Cosgrove et al., 2015; Karlsson et al., 2015; Tuominen et al., 2015). Similarly, the reduced function D2R variant Taq1A has been frequently associated with obesity (Barnard et al., 2009; Chen et al., 2012; Carpenter et al., 2013), though a recent large, prospective study did not find any association (Hardman et al., 2014). None of these studies, however, have examined changes in physical activity potentially associated with reduced D2R, despite the known role dopamine and D2R in regulating activity (Beeler et al., 2012a; Kravitz et al., 2016; Beeler and Mourra, 2018).
Earlier studies using pharmacological blockade of D2R suggested that reduced D2R signaling diminished rather than increased appetitive motivation (Salamone et al., 2007; Wise, 2008). In classic studies in the 1970s and 80s, Wise and colleagues (Wise et al., 1978; Wise, 2008) administered neuroleptics (predominantly antagonizing D2R) to rats well-trained in an operant task. Rather than observing an immediate effect on their motivation to lever press, a decrease in effort was observed progressively across days, interpreted that dopamine was mediating reinforcement learning. The progressive decrease across days has been called ‘extinction mimicry.’ Here we revisit these classic studies in a homecage foraging paradigm and further investigate the role of D2R regulating consumption and activity using mice with a selective deletion of D2R in iMSNs (Friend et al., 2016). We demonstrate that reduced D2R signaling shifts behavioral strategy toward greater cost sensitivity and behavioral energy conservation. We suggest that D2R regulates costs by gating the degree to which dopamine release can disinhibit the indirect pathway.
METHODS
Animals
For haloperidol studies, wild-type C57BL/6 mice of both sexes were used at approximately 120 days of age. To generate the D2R iMSN KO mice, mice expressing cre-recombinase regulated by the adenosine 2A receptor promoter (Adora2a; B6.FVB(Cg)-Tg(Adora2a-Cre)KG139Gsat/Mmucd; GENSAT, 036158-UCD) were crossed with mice carrying a conditional Drd2 null alleles (B6.129S4 (FVB)-Drd2tm1.1Mrub/J, JAX 020631, hereafter fdrd2) and bred to homozygozity for the Drd2 null allele, with either cre+/− (experimental animals) or cre−/− (littermate controls), as in Friend et al (2016). The ablation of D2R in iMSNs with these genotypes have been previously validated in published accounts (Dobbs et al., 2016; Friend et al., 2016; Lemos et al., 2016). Genotypes were verified using PCR. For experiments, mice were singly housed in homecage operant chambers (below) and maintained on 12:12 light-dark cycles with ad libitum access to water. All procedures were approved by the Queens College Institutional Animal Care and Use Committee.
Behavioral Tests
Homecage operant paradigms:
mice were singly housed in standard mouse polycarbonate mouse cages equipped with two levers, pellet dispenser and food hopper (Med-Associates, Fairfax, VT). No food restriction was employed and the only source of food was through lever pressing (closed economy) except in the concurrent choice test. 20 mg grain pellets (Bio-Serv, Flemington, NJ) were dispensed on a progressive ratio schedule with an increment of two such that the first pellet cost 2 lever presses, the next 4, the next 6 and so on (PR2). After 30 minutes of inactivity (no lever press on active or inactive lever), the ratio reset to 2 to start the sequence again. This allows the mice to titrate their average cost per pellet by balancing the size of meals (with larger meals proportionally more expensive) against the number and frequency of meals (Fig 1A). In the homecage concurrent choice, mice were provided free chow in their cage and could earn sucrose pellets (Bio-Serv, Flemington, NJ) through lever pressing on a PR2 schedule. In experiments with running wheels, radio-telemetry running wheels (Med-Associates, Fairfax, VT) were fit in the operant-equipped cages and running was monitoring 24/7 in 1 min bins.
Fig. 1.
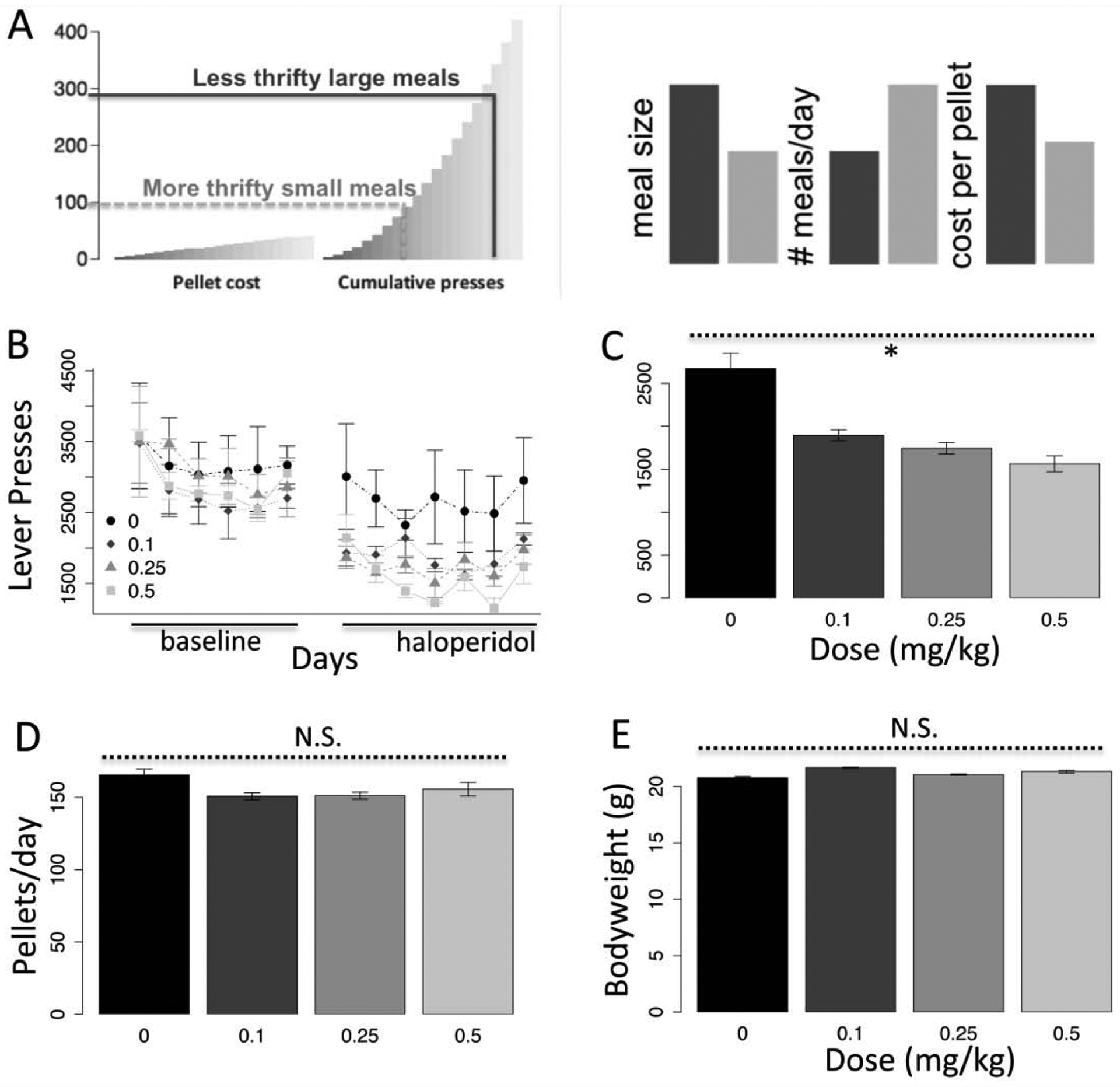
Haloperidol decreases responding without altering consumption or body weight. a) Illustration of thrift in homecage PR paradigm comparing more and less thrifty strategies in balancing meal size and frequency, b) Dose-response effect of haloperidol on active lever presses showing 6 days of pre-treatment baseline and 7 days of haloperidol administration at the indicated doses. c) Average responding across treatment days by dose (black bar represents average baseline across groups). d) Average consumption by dose (black bar, baseline), e) Average body weight by dose (black bar, baseline). N=6. *p < .05. Error bars, S.E.M.
Open field:
mice were tested in a rat-sized open field (40 cm long × 40 cm wide × 37 cm high) using photobeams to detect and quantify movement (Med-Associates, Fairfax, VT). Three 90-minute sessions on consecutive days were averaged.
Sucrose preference:
mice were singly housed and provided with three bottles, one containing water and two containing sucrose at concentrations that increased across days with each concentration applied for two days. The position of the bottles were switched daily to counter-balance for any side preference. Consumption was measured daily.
Two-bottle conditioning:
Mice were habituated to the test chambers for three days, 30 minutes per day. For training, mice were placed in chambers with a single bottle containing either water or 0.8M sucrose located in the center of the chamber wall. Water and sucrose tubes marked with either white or red tape around the sipper to serve as differentiating cues, counter-balanced between the tastants. Mice were exposed to tastant for 60 minute sessions with sucrose and water on alternating days for a total of 6 training days, 3 for each tastant. On test day, mice were placed in the chambers for 10 minutes with both sucrose- and water-cued bottles on either side of the cage, both containing water. Preference is calculated as the amount consumed on test day from the sucrose-paired bottle divided by consumption from both bottles.
Drugs
Pharmaceutical grade haloperidol solution was diluted in saline to indicated concentrations prior to 1x daily intraperitoneal administration at 5:30 pm, 30 min prior to onset of active cycle.
Fitting data to exponential demand function
The data from each mouse was individually fit and plotted and used to calculate averages of elasticity coefficient for the two genotypes tested. Additionally, the genotype data were fit by group to generate the average fit. We used the exponential demand function of Hursh et al (Hursh and Silberberg, 2008) as follows:
Where Q is maximal consumption estimated at zero cost, k sets the range (here, k=1), p is the price (cost in lever presses) set as the progressive ratio increment active during each day (ie., PR2, PR4, PR6 etc) and alpha is the elasticity coefficient. The data were fit in R (https://www.r-project.org, v. 3.4.3) minimizing least squares using lsqcurvefit in package pracma (v. 2.1.1) with Q and alpha as free parameters, with initial parameterset to log10(150) for Q and .0012 for alpha.
Statistics
Analysis of variance (ANOVA) was used to test significance using R (v. 3.4.3). Error bars represent standard error of the mean (S.E.M.).
RESULTS
Haloperidol reduces effort without altering consumption or bodyweight
To revisit extinction mimicry studies, we used a homecage operant paradigm (Beeler et al., 2012b). In this closed-economy, mice obtained food on a progressive ratio schedule (HCPR-2, see methods) with the cost resetting if the mouse stops pressing for 30 minutes (end of a bout of pressing or ‘meal’). Under these contingencies, larger meals are more costly, but mice can maintain equivalent consumption with less effort by eating shorter but more frequent meals (Fig. 1a). For example, hyperdopaminergic mice eat larger but fewer meals and, as a consequence, exert much more effort for the same total amount of food, reflected in a higher average cost per pellet (Beeler et al., 2012b, 2012c). Wild-type C57BL/6 mice were allowed to establish stable pressing (Fig. 1b, left) before haloperidol administration for seven days (Fig. 1b, right). No baseline differences were observed between groups (F(1,10) = .17, p = .69). Haloperidol induced a dose-dependent reduction in lever pressing (Fig. 1b/c, dose, F(1,10) = 6.02, p < .05), though we did not observe a gradual, progressive decrease in pressing. Despite clear reduction in lever pressing, consumption was not significantly reduced (Fig. 1d, F(1,10) = .72, p = .41) and there was no change in body weight (Fig. 1e, F(1,10) = .39, p = .54). Though haloperidol does not impair motor capacity for lever pressing, it can slow the rate of pressing (Salamone et al., 1993). In a conventional, time-limited session-based paradigm, this could lead to conflating decreased consumption with slower pressing. In the homecage paradigm, however, there are no time limits and so reduced rate of pressing is not conflated with motivation: the mice can take as much time as they need.
Haloperidol dose-dependently induces greater behavioral thrift
To examine how mice reduced their effort but maintained consumption and bodyweight, we analyzed meal patterns. Upon administration of haloperidol, mice decrease the size (and cost) of individual meals (Fig. 2a, F(1,10) = 6.12, p < .05) but compensate by increasing the frequency and number of meals (Fig. 2b, F(1,10) = 10.76, p < .01), such that a decreased breakpoint (Fig. 2c, F(1,10) = 6.15, p < .05) results in overall lower average cost per pellet (Fig. 2d, F(1,10) = 8.64, p < .05). To ensure that the maintenance of consumption was not the result of compensatory pressing after haloperidol clearance, we plotted pressing across 24 hours averaged across haloperidol days. We observe an obvious effect of haloperidol in the first half of the dark cycle that wears off in the second half; however, during this latter period there is no compensatory increased consumption (Fig. 3a). Consumption (Fig. 3b) is greater during the first half for all mice and unaffected by haloperidol, though cost-per–pellet (Fig. 3c) is decreased by haloperidol during this period of greater consumption. These data suggest haloperidol induces increased behavioral thrift, shifting to a strategy that reduces cost while sustaining similar consumption, exhibiting a pattern exactly the opposite of hyperdopaminergic mice (Beeler et al., 2012b, 2012c).
Fig. 2.
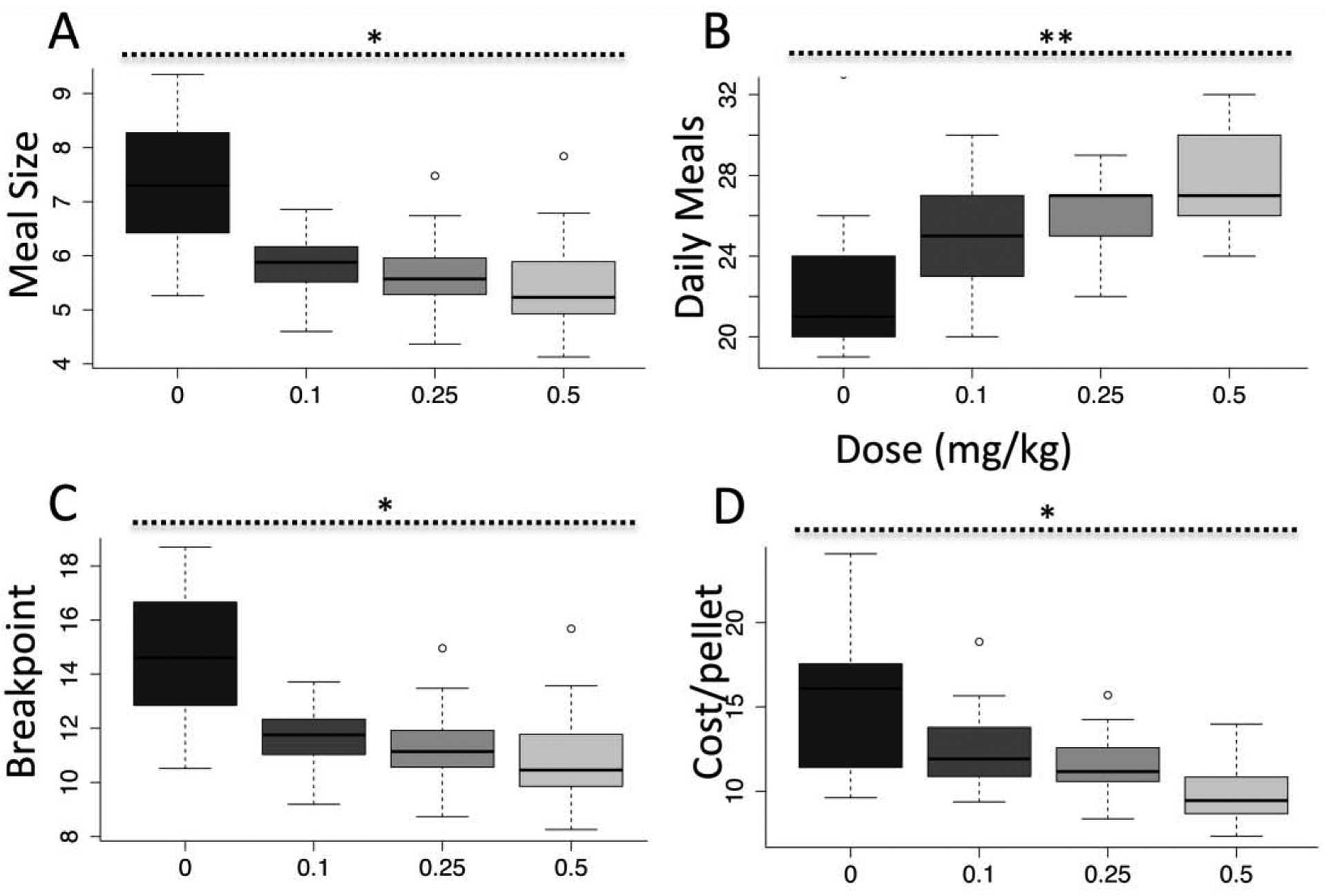
Meal patterning in response to haloperidol. Dose-response (black bar indicates mean of baseline) in a) meal size and b) number of meals per day, c) breakpoint and d) average cost per pellet. N = 6. * p < .05; ** p < .01. Error bars, S.E.M.
Fig. 3.
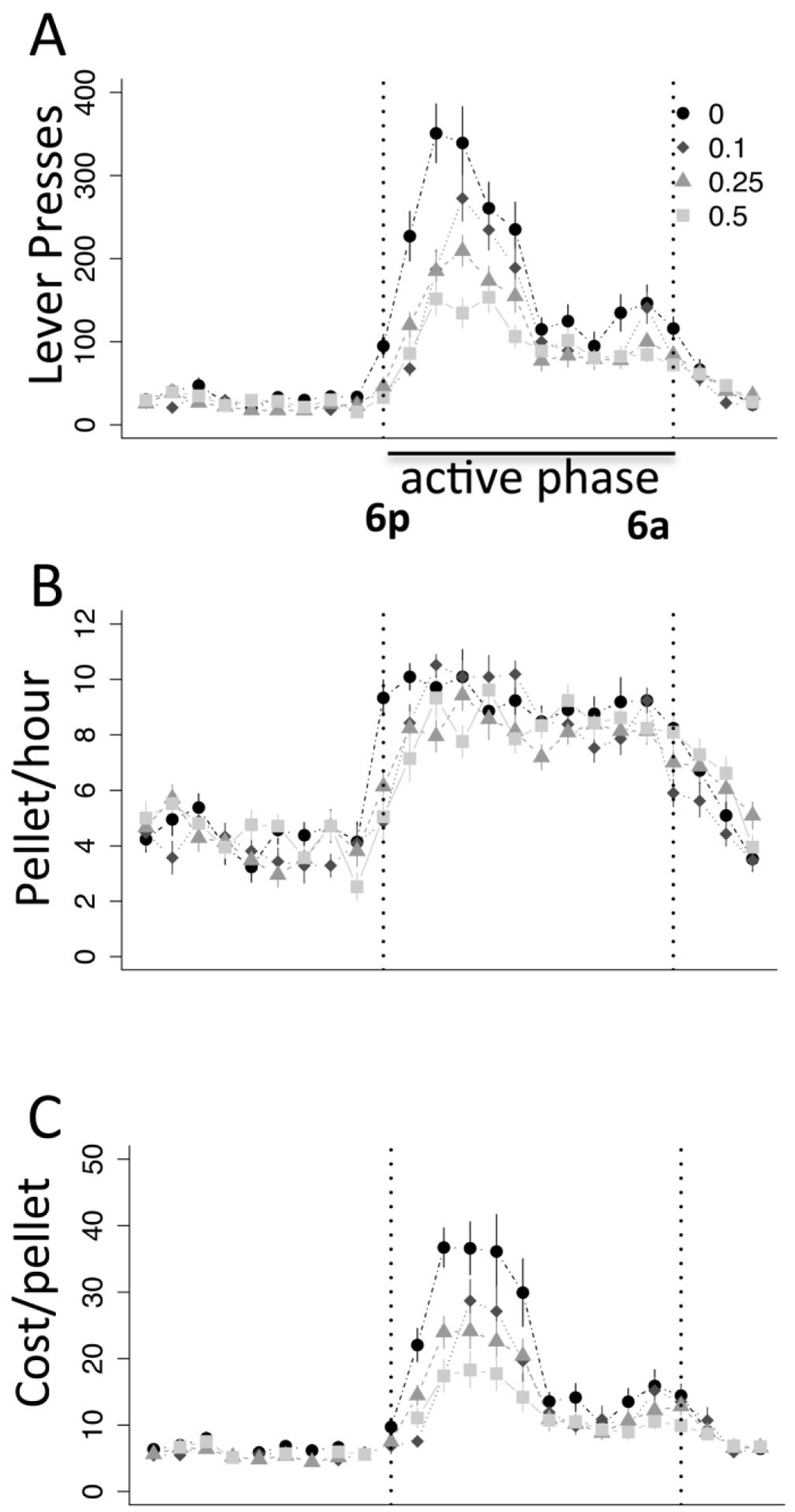
Haloperidol effects on food pursuit across 24 hr period. All haloperidol days were average and plotted binned by hour, showing a) lever pressing, b) pellets earned and c) average cost per pellet. Statistics on dose effects presented in Figs. 2 and 3. N = 6. Error bars, S.E.M.
Selective deletion of iMSN D2R induces greater behavioral thrift
D2R is ubiquitously expressed across multiple brain regions and cell types (Dobbs et al., 2017). To selectively examine the role of D2R expressed on indirect pathway medium-spiny neurons (iMSNs), we crossed floxed drd2 mice with Adora-cre mice that express cre recombinase under the control of the A2A promoter, a strategy validated and used successfully in previously published reports (Dobbs et al., 2016; Friend et al., 2016; Lemos et al., 2016). Consistent with prior published studies of these mice as well as a global knockdown (Beeler et al. 2016), we observed substantially reduced running wheel activity (Fig. 4a–b; a, F(1,8) = 10.92, p < .01; b, F(1,9) = 6..97, p < .05) and reduced activity in the open field (Fig. 4 c–d;distance, F(1,9) = 7.31, p < .05; ambulatory episodes, F(1,9) = 15.16, p < .01; ambulatory time, F(1,9) = 13.35, p < .01; resting time, F(1,9) = 11.43, p < .01; velocity, F(1,9) = .001, p = .98), confirming the reduced activity phenotype previously observed.
Fig. 4.
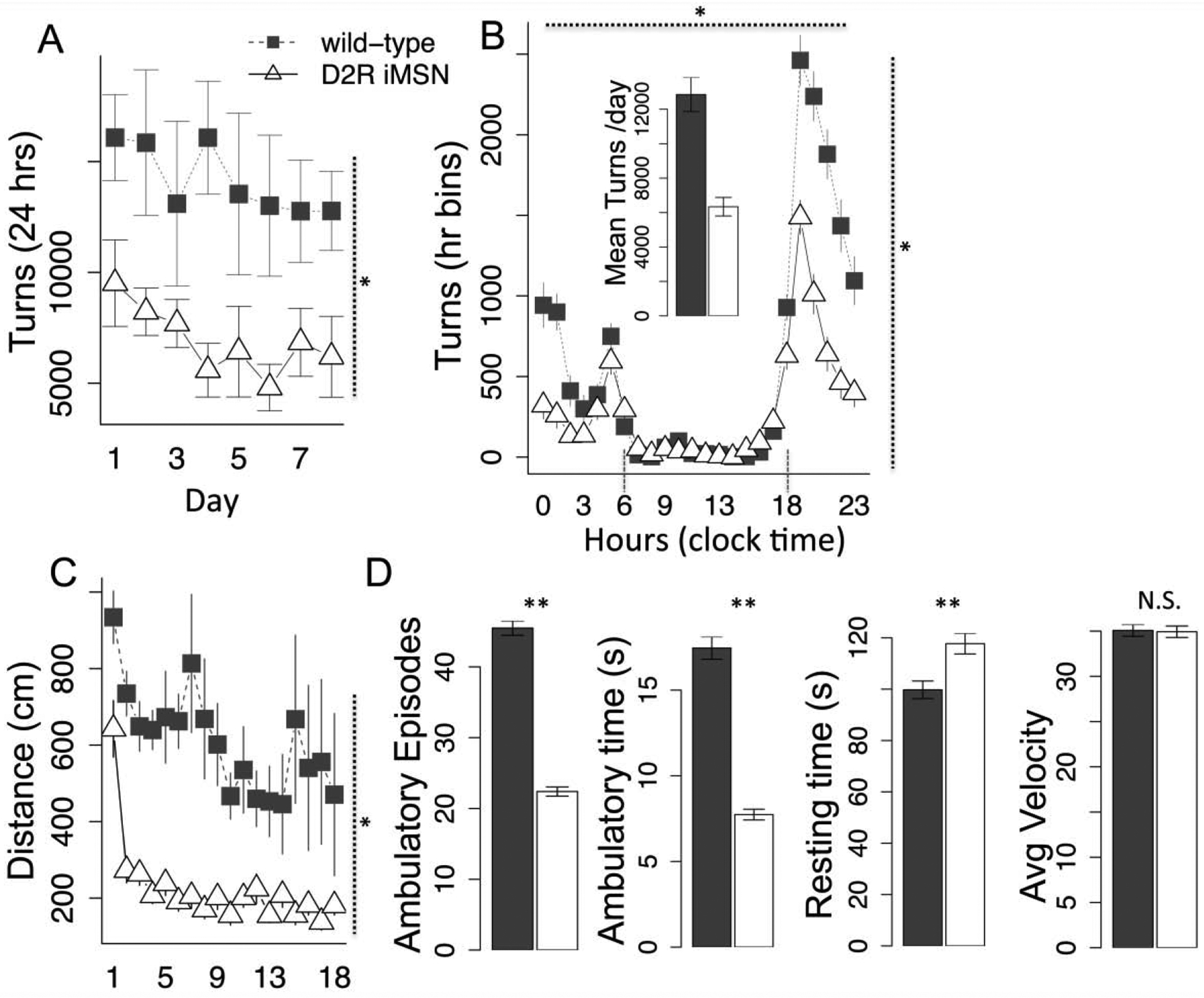
Activity phenotype of D2R iMSN KO mice. a) total running per day across experiment, b) running binned by hour across 24 hour period (averaged across days), dotted lines demarcating active and inactive periods. Inset, total daily running averaged across experiment, c) distance traveled in open field, d) genotype comparison in ambulatory episodes, ambulatory time, resting time and average velocity. Vertical dotted lines indicate statistic associated with main effect while horizontal dotted line indicate genotype × repeated measure (day, hours) statistic. N = 5, * < .05, **, < .01. Error bars, S.E.M.
Mice were housed in operant homecages on the progressive ratio schedule (PR2) as above. In contrast to wild-type littermates that maintain or slightly gain weight, the D2R iMSN KOs lose weight (Fig. 5a, geno × day, F(1,9) = 8.25, p < .05) and consume less (Fig. 5b, F(1,9) = 19.75, p < .01). They exhibit substantially reduced lever pressing and breakpoint (Fig. 5c–d, lever presses, F(1,9) = 34.82, p < .001; breakpoint, F(1,9) = 15.54, p < .01) and, as a consequence maintain a much lower average cost-per-pellet than their wild-type littermates (Fig. 5e, F(1,9) = 17.07, p < .01). Curiously, unlike the haloperidol studies, they do not exhibit a compensatory increase in the frequency of smaller meals. As a consequence, they weigh less than wild-type controls, consistent with prior observations with global D2R knockout mice (Kim et al., 2010). Friend et al (2016) demonstrated the D2R iMSN KOs have no alterations in their basal metabolic rates. These data suggest that the D2R iMSN KOs expend less energy in pursuit of food and consequently maintain a lower bodyweight when there is a procurement cost.
Fig. 5.
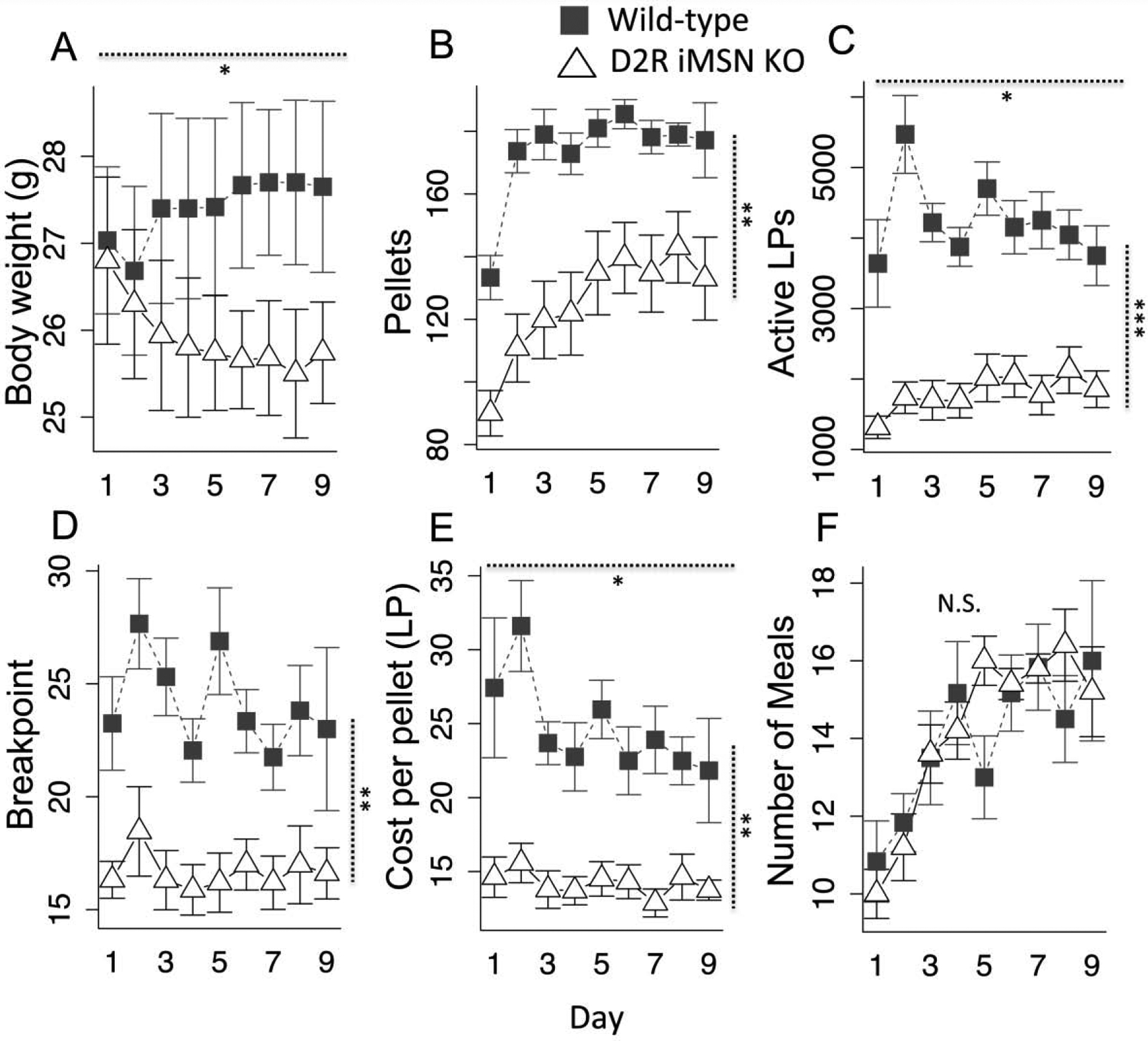
D2R iMSN KO in homecage progressive ratio. a) daily body weight b) daily consumption, c) daily lever presses, d) daily breakpoint, e) daily average cost per pellet, f) daily total number of meals. Vertical dotted lines indicate genotype main effect statistic, horizontal dotted lines indicate genotype × day statistic. N = 5, * p < .05, ** p < .01, *** p < .001. Error bars, S.E.M.
Selective deletion of iMSN D2R confers greater elasticity in food demand under conditions of escalating costs and scarcity
To further probe the impact of D2R deletion in iMSNs, we placed mice in an escalating progressive ratio homecage paradigm where the increment determining the progression in pellet costs is increased each day, making food increasingly expensive across days and implementing a form of food scarcity associated with cost. All mice reduced bodyweight as food costs escalated. At higher ratios the wild-type mice increased their effort, somewhat mitigating weight loss while the D2R iMSN KO mice did not (Fig. 6a, geno × day, F(1,9) = 6.45, p < .05), reflecting in the wild-type ability to maintain stable consumption across cost increments while the D2R iMSN KO mice reduced their consumption with increased cost (Fig. 6b, geno × day, F(1,9) = 26.52, p < .001), dramatically reflected in the total lever presses per day in the two groups (Fig. 6c, F(1,9) = 21.08, p < .01). This failure to respond adequately to escalating costs with increased effort is observed where both genotypes increase their breakpoint, but the increase in the KO mice is greatly diminished (Fig. 6d, geno main effect, F(1,9) = 26.1, p < .001, geno × day, F(1,9) = 19.47, p < .01). The fact that the KO more than double their breakpoint indicates their failure to expend energy via lever pressing at lower costs does not reflect a motor impairment. While the average cost of pellets increases nearly 7-fold for wild-type mice, the KO mice constrain these costs to only a 3-fold increase (Fig. 6e, F(1,9) = 23.24, p < .001, geno × day, F(1,9) = 16.06, p < .01). The D2R iMSN KO mice do not compensate for reduced effort with increased meal frequency.
Fig. 6.
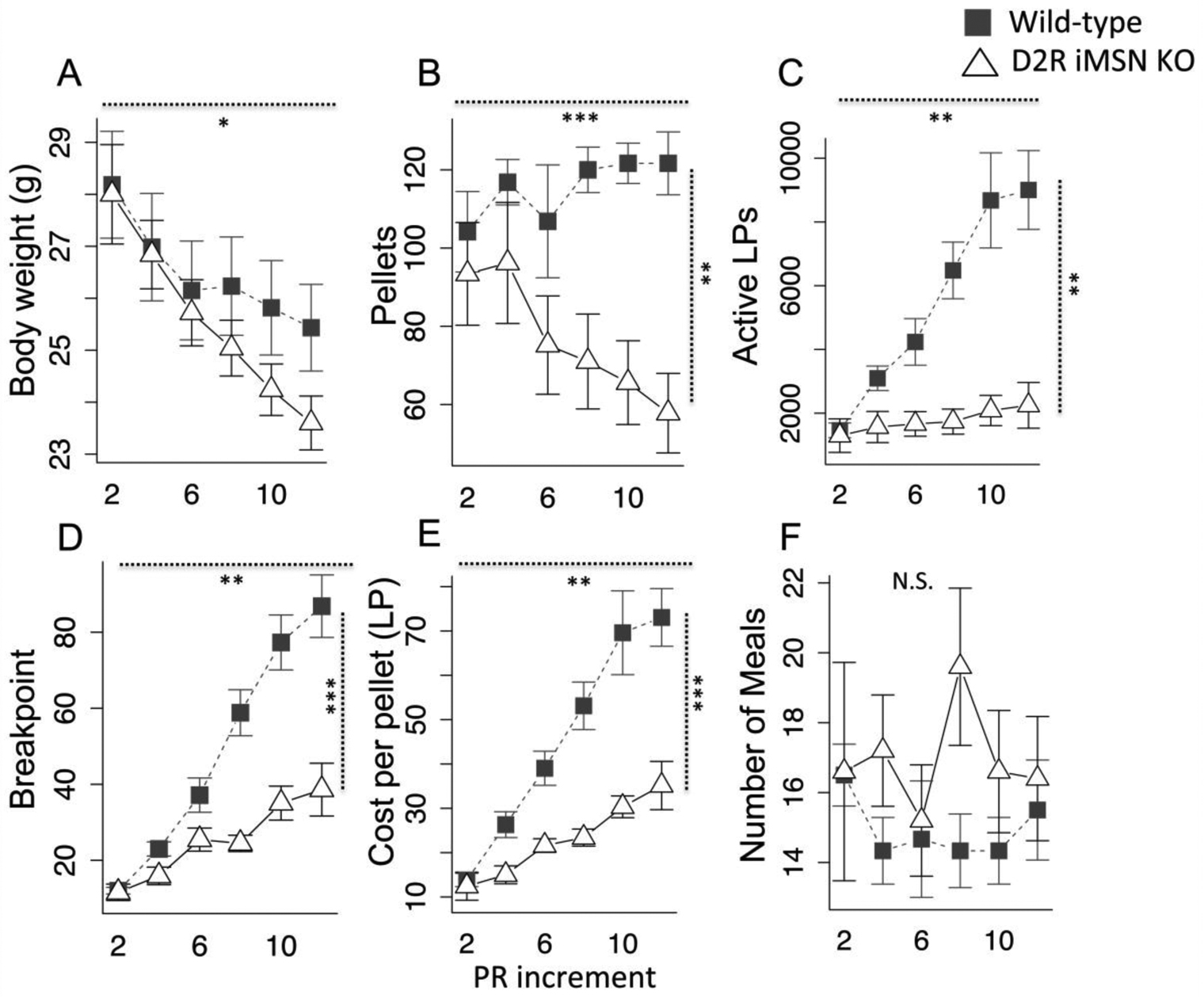
D2R iMSN KO in escalating homecage progressive ratio (scarcity). a) daily body weights, b) daily consumption, c) daily lever presses, d) daily breakpoint, e) daily average cost per pellet, f) daily total number of meals. Vertical dotted lines indicate genotype main effect statistic, horizontal dotted lines indicate genotype × day statistic. N = 5, * p < .05, ** p < .01, *** p < .001. Error bars, S.E.M.
We fit consumption data to an exponential model of demand across cost increments to assess elasticity in demand (Hursh and Silberberg, 2008). The wild-type mice exhibit no elasticity, with a slight increase in demand (Fig. 7), perhaps reflecting compensatory consumption in response to increased energy expenditure to obtain pellets. In contrast, as costs escalate, the D2R iMSN KO mice reduce their demand, showing greater elasticity (Fig. 7, comparing elasticity coefficients, F(1,10) = 24.88, p < .001). The strong inelasticity observed in the wild-type may seem anomalous; however, it should be noted that because this is progressive ratio, the mice experience a range of prices in every meal. In this situation, ‘price’ is a moving target and demand can be construed as response to the average cost of pellets across a 24 hour period. In this case, the average cost per pellet in wild-type asymptotes at approximately 70 lever-presses (Fig. 6e). In more traditional paradigms with escalating fixed ratio costs, the drop in demand is modest at this cost range (e.g., Soto et al., 2011; Beeler et al., 2012b); the effect may be further reduced in progressive ratio where a substantial proportion of pellets are acquired at low cost such that overall demand is only modestly impacted by higher costs further in the incrementing progression. In these circumstances, the elasticity observed in the D2R iMSN KOs is even more notable.
Fig. 7.
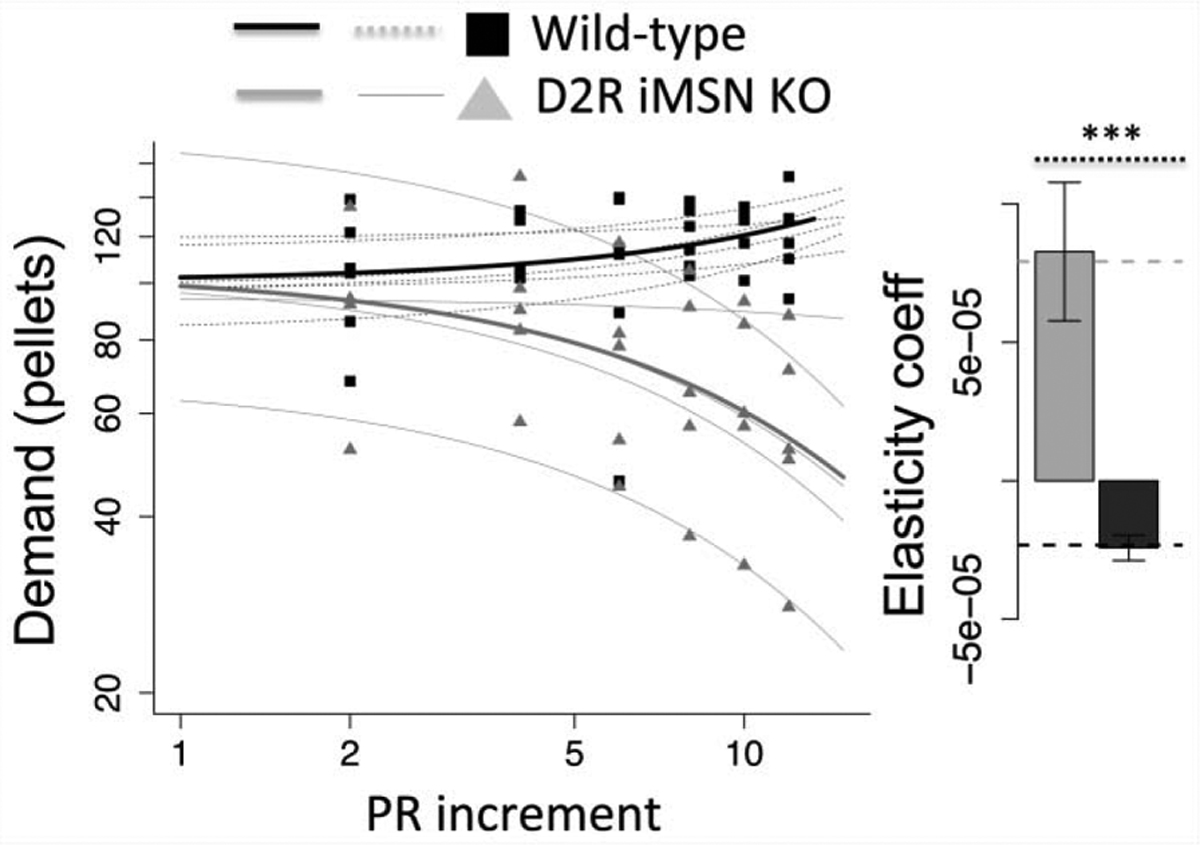
Exponential demand curve fit to D2R iMSN KO and wild-type littermate controls. Data were fit for each individual mouse to exponential demand model (methods), yielding a best fit alpha parameter that describes elasticity. Actual data plotted as points (gray triangles, KOs; black squares, WT) with thin traces displaying fits for individual mice (gray, KOs; black, WT). Bold lines show best fits to genotype group data. The elasticity coefficient (alpha) for individual fits were averaged by genotype, shown in bar graph to right. N = 5, *** p < .001. Error bars, S.E.M.
Selective deletion of iMSN D2R has no impact on sucrose preference or reinforcement
Reduced lever pressing effort in pursuit of food described above (Figs. 5–6) could arise from either increased sensitivity to the cost (effortful energy expenditure in pursuit of reward) or to decreased reinforcement efficacy of the reward. To assess preference and reinforcer efficacy, we conducted concurrent choice, sucrose preference and two-bottle conditioning tests.
In a concurrent choice paradigm, mice have free access to chow but can also choose to lever press for a more preferred food, such as sucrose pellets. In a prior study with global D2R knockdown (Beeler et al., 2016), we observed no genotype difference in lever pressing for sucrose pellets in a homecage concurrent choice paradigm. Here we tested the selective KO mice in concurrent choice and observe no difference in body weight (Fig. 8a, F(1,9) = .03, p = .86), nor any reduction in earned sucrose pellets or breakpoint (Fig. 8a, pellets, F(1,9) = .1, p = .76, breakpoint, F(1,9) = .05, p = .82). These data are consistent with prior literature demonstrating that decreased dopamine function does not affect effort when costs are low (Aberman and Salamone, 1999). As all mice eat relatively little sucrose, the costs of obtaining this in a progressive ratio schedule are also relatively low. No difference between the genotypes indicates that there is no change in preference for or reinforcement efficacy of sucrose evident in this paradigm.
Fig. 8.
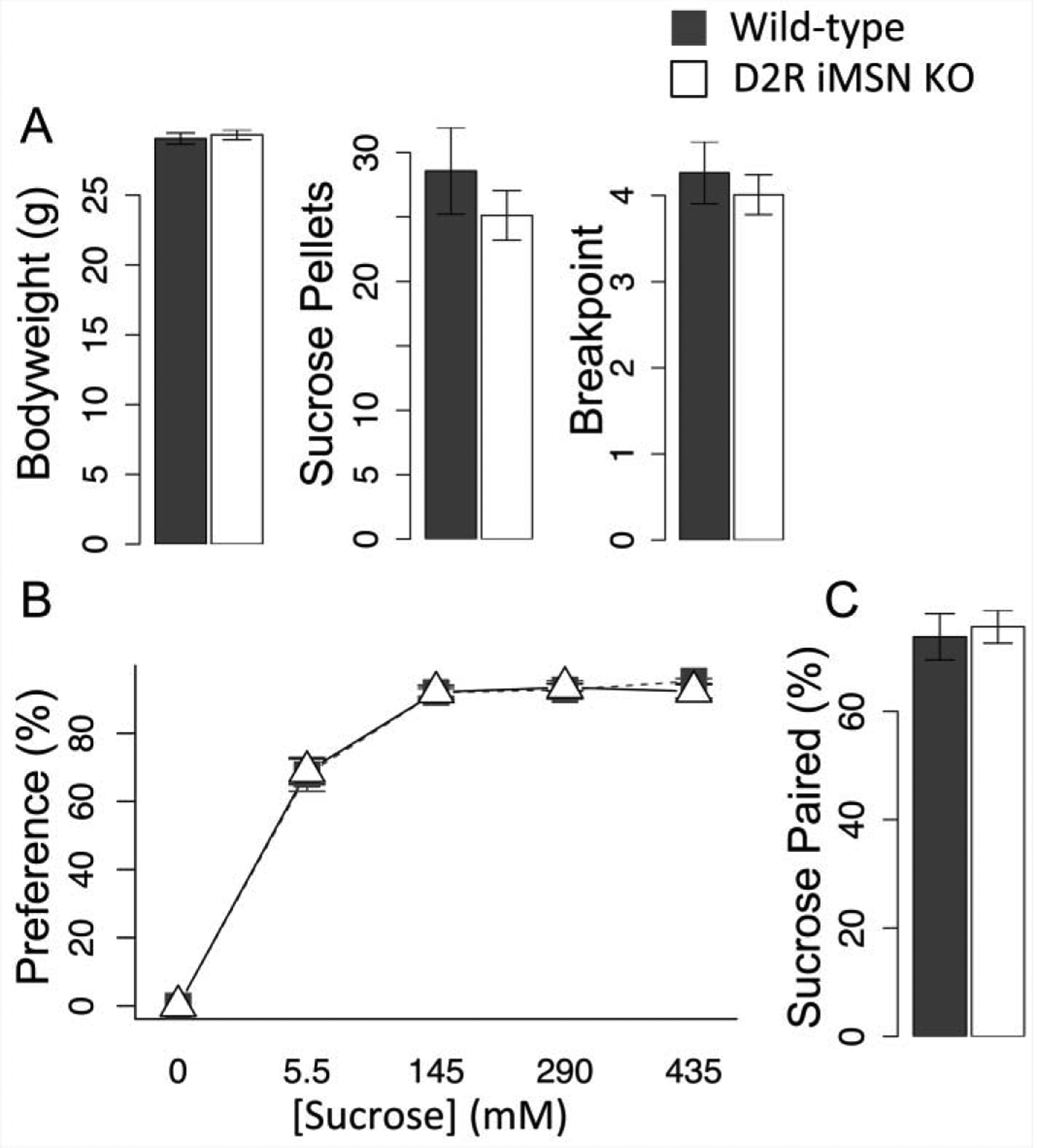
Sucrose preference and reinforcement in D2R iMSN KO mice. a) Concurrent choice: body weight, sucrose pellets earned and breakpoint, N = 5, b) homecage sucrose preference, N = 11/13 (WT/KO) c) Two-bottle conditioning, percent preference for bottle previously paried with sucrose in choice test with water in both bottles, N = 10/12 (WT/KO). Error bars, S.E.M.
To test for preference in a non-effortful task, we conducted homecage sucrose preference tests. The KO mice do not exhibit any decrease in sucrose preference (Fig. 8b, F(1,20) = .004, p = .95), suggesting the loss of iMSN D2R does not diminish how much they like sucrose or its hedonic value. In a two-bottle conditioning task, the KO do not exhibit any reduction of conditioning for the bottle previously paired with sucrose (Fig. 8c, F(1,18) = .125, p = .72), suggesting that loss of iMSN D2R does not reduce the ability of sucrose to act as a reinforcer in associative learning in these mice. Together, data in these three tests suggest that the decrease in lever pressing for food observed in the KO mice above is not the result of decreased hedonic value or a reduction in reinforcement efficacy; rather, the decrease appears to specifically reflect increased sensitivity to costs and a predisposition to conserve energy and constrain effort in pursuit of reward.
DISCUSSION
We previously demonstrated that genetically induced hyperdopaminergia causes animals to be less thrifty in their behavioral energy expenditure: increasing how hard they work for reward without altering their overall consumption (Beeler et al., 2012b). Here we demonstrate the complementary, reverse pattern, that pharmacological blockade with haloperidol, a dopamine antagonist that acts primarily on D2R, increases thriftiness, reducing effort expended in pursuit of food without altering consumption and bodyweight.
Reduced willingness to work under haloperidol administration has been demonstrated previously using conventional session-based test paradigms (reviewed in Salamone et al., 2007). In the homecage paradigm used here, the mouse can adapt its foraging strategy to minimize costs while maintaining consumption. Haloperidol shifts foraging strategy to favor shorter, less expensive bouts of lever pressing where smaller meals are compensated by an increase in the number of meals, resulting in more economical foraging; that is, haloperidol induces greater behavioral thrift without inducing extinction or reducing consumption.
Hypothesizing that postsynaptic D2R on iMSNs specifically regulate cost sensitivity, we selectively deleted this population of D2Rs. Consistent with the haloperidol study, we observe decreased effort expended to obtain food, an effect more pronounced as costs escalate. Deletion of D2R on iMSNs confers greater demand elasticity compared to wild-type littermates. These results extend earlier findings by Soto et al (Soto et al., 2011) who found that mice with a global D2R knockout exhibited increased elasticity. Our data demonstrate that postsynaptic D2R on iMSNs are sufficient to mediate this effect. The D2R iMSN KO mice could compensate for smaller, less expensive meals by increasing meal number, as observed with haloperidol, but they do not; instead, they reduce consumption as costs increase. While these data demonstrate that reduced D2R signaling decreases appetitive motivation in response to costs, the question is whether this arises from decreased reinforcement efficacy of food reward or diminished willingness to expend energy in appetitive pursuit. The sucrose preference and two-bottle conditioning tests with the selective knockouts demonstrate that the hedonic value of sucrose and its efficacy in reinforcing learned associations is unchanged, arguing against a loss of reinforcement efficacy, consistent with prior studies of haloperidol by Salamone and colleagues (Salamone et al., 1993).
These studies suggest that D2R expressed on iMSNs regulate behavioral thrift; that is, how much energy and effort an animal is willing to expend in the pursuit of reward. We propose a simple model by which the level of D2R expression on iMSNs gates the extent to which dopamine can inhibit the indirect pathway and relieve inhibition on cortical activity, regulating an inhibitory tone that must be overcome to initiate and sustain action. In response to dopamine release, reduced D2R disinhibits inhibitory tone on cortical activity to a lesser extent, increasing the activity required to overcome that inhibition; for example, requiring greater facilitation via direct pathway activation. In effect, this requires greater incentive motivation to overcome this gate and expend energy in pursuit of reward, which effectively increases the value of energy expended in work relative to the reward. We propose that striatal D2R determines how much value is required to initiate and sustain work; alternatively stated, D2R gates the value of the energy expended in work (Beeler and Mourra, 2018).
Role of learning, synaptic plasticity and reinforcement
D2R regulates both synaptic plasticity (Kreitzer and Malenka, 2008; Shen et al., 2008; Lovinger, 2010) and cell excitability in indirect pathway MSNs (Surmeier et al., 2007), modulating both learning and on-going neurotransmission. As D2R modulates both indirect pathway inhibition and corticostriatal plasticity, both presumably contribute to the decreased willingness to expend energy in the pursuit of food observed here. While considerable evidence suggests that dopamine can modulate behavioral response to costs, as demonstrated here, a recent study has demonstrated that costs modulate dopamine signals that arise from and mediate learning (Tanaka et al., 2019). Specifically, dopamine signals in response to both cues that predict reward and in response to received reward are greater when the costs associated with those stimuli are greater. This could potentially describe a mechanism by which escalating costs facilitate learning that effectively increases the value of reward (see also Kacelnik and Marsh, 2002) and facilitates the increased effort necessary to obtain that reward, as observed here in wild-type. That is, while dopamine can modulate effort, i.e. signal the value of work (Hamid et al., 2015), the Tanaka et al (2019) study suggest that learning can scale that dopamine-signaled value to adapt to environmental conditions and contingencies, suggesting one way in which dopamine modulation of MSN excitability and corticostriatal plasticity—current and future behavior—may be integrated to achieve adaptation in response to an environmental economy.
Our study and those of Wise and colleagues (e.g., Wise et al., 1978) are in agreement in that we both show that D2 blockade substantially reduces effort to obtain reward. Our studies differ in that Wise et al (1978) observed a gradual decrease across sessions consistent with learning, i.e., extinction mimicry, while we did not observe a gradual decrease but an adjustment in behavioral strategy that reduced costs but maintained consumption/reward. We suggest the different paradigms (session-based vs. homecage) capture different aspects of the same D2R-related phenomenon. The session-based experiments of Wise and colleagues breaks experience and learning into discrete blocks (sessions) distributed across time that allows observation of incremental changes as experience accrues, but does not provide insight into how these changes affect adaptive behavior in a naturalistic foraging environment. Our closed-economy, homecage paradigm precludes this block-by-block observation of experience and learning as a 24 hour period collapses the equivalent of 32 45-minute sessions time-wise into a single data point. However, our paradigm allows observation of how the effects of haloperidol alter self-regulated, adaptive foraging-like behavior, demonstrating no change in appetitive motivation or reduction in consumption, only an increase in the energetic efficiency in food pursuit.
It is interesting that faced with escalating costs, the selective knockouts, unlike the mice administered haloperidol, do not compensate for smaller meals with increased meal frequency. The deletion of D2R in iMSNs likely impairs or even inverts corticostriatal plasticity (Calabresi et al., 1997). LTD in corticostriatal synapses onto the indirect pathway can allow learning to selectively disinhibit specific stimuli and responses. In the haloperidol studies, because of the continuous nature of the homecage paradigm, blockade of disinhibitory learning is not complete, i.e., as the drug is metabolized, continued pressing and reward can reinstate prior learning. In the knockouts, the loss of D2R is absolute and imposes an inflexible, non-selective inhibitory gate that operates independently of dopamine, modulating both iMSN excitability and synaptic plasticity. If dopamine signals ‘the value of work’ (Hamid et al., 2015), iMSNs without D2R don’t get the message, or rather they overvalue the energy required for work relative to the value of the reward.
Does reduced D2R drive overconsumption in obesity?
A prominent theory proposes that D2R is reduced in obesity, which generates compulsive overeating, borrowing the reward deficiency hypothesis from the addiction literature (Blum et al., 2014). Whether D2R is reduced in obesity is increasingly contested as more studies emerge (Hardman et al., 2014). Nevertheless, the data reported here suggest that reduced D2R signaling does not increase appetitive motivation nor induce compulsive over-consumption. On the contrary, if pursuit of food requires effort, reduced D2R decreases willingness to exert effort in the service of food motivation, even when hunger is increased, as in the scarcity paradigm. Our findings are consistent with a rich literature dating back decades from Wise (review, Wise, 2004), Salamone (review, (Salamone et al., 2007) and others showing that reduced dopamine or D2R blockade decreases appetitive motivation. More recently, two studies using mouse genetics to reduce D2R have demonstrated that D2R reduction does not increase consumption, even under palatable, high fat, dietary induced obesity conditions; instead, activity is dramatically reduced across multiple measures, including voluntary wheel-running, homecage activity and open field activity (Beeler et al., 2016; Friend et al., 2016). The one study, to our knowledge, that putatively runs counter to this is from Johnson and Kenny (2010) where the authors knockdown D2R in rats using lentivirus and show that the rats are less affected by aversive stimuli in pursuit of food reward. However, there was no difference between the knockdowns and controls in bodyweight or consumption (Johnson and Kenny, 2010, Fig 6c–d).
None of the theorizing about reduced D2R driving compulsive consumption takes into account the role of D2R in regulating activity and behavioral energy expenditure. The work reported here, as well as other recent mouse genetic studies (Beeler et al., 2016; Friend et al., 2016), suggest that the effect of D2R likely lies more in reducing willingness to expend energy in voluntary activity than in driving compulsive appetitive motivation. Activity levels are reduced in obesity (Friend et al., 2016). Putative obesity related reductions in D2R may gate behavioral activation and decrease physical activity, making it difficult to sustain voluntary exercise regimens important both for health and maintaining weight loss (Levin and Dunn-Meynell, 2004; MacLean et al., 2009, 2015; DeLany et al., 2014). These data suggest that studies of D2R in obesity, in both human and animal subjects, should assess physical activity and voluntary exercise.
Limitations and caveats
Both haloperidol and selective genetic deletion of D2R do not isolate D2R in the dorsal versus ventral striatum. Recent studies have suggested that D2R may be differently affected in these regions in obesity and may, therefore, contribute differently (Guo et al., 2014), though our studies indicate the net effect when D2R is deleted in both regions. The genetic deletion is constitutive, thus potential developmental effects could come into play. However, there are no indications of gross abnormalities in the mutant mice and a similar phenotype between the pharmacological and selective genetic manipulation strengthen the argument that observed behavioral alterations arise from altered D2R. The haloperidol results argue against developmental causes in knockouts while D2R selectivity in the knockouts argue against non-specific effects in haloperidol. Finally, both haloperidol and selective deletion of D2R can impair motor learning (Beeler et al., 2016); however, we see no evidence of impaired learning in acquisition of lever pressing. Within the scarcity paradigm, the D2R iMSN KOs increase their lever pressing as costs increase; each increase indicates they were capable of pressing more at the prior cost but, effectively, chose not to. This is mirrored in the open field where their reduced activity arises from fewer ambulatory episodes (fewer initiations), shorter episodes (earlier termination of locomotion) and greater time resting but no difference in velocity of movement, again indicating a motivational rather than motor impairment.
D2R has been implicated in numerous behavioral functions, including the regulation of appetitive motivation, voluntary activity/behavioral energy expenditure, behavioral flexibility and learning. Our thrift hypothesis posits dopamine as a primary substrate regulating allocation of resources, with D2R serving as a gate for energy (resource) expenditure—or ‘cost controller’ (Beeler and Mourra, 2018). The studies reported here are consistent with striatal D2R gating behavioral energy expenditure and regulating cost-sensitivity in decision-making.
HIGHLIGHTS.
Haloperidol shifts foraging strategy toward greater thriftiness without altering consumption or body weight
Selective dopamine D2 receptor (D2R) deletion on indirect pathway medium spiny neurons (iMSNs) increases cost sensitivity
D2R deletion on iMSNs does not increase appetitive drive
Selective D2R knockouts exhibit greater elasticity in food demand favoring energy conservation
Postsynaptic striatal D2R gates effort and behavioral energy expenditure in response to costs associated with reward
ACKNOWLEDGEMENTS
Funding: This work was supported by the National Institutes for Drug Abuse NIDA [JB, DA046058] and a PSC-CUNY Award, jointly funded by The Professional Staff Congress and The City University of New York (JB). The authors declare no conflicts of interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aberman JE, Salamone JD (1999) Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience 92:545–552. [DOI] [PubMed] [Google Scholar]
- Barnard ND, Noble EP, Ritchie T, Cohen J, Jenkins DJA, Turner-McGrievy G, Gloede L, Green AA, Ferdowsian H (2009) D2 dopamine receptor Taq1A polymorphism, body weight, and dietary intake in type 2 diabetes. Nutr Burbank Los Angel Cty Calif 25:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, Faust RP, Turkson S, Ye H, Zhuang X (2016) Low Dopamine D2 Receptor Increases Vulnerability to Obesity Via Reduced Physical Activity, Not Increased Appetitive Motivation. Biol Psychiatry 79:887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, Frazier CRM, Zhuang X (2012a) Putting desire on a budget: dopamine and energy expenditure, reconciling reward and resources. Front Integr Neurosci 6 Available at: http://journal.frontiersin.org/article/10.3389/fnint.2012.00049/abstract [Accessed February 4, 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, Frazier CRM, Zhuang X (2012b) Dopaminergic enhancement of local food-seeking is under global homeostatic control: Dopamine modulates responding locally. Eur J Neurosci 35:146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, McCutcheon JE, Cao ZFH, Murakami M, Alexander E, Roitman MF, Zhuang X (2012c) Taste uncoupled from nutrition fails to sustain the reinforcing properties of food. Eur J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, Mourra D (2018) To Do or Not to Do: Dopamine, Affordability and the Economics of Opportunity. Front Integr Neurosci 12 Available at: http://journal.frontiersin.org/article/10.3389/fnint.2018.00006/full [Accessed February 13, 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Hyman SE (2000) Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25:515–532. [DOI] [PubMed] [Google Scholar]
- Blum K, Thanos PK, Gold MS (2014) Dopamine and glucose, obesity, and reward deficiency syndrome. Front Psychol 5 Available at: http://journal.frontiersin.org/article/10.3389/fpsyg.2014.00919/abstract [Accessed November 25, 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Saiardi A, Pisani A, Baik J-H, Centonze D, Mercuri NB, Bernardi G, Borrelli E (1997) Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors. J Neurosci 17:4536–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter CL, Wong AM, Li Z, Noble EP, Heber D (2013) Association of dopamine D 2 receptor and leptin receptor genes with clinically severe obesity: Genes, Addiction, and Obesity. Obesity:n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput J-P, Klingenberg L, Rosenkilde M, Gilbert J-A, Tremblay A, Sjödin A (2011) Physical Activity Plays an Important Role in Body Weight Regulation. J Obes 2011:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ALC, Blum K, Chen TJH, Giordano J, Downs BW, Han D, Barh D, Braverman ER (2012) Correlation of the Taq1 dopamine D2 receptor gene and percent body fat in obese and screened control subjects: A preliminary report. Food Funct 3:40–48. [DOI] [PubMed] [Google Scholar]
- Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP, Rodarte RQ, Martin CK, Blair SN, Bouchard C (2011) Trends over 5 Decades in U.S. Occupation-Related Physical Activity and Their Associations with Obesity Lucia A, ed. PLoS ONE 6:e19657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Veldhuizen MG, Sandiego CM, Morris ED, Small DM (2015) Opposing relationships of BMI with BOLD and dopamine D2/3 receptor binding potential in the dorsal striatum: RELATIONSHIPS OF BMI, BOLD, AND D2R/D3R BP. Synapse 69:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weijer BA, van de Giessen E, Janssen I, Berends FJ, van de Laar A, Ackermans MT, Fliers E, la Fleur SE, Booij J, Serlie MJ (2014) Striatal dopamine receptor binding in morbidly obese women before and after gastric bypass surgery and its relationship with insulin sensitivity. Diabetologia 57:1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weijer BA, van de Giessen E, van Amelsvoort TA, Boot E, Braak B, Janssen IM, van de Laar A, Fliers E, Serlie MJ, Booij J (2011) Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res 1:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLany JP, Kelley DE, Hames KC, Jakicic JM, Goodpaster BH (2014) Effect of physical activity on weight loss, energy expenditure, and energy intake during diet induced weight loss: Effects of Physical Activity During Intervention. Obesity 22:363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D (2004) Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology 47:227–241. [DOI] [PubMed] [Google Scholar]
- Ding D, Lawson KD, Kolbe-Alexander TL, Finkelstein EA, Katzmarzyk PT, van Mechelen W, Pratt M, Committee LPAS 2 E (2016) The economic burden of physical inactivity: a global analysis of major non-communicable diseases. The Lancet 388:1311–1324. [DOI] [PubMed] [Google Scholar]
- Dobbs LK, Kaplan AR, Lemos JC, Matsui A, Rubinstein M, Alvarez VA (2016) Dopamine Regulation of Lateral Inhibition between Striatal Neurons Gates the Stimulant Actions of Cocaine. Neuron 90:1100–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs LK, Lemos JC, Alvarez VA (2017) Restructuring of basal ganglia circuitry and associated behaviors triggered by low striatal D2 receptor expression: implications for substance use disorders: Restructuring of basal ganglia circuitry and associated behaviors. Genes Brain Behav 16:56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JP, Kessler RM, Feurer ID, Volkow ND, Patterson BW, Ansari MS, Li R, Marks-Shulman P, Abumrad NN (2012) Relationship of Dopamine Type 2 Receptor Binding Potential With Fasting Neuroendocrine Hormones and Insulin Sensitivity in Human Obesity. Diabetes Care 35:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein SA, Antenor-Dorsey JAV, Gredysa DM, Koller JM, Bihun EC, Ranck SA, Arbeláez AM, Klein S, Perlmutter JS, Moerlein SM, Black KJ, Hershey T (2013) A comparison of D2 receptor specific binding in obese and normal-weight individuals using PET with (N-[(11)C]methyl)benperidol. Synap N Y NY 67:748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend DM, Devarakonda K, O’Neal TJ, Skirzewski M, Papazoglou I, Kaplan AR, Liow J-S, Guo J, Rane SG, Rubinstein M, Alvarez VA, Hall KD, Kravitz AV (2016) Basal Ganglia Dysfunction Contributes to Physical Inactivity in Obesity. Cell Metab Available at: http://linkinghub.elsevier.com/retrieve/pii/S1550413116305964 [Accessed January 7, 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Simmons WK, Herscovitch P, Martin A, Hall KD (2014) Striatal dopamine D2-like receptor correlation patterns with human obesity and opportunistic eating behavior. Mol Psychiatry 19:1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM, Kennedy RT, Aragona BJ, Berke JD (2015) Mesolimbic dopamine signals the value of work. Nat Neurosci 19:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman CA, Rogers PJ, Timpson NJ, Munafò MR (2014) Lack of association between DRD2 and OPRM1 genotypes and adiposity. Int J Obes 38:730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy GN, Matthews CE, Dunstan DW, Winkler EAH, Owen N (2011) Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur Heart J 32:590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A (2008) Economic demand and essential value. Psychol Rev 115:186–198. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ (2010) Addiction-like reward dysfunction and compulsive eating in obese rats: Role for dopamine D2 receptors. Nat Neurosci 13:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacelnik A, Marsh B (2002) Cost can increase preference in starlings. Anim Behav 63:245–250. [Google Scholar]
- Karlsson HK, Tuominen L, Tuulari JJ, Hirvonen J, Parkkola R, Helin S, Salminen P, Nuutila P, Nummenmaa L (2015) Obesity Is Associated with Decreased - Opioid But Unaltered Dopamine D2 Receptor Availability in the Brain. J Neurosci 35:3959–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ (2011) Reward Mechanisms in Obesity: New Insights and Future Directions. Neuron 69:664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Voren G, Johnson PM (2013) Dopamine D2 receptors and striatopallidal transmission in addiction and obesity. Curr Opin Neurobiol 23:535–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RM, Zald DH, Ansari MS, Li R, Cowan RL (2014) Changes in dopamine release and dopamine D2/3 receptor levels with the development of mild obesity: Changes in Dopamine Release with Mild Obesity. Synapse:n/a–n/a. [DOI] [PubMed] [Google Scholar]
- Kim KS, Yoon YR, Lee HJ, Yoon S, Kim SY, Shin SW, An JJ, Kim MS, Choi SY, Sun W, Baik JH (2010) Enhanced Hypothalamic Leptin Signaling in Mice Lacking Dopamine D2 Receptors. J Biol Chem 285:8905–8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, O’Neal TJ, Friend DM (2016) Do Dopaminergic Impairments Underlie Physical Inactivity in People with Obesity? Front Hum Neurosci 10 Available at: http://journal.frontiersin.org/article/10.3389/fnhum.2016.00514/full [Accessed October 20, 2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC (2008) Striatal Plasticity and Basal Ganglia Circuit Function. Neuron 60:543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladabaum U, Mannalithara A, Myer PA, Singh G (2014) Obesity, Abdominal Obesity, Physical Activity, and Caloric Intake in US Adults: 1988 to 2010. Am J Med 127:717–727.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Friend DM, Kaplan AR, Shin JH, Rubinstein M, Kravitz AV, Alvarez VA (2016) Enhanced GABA Transmission Drives Bradykinesia Following Loss of Dopamine D2 Receptor Signaling. Neuron 90:824–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA (2004) Chronic exercise lowers the defended body weight gain and adiposity in diet-induced obese rats. Am J Physiol-Regul Integr Comp Physiol 286:R771–R778. [DOI] [PubMed] [Google Scholar]
- Lovinger DM (2010) Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology 58:951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke A, Cooper RS (2013) Physical activity does not influence obesity risk: time to clarify the public health message. Int J Epidemiol 42:1831–1836. [DOI] [PubMed] [Google Scholar]
- Luke A, Dugas LR, Ebersole K, Durazo-Arvizu RA, Cao G, Schoeller DA, Adeyemo A, Brieger WR, Cooper RS (2008) Energy expenditure does not predict weight change in either Nigerian or African American women. Am J Clin Nutr 89:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PS, Higgins JA, Wyatt HR, Melanson EL, Johnson GC, Jackman MR, Giles ED, Brown IE, Hill JO (2009) Regular exercise attenuates the metabolic drive to regain weight after long-term weight loss. Am J Physiol-Regul Integr Comp Physiol 297:R793–R802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PS, Wing RR, Davidson T, Epstein L, Goodpaster B, Hall KD, Levin BE, Perri MG, Rolls BJ, Rosenbaum M, Rothman AJ, Ryan D (2015) NIH working group report: Innovative research to improve maintenance of weight loss: Improving Weight Loss Maintenance. Obesity 23:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naef L, Pitman KA, Borgland SL (2015) Mesolimbic dopamine and its neuromodulators in obesity and binge eating. CNS Spectr 20:574–583. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Hiver A, Lüscher C (2015) Sufficiency of Mesolimbic Dopamine Neuron Stimulation for the Progression to Addiction. Neuron 88:1054–1066. [DOI] [PubMed] [Google Scholar]
- Pontzer H, Durazo-Arvizu R, Dugas LR, Plange-Rhule J, Bovet P, Forrester TE, Lambert EV, Cooper RS, Schoeller DA, Luke A (2016) Constrained Total Energy Expenditure and Metabolic Adaptation to Physical Activity in Adult Humans. Curr Biol 26:410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM (2007) Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 191:461–482. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Kurth PA, McCullough LD, Sokolowski JD, Cousins MS (1993) The role of brain dopamine in response initiation: effects of haloperidol and regionally specific dopamine depletions on the local rate of instrumental responding. Brain Res 628:218–226. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ (2008) Dichotomous Dopaminergic Control of Striatal Synaptic Plasticity. Science 321:848–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook RP, Hand GA, Drenowatz C, Hebert JR, Paluch AE, Blundell JE, Hill JO, Katzmarzyk PT, Church TS, Blair SN (2015) Low levels of physical activity are associated with dysregulation of energy intake and fat mass gain over 1 year. Am J Clin Nutr 102:1332–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto PL, Grandy DK, Hursh SR, Katz JL (2011) Behavioral economics of food reinforcement and the effects of prefeeding, extinction, and eticlopride in dopamine D2 receptor mutant mice. Psychopharmacology (Berl) 215:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W (2007) D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 30:228–235. [DOI] [PubMed] [Google Scholar]
- Tanaka S, O’Doherty JP, Sakagami M (2019) The cost of obtaining rewards enhances the reward prediction error signal of midbrain dopamine neurons. Nat Commun 10:3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataranni PA, Harper IT, Snitker S, Parigi AD, Vozarova B, Bunt J, Bogardus C, Ravussin E (2003) Body weight gain in free-living Pima Indians: effect of energy intake vs expenditure. Int J Obes 27:1578–1583. [DOI] [PubMed] [Google Scholar]
- Tuominen L, Tuulari J, Karlsson H, Hirvonen J, Helin S, Salminen P, Parkkola R, Hietala J, Nuutila P, Nummenmaa L (2015) Aberrant mesolimbic dopamine–opiate interaction in obesity. NeuroImage 122:80–86. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Baler RD (2011) Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci 15:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding Y-S, Wong C, Ma Y, Pradhan K (2008) Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: Possible contributing factors. NeuroImage 42:1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA (2005) How can drug addiction help us understand obesity? Nat Neurosci 8:555–560. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA, Baler R (2017) The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci 18:741–752. [DOI] [PubMed] [Google Scholar]
- Wang G-J, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusll N, Fowler JS (2001) Brain dopamine and obesity. The Lancet 357:354–357. [DOI] [PubMed] [Google Scholar]
- Westerterp KR, Speakman JR (2008) Physical activity energy expenditure has not declined since the 1980s and matches energy expenditures of wild mammals. Int J Obes 32:1256. [DOI] [PubMed] [Google Scholar]
- Wise R (2008) Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res 14:169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5:483–494. [DOI] [PubMed] [Google Scholar]
- Wise RA, Spindler J, DeWit H, Gerber GJ (1978) Neuroleptic-Induced “Anhedonia” in Rats: Pimozide Blocks Reward Quality of Food. Science 201:262–264. [DOI] [PubMed] [Google Scholar]


