Abstract
Inappropriate antibiotic use is common in older adults (>65 years of age) and these individuals are particularly vulnerable to serious antibiotic-associated adverse effects such as cardiac arrhythmias, delirium, aortic dissection, drug-drug interactions, and Clostridioides difficile. Antibiotic prescribing improvement efforts in older adults have been primarily focused on inpatient and long-term care settings. However, the ambulatory care setting is where the vast majority of antibiotic prescribing to older adults occurs. To help improve the clinical care of older adults, we review drivers of antibiotic prescribing in this population, explore system aspects of ambulatory care that care create barriers to optimal antibiotic use, discuss existing stewardship interventions, and provide guidance on priority areas for future inquiry.
Inappropriate antibiotic use in healthcare settings is an important driver of antibiotic resistance. While the increasing prevalence of multidrug-resistant bacteria represents a global public health crisis, unnecessary antibiotic prescriptions also pose a direct threat to patient safety due to risk of adverse drug events (ADE). The last two decades have seen antimicrobial stewardship program (ASP) implementation in acute and long-term care settings. However, available reports indicate that over 80% of human antibiotic use and 60% of costs occur in ambulatory care settings.1–3 Estimates also suggest that at least 30% of ambulatory antibiotic prescriptions among American adults are inappropriate.4 Consequently, the Centers for Disease Control and Prevention (CDC) has called for actions to enhance antibiotic stewardship in ambulatory settings.5
Most ambulatory care antibiotic stewardship research has focused on either pediatric or younger adults populations.6 However, in 2014–2016, older adults (>65 years of age) had the highest ambulatory care antibiotic prescribing rate of any age group, over 1,100 prescriptions per 1,000 persons.7 Older adults are 1.5 times more likely to receive antibiotics in a given year than younger adults and, in 2014, received 51.6 million prescriptions for antibiotics.4,8 In the ambulatory clinic setting, adults ≥65 years of age receive unnecessary antibiotics at over 46% of visits involving non-bacterial respiratory tract infections (e.g., upper respiratory infection, acute bronchitis, sinusitis, and non-suppurative otitis media), the majority of which are broad spectrum agents.9,10
This high rate of inappropriate prescribing is particularly concerning for older adults who are at increased risk for a serious ADEs due to age-related physiologic changes, polypharmacy, and comorbidities.11 Antibiotics as a class are the third most common cause of ADEs among older adults and nearly 15% of all emergency department (ED) visits for antibiotic-associated ADEs occur in older adults.12 Antibiotic dosing errors are more common in older adults and this population is also at increased risk for neurotoxicity (e.g. delirium) related to a variety of antibiotic classes.13,14 This population is also more likely to develop Clostridioides difficile infection (CDI) and suffer adverse outcomes as a result; 80% of CDI deaths occur in patients age 65 years and older.15
Specific classes of antibiotics pose increased safety risks for older adults. For instance, the Food and Drug Administration has placed a boxed warning on fluoroquinolone antibiotics which highlights older adults as being at an elevated risk of serious side effects, including tendon rupture, delirium, peripheral neuropathy, blood sugar disturbances, and aortic dissection.16 Fluoroquinolones also increase the risk of CDI. Yet, ciprofloxacin was recently found to be the most commonly prescribed antibiotic in patients age 75 years and older in ambulatory settings.8 Macrolide antibiotics are commonly prescribed to older adults, were associated with an elevated risk of cardiac arrhythmia and death in a predominantly male, older adult cohort (average age 56 years).17 This relationship was not observed in other cohort studies involving younger adults. Trimethoprim/sulfamethoxazole (TMP/SMX) merits particular close monitoring in older adults given the risk of preexisting renal insufficiency necessitating dose adjustment and the potential for drug-drug interactions causing hyperkalemia (e.g. angiotensin converting enzyme inhibitors) or increased bleeding risk (e.g. supratherapeutic warfarin levels).
Understanding the barriers to optimal antibiotic use for older adults in ambulatory settings, including drivers of unnecessary and inappropriate prescribing, is essential in combatting antibiotic resistance and preventing harm. We review aspects of ambulatory care that can create barriers to optimal antibiotic use, discuss existing stewardship interventions, and identify priority areas for future inquiry.
Ambulatory Care Settings
Ambulatory care refers to any medical services performed in an outpatient setting without admission to a hospital or facility (i.e., long-term care). This term encompasses a variety of healthcare settings with diverse organizational structures and care processes, each of which may have distinct influences on antibiotic stewardship efforts. For instance, emergency department, urgent care, and retail clinic encounters are unscheduled and do not involve a preexisting patient-clinician relationship. This contrasts with other ambulatory care settings that schedule encounters and often involve longitudinal care including primary and specialty care clinics, ambulatory surgical centers, hemodialysis centers, and home care. Dentistry offices, where 10% of all ambulatory care antibiotics are prescribed, also fall under the umbrella of ambulatory care.18 Finally, ehealth-based ambulatory encounters, which may include asynchronous (e.g., telephone calls or electronic messages) or synchronous encounters (e.g., televisits) are increasingly common patient care delivery modalities. There is a paucity of research examining patient-clinician communication in non-clinic settings and its role in antibiotic prescribing, as no billing codes are typically filed for these brief interactions. However, preliminary data suggests that in a general adult population, these contacts may account for a significant percentage (~10%) of antibiotic prescriptions.19
A recently published national study of over 150 million visits involving adults <65 years of age with employer-sponsored health care revealed important differences in antibiotic prescribing between the different ambulatory care settings. By volume, the overwhelming majority of antibiotic prescriptions originated in primary care clinics (95%), followed by the ED (3%), urgent care (1.7%) and retail clinics (0.3%). However, the percentage of inappropriate prescriptions for respiratory tract conditions was highest among visits to urgent care (45.7%), followed by EDs (24.6%), primary care clinics (17.0%) and retail clinics (14.4%).20 Whether these findings would apply to older adults is unknown; however, they do suggest a need for customized antibiotic stewardship interventions to address healthcare setting-specific barriers to appropriate antibiotic prescribing. Additionally, although non-clinic settings may account for only a small proportion of all inappropriate antibiotics, national trend data indicate an increasing portion of care related to acute infections occur in these settings.21 As such, they need to be included in comprehensive efforts to improve antibiotic stewardship for older adults.
Drivers of Inappropriate Antibiotics for Older Adults in Ambulatory Settings
While our understanding of the factors that drive antibiotic prescribing decisions in older adults is limited, a recent qualitative study of general practitioners yields some important insights into the clinical decision process. Among the main identified themes were an acknowledgement of diagnostic uncertainty and associated concern for potential deterioration resulting in hospital admission or death.22 This finding is congruent with the increased risk of both atypical presentations and adverse outcomes among older adults with urinary tract infections and other serious bacterial illnesses, including sepsis.23,24 These concerns translated into overall more aggressive antibiotic utilization including a lower threshold to initiate antibiotics without a clear indication, preferential use of broad-spectrum agents, longer treatment courses, and more frequent hospital referrals for initiation of intravenous antibiotics.22
Although not specific to older adults, a wide variety of additional factors that influence antibiotic prescribing in ambulatory care have been identified.25 Individual provider antibiotic prescribing patterns vary substantially and may be driven by their beliefs about antibiotics, including whether they perceive them as low risk and adopt a ‘better safe than sorry’ approach.25,26 Time pressures may lead clinicians to perceive that prescribing antibiotics is faster than explaining why a patient does not need antibiotics. Patients may see clinicians with different practice patterns regarding antibiotic prescription (either in the same practice or when visiting multiple practices), leading to an expectation for antibiotics. In response to a desire to improve patient satisfaction scores tied to reimbursement, or to improve patient critiques on social media sites tied to patient recruitment, clinicians may prescribe antibiotics in an attempt to improve patient satisfaction.25
Within the broader categorization of ambulatory care, the unscheduled care settings (e.g., ED, UC, retail clinics) share important system-level barriers. Time pressures related to overcrowding in settings where patient arrivals are not scheduled and lack of familiarity with the patients have been identified as potentially important drivers of inappropriate prescribing. This effect is likely magnified for older adults given their increased medical complexity and higher incidence of cognitive impairment, which can restrict optimal patient-clinician communication. These care settings may also serve a population of older adults who do not have reliable followup care, which may also drive overprescribing.
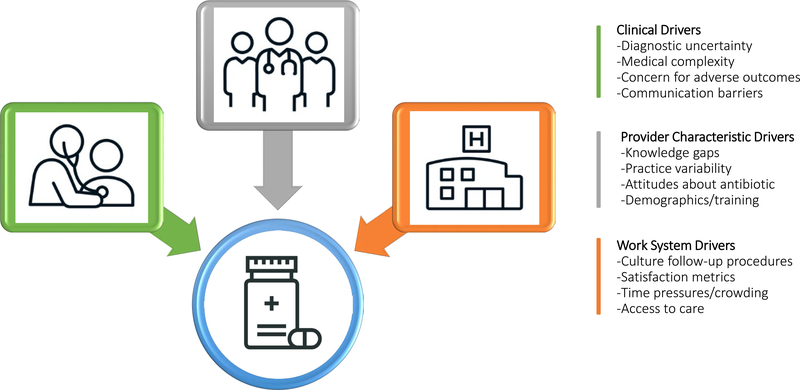
One study involving nearly 500,000 ambulatory care encounters, including UC, family medicine, pediatric, and internal medicine clinics, demonstrated significant variation in antibiotic prescription by clinician degree. Specifically, advanced practice providers were more likely to prescribe antibiotics for inappropriate conditions than physicians. Furthermore, clinicians over the age of 60 were more likely to prescribe antibiotics for inappropriate indications than clinicians under the age of 30.9 A national study of antibiotic prescribing for older adults also demonstrated significant variation based on region of the country, with clinicians in the South more likely to prescribe antibiotics than those in other regions.8 The factors most likely to drive inappropriate antibiotic prescribing for older adults in ambulatory care settings are presented in Figure 1 with corresponding descriptive summaries provided in Table 1.
Figure 1.
Drivers of Inappropriate Antibiotic Prescribing for Older Adults in Ambulatory Care Settings
Table 1.
Descriptions of Inappropriate Antibiotic Prescribing Drivers for Older Adults in Ambulatory Care Settings
| Driver | Description |
|---|---|
| Clinically Based | |
| Diagnostic uncertainty | Older adults can present atypically with serious bacterial illness, including sepsis (e.g., absence of classic symptoms such as fever). |
| Medical complexity | Older adults have a higher prevalence of comorbidities and polypharmacy |
| Concern for adverse outcome | Clinicians apply a lower threshold to initiate antibiotics, use broad-spectrum agents, and prescribe longer treatment courses for older adults in an attempt to mitigate the risk of hospitalization or death. |
| Communication barriers | Cognitive impairment, hearing loss, aphasia, and similar conditions can hinder clinical data acquisition |
| Work System Based | |
| Insufficient culture follow-up | Non-pharmacist clinical staff tasked with modifying antibiotic therapy based on culture results. |
| Patient satisfaction metrics | Perception that antibiotics improve patient satisfaction scores which may be tied to reimbursement. |
| Time pressures | Overcrowding or high patient volume leading to default antibiotic prescribing versus using time to educate patients about appropriate indications. |
| Access to care | Antibiotic prescribing threshold is lower for patients perceived as vulnerable due to unreliable access to follow-up care (e.g., undomiciled, uninsured) |
| Provider Based | |
| Knowledge gaps | Difficult for ambulatory care providers to keep up with constantly evolving guidelines and literature around appropriate antibiotic prescribing. |
| Practice variability | Individual provider practice patterns can influence antibiotic expectations at future encounters with different providers. |
| Attitudes about antibiotics | Providers who weigh the potential for any clinical benefit more heavily than the potential for adverse drug events related to antibiotics are more likely to prescribe. This includes antibiotic prescribing described as “just in case”. |
| Demographics | Variability in antibiotic prescribing has been observed based on clinical degree type, age, and geographic location. |
Ambulatory Care Stewardship Interventions
Ambulatory Care Clinics
A number of studies have investigated interventions to improve antibiotic prescribing in ambulatory clinics. Although not focused on age, studies in ambulatory care settings have found that the following interventions show promise in improving antibiotic stewardship: clinician education (active, in-person); public displays of antibiotic stewardship support; clinical decision support, including best practice alerts in the electronic health record; audit and feedback; communication skills training; requiring clinicians to justify in the medical chart why they are prescribing an antibiotic; delayed prescribing; point-of-care diagnostics; and academic detailing.6,27
In one study that specifically focused on older adults with acute respiratory tract infections in ambulatory clinics, the study team examined the impact of patient education materials (clinic posters and mailed education materials on appropriate antibiotic use) on antibiotic prescribing rates. The intervention had no impact on prescribing rates, however, the investigators did observe wide variation in antibiotic prescribing rates across the unique practices (21–88%).28 This finding is consistent with another study that identified substantial variations in individual-provider outpatient antibiotic prescribing practices.29 These results suggest that provider-focused interventions may be more effective than those aimed at patients.
Emergency Department
The ED has been the focus for a number of antibiotic stewardship interventions primarily focused on patients being admitted or general adult and pediatric populations.30 Among the ED studies identified in a recent systematic review, none specifically reported on outcomes for the subgroup of older adults. However, the identified studies that focus on improving prescribing for community-acquired pneumonia (CAP) and urinary tract infections (UTI) are particularly relevant for older adults. Several studies were able to demonstrate some degree of improved empirical antibiotic selection for CAP and UTI using multifaceted intervention bundles including standardized care pathways, guideline-focused educational programs, audit and feedback, and computerized decision support. Notably, studies using single interventions generally showed a lower impact on appropriate prescribing rates. Also relevant to ambulatory patients in the ED, multiple studies identified in the review demonstrated that clinical pharmacists reviewing culture results post-discharge have improved appropriateness of antibiotic prescribing. Regarding the quality of the available evidence, all but one of the studies assessed had a high risk of bias or failed to provide sufficient methodologic information to complete the assessment.30 This highlights the need for improved study design, including reporting antibiotic utilization and clinical outcomes by age group, in future ED-based antibiotic stewardship intervention studies.
Although not directly aimed at improving antibiotic stewardship, a variety of care delivery models aimed at improving care for older adults in the ED have been developed. For instance, the Geriatric Emergency Department Intervention (GEDI) is a “nursing-led, physician-championed” model that involves specially trained staff who aim to expedite, modify, and coordinate care for frail older adults in the ED setting. When examined in a pragmatic trial, the GEDI model was able to demonstrate sustainable reductions in ED length of stay, hospital admissions, and hospital length of stay without increasing the risk of mortality or recidivism.31 A logical evolution of GEDI and similar proposed models would be to incorporate antibiotic stewardship specific training with an emphasis on high-risk prescribing scenarios for older adults (e.g., asymptomatic bacteriuria, empirical antibiotics for delirium).
Urgent and Retail Care
Similarly, few antibiotic stewardship studies have addressed urgent care and retail clinics and none focus on older adults. In a network of urgent care centers in North Carolina, an educational intervention specifically addressing communication with patients was associated with fewer inappropriate antibiotic prescriptions for upper respiratory infections.32 Another study found moderate success with pharmacist-led culture follow-up for patients with pending urine and wound cultures.33 However, the average age of patients in these studies was 40 years. One national chain of retail clinics has reported on the implementation of a centralized, multifaceted stewardship program across over 1,100 sites with associated low rates of inappropriate antibiotic prescribing.34 However, critics have pointed out that the ease of accessibility of retail clinics may promote healthcare usage and subsequent antibiotic prescriptions for patients who have low acuity conditions and require only supportive care.35
Telemedicine
Direct-to-consumer telemedicine companies provide patients with around-the-clock remote access to care via personal computers and smart phones. A large percentage of these visits are for upper respiratory infections. Telemedicine users tend to be younger than non-users and one study found that pediatric patients accessing these services are more likely to be prescribed antibiotics than those seeking care in clinic or urgent care settings.36 Interestingly, patients accessing telemedicine services are also more likely to be prescribed broad-spectrum antibiotics such as azithromycin and fluoroquinolones.37 Understanding the factors that drive antibiotic prescribing in these settings is key: a recent report concluded that telemedicine providers who infrequently prescribed antibiotics had lower patient satisfaction scores, thus potentially increasing the motivation of telemedicine clinicians to prescribe antibiotics.38
Future Directions
Older adults receiving care in ambulatory settings are at high risk of inappropriate antibiotic prescribing and increased risk for associated patient harms. We need a better understanding of the setting-specific drivers of unnecessary or suboptimal (selection/spectrum, dose, duration) antibiotic prescribing in this vulnerable population along with evidence-based interventions to improve their quality of care. We propose several steps to address the current knowledge gaps. First, national data outlining antibiotic prescribing by ambulatory care setting, indication, and age group is desperately needed in order to quantify the magnitude of this issue. This is especially critical for emerging healthcare delivery systems such as direct-to-consumer commercial telehealth entities that target their marketing to low-acuity infectious conditions. Second, additional rigorous qualitative work is needed to explore both behavioral and system level drivers of antibiotic prescribing for older adults across ambulatory care settings. This will provide critical data for intervention mapping, as has been called for in the literature, such that effectiveness trials are targeting high impact barriers specific to both infection type and care setting.39 Third, subgroup reporting by age in interventional antibiotic stewardship trials is needed to understand the differential impact on prescribing for older adults. As the literature suggests unique drivers of overprescribing in this population, a “one size fits all” intervention implementation following demonstrated effectiveness in the general adult population, may not be optimal. In Table 2, we present potential modifications to existing, evidence-based ambulatory care stewardship interventions to enhance their application to older adults. Finally, novel care delivery systems aimed at improving care for frail older adults in ambulatory care settings, such as GEDI,31 should expand their scope to include diagnostic and antibiotic stewardship for this high-risk population.
Table 2.
Ambulatory Care Antibiotic Stewardship Interventions and Potential Applications for Older Adult Populations
| Ambulatory Care Stewardship Interventions | Application for Older Adults |
|---|---|
| Clinical Decision Support | |
| Best Practice Alerts (electronic health record) | Target high risk conditions specific to older adults (e.g., asymptomatic bacteriuria) |
| Care Pathways | Incorporate evidence-based diagnostic criteria into care pathways for common infections in older adults (e.g., pneumonia, cellulitis, urinary tract infection) |
| Pharmacist Medication Review | Particular attention to dosing adjustments, drug-drug interactions and known adverse effects of antibiotics in older adults (e.g., neuro- and cardiac toxicities) |
| Behavior Modification | |
| Audit and Feedback/Academic Detailing | Focus on high impact clinical scenarios (e.g., bronchitis, asymptomatic bacteriuria) |
| Justifying Prescriptions | Mandate that all antibiotic prescriptions, including those given “just in case”, include a syndrome-specific indication (e.g., cystitis, pneumonia) |
| Clinician Education | Include topics specific to older adults (e.g., atypical presentations, stepwise workup for delirium, when to obtain cultures) |
| Communication Skills Training | Ensure training incorporates scenarios related to older adults (e.g. diagnostic uncertainty around presence of pneumonia or cellulitis) and emphasizes contingency planning |
| Patient Education | Provide educational materials specific to common conditions (e.g., upper respiratory tract infections) that suggest supportive care measures and detail the potential adverse consequences of antibiotics (e.g., drug-drug interactions, Clostridiodes difficile colitis) Public Commitment to Stewardship Posters |
| Public Commitment to Stewardship Posters | Specifically mention stewardship commitment for conditions common among older adults (e.g., asymptomatic bacteriuria) and ensure font size on posters is legible to individuals with visual impairments |
| Diagnostic Tools | |
| Pharmacist Culture Review | Modifying therapy to narrow-spectrum agents and shortest duration possible based on culture results |
| Biomarkers | Ensure availability of real-time C-reactive protein or procalcitonin to help guide antibiotic prescribing for respiratory tract infections |
| Rapid Diagnostic Tests | Ensure availability and provide education about low value of antibiotics for stable patients with negative rapid strep tests or confirmed influenza, consider using respiratory antigen panels to identify serious respiratory illnesses caused by viral infections other than influenza (e.g., respiratory syncytial virus, human metapneumovirus, parainfluenza) |
ACKNOWLEDGEMENTS
This work was supported in part by funds and facilities provided by the Agency for Healthcare Research and Quality (K08HS024342), the Cleveland Geriatric Research, Education, and Clinical Center (GRECC) and the Specialty Care Center of Innovation at the VA Northeast Ohio Healthcare System. The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of the Agency for Healthcare Research and Quality, the VA or of the United States Government.
Footnotes
Conflict of Interest:
MP has served as Co-Investigator on a research study funded by Roche. RJ is the Principal Investigator on research grants from Pfizer and Accelerate; she has also participated in advisory boards for Pfizer and Merck. None of the other authors have relevant conflicts of interest to disclose.
REFERENCES
- 1.Suda KJ, Hicks LA, Roberts RM, Hunkler RJ, Danziger LH. A national evaluation of antibiotic expenditures by healthcare setting in the United States, 2009. J Antimicrob Chemother. 2013;68(3):715–718. doi: 10.1093/jac/dks445 [DOI] [PubMed] [Google Scholar]
- 2.Public Health Agency of Sweden, National Veterinary Institute. Swedres-Svarm 2014: Consumption of Antibiotics and Occurrence of Antibiotic Resistance in Sweden. Solna and Uppsala, Sweden: Public Health Agency of Sweden, National Veterinary Institute; 2015. https://www.folkhalsomyndigheten.se/contentassets/7bb3429f570c4ca0aa5dad4be3c1b58b/swedres-svarm-2014-14027.pdf. Accessed September 26, 2019. [Google Scholar]
- 3.Public Health England. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR): Report 2014. London, England: Public Health England; 2014. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_d300ata/file/362374/ESPAUR_Report_2014__3_.pdf. Accessed September 26, 2019. [Google Scholar]
- 4.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011. JAMA. 2016;315(17):1864303–1873. doi: 10.1001/jama.2016.4151 [DOI] [PubMed] [Google Scholar]
- 5.Sanchez GV. Core Elements of Outpatient Antibiotic Stewardship. MMWR. Recommendations and Reports. https://www.cdc.gov/mmwr/volumes/65/rr/rr6506a1.htm. Published 2016. Accessed April 26, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Drekonja DM, Filice GA, Greer N, et al. Antimicrobial stewardship in outpatient settings: A systematic review. Infect Control Hosp Epidemiol. 2015;36(2):142–152. doi: 10.1017/ice.2014.41 [DOI] [PubMed] [Google Scholar]
- 7.King LM, Bartoces M, Fleming-Dutra KE, Roberts RM, Hicks LA. Changes in US Outpatient Antibiotic Prescriptions From 2011–2016. Clin Infect Dis. 2019;(ciz225). doi: 10.1093/cid/ciz225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabbani S, Palms D, Bartoces M, Stone N, Hicks LA. Outpatient Antibiotic Prescribing for Older Adults in the United States: 2011 to 2014. J Am Geriatr Soc. 2018;66(10):1998315 2002. doi: 10.1111/jgs.15518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt ML, Spencer MD, Davidson LE. Patient, Provider, and Practice Characteristics Associated with Inappropriate Antimicrobial Prescribing in Ambulatory Practices. Infect Control Hosp Epidemiol. 2018;39(3):307–315. doi: 10.1017/ice.2017.263 [DOI] [PubMed] [Google Scholar]
- 10.Silverman M, Povitz M, Sontrop JM, et al. Antibiotic Prescribing for Nonbacterial Acute Upper Respiratory Infections in Elderly Persons. Ann Intern Med. 2017;166(11):765–774. doi: 10.7326/M16-1131 [DOI] [PubMed] [Google Scholar]
- 11.Tanner LA, Baum C. Spontaneous adverse reaction reporting in the elderly. Lancet. 1988;2(8610):580. doi: 10.1016/s0140-6736(88)92713-4 [DOI] [PubMed] [Google Scholar]
- 12.Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibioticassociated adverse events. Clin Infect Dis. 2008;47(6):735. [DOI] [PubMed] [Google Scholar]
- 13.Mattappalil A, Mergenhagen KA. Neurotoxicity with antimicrobials in the elderly: a review. Clin Ther. 2014;36(11):1489–1511.e4. doi: 10.1016/j.clinthera.2014.09.020 [DOI] [PubMed] [Google Scholar]
- 14.Clark CM, White AT, Sellick JA, Mergenhagen KA. Evaluation of Antibiotic Prescribing in a Veterans Affairs Outpatient Setting: Identification of Stewardship Targets. Sr Care Pharm. 2019;34(4):268–278. doi: 10.4140/TCP.n.2019.268. [DOI] [PubMed] [Google Scholar]
- 15.Pechal A, Lin K, Allen S, Reveles K. National age group trends in Clostridium difficile infection incidence and health outcomes in United States Community Hospitals. BMC Infect Dis. 2016;16(1):682. doi: 10.1186/s12879-016-2027-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakoulas G. Adverse Effects of Fluoroquinolones: Where Do We Stand? NEJM Journal Watch Infectious Diseases. https://www.jwatch.org/na48248/2019/02/13/adverse-effectsfluoroquinolones-where-do-we-stand. Published February 13, 2019. Accessed September 27, 2019. [Google Scholar]
- 17.Rao GA, Mann JR, Shoaibi A, et al. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Ann Fam Med. 2014;12(2):121–127. doi: 10.1370/afm.1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60(9):1308–1316. doi: 10.1093/cid/civ076 [DOI] [PubMed] [Google Scholar]
- 19.Linder JA, Brown T, Lee JY, Chua K-P, Fischer MA. 1632. Non-Visit-Based and Non-Infection-Related Ambulatory Antibiotic Prescribing. Open Forum Infect Dis. 2018;5(Suppl 1):S43. doi: 10.1093/ofid/ofy209.102 [DOI] [Google Scholar]
- 20.Palms DL, Hicks LA, Bartoces M, et al. Comparison of Antibiotic Prescribing in Retail Clinics, Urgent Care Centers, Emergency Departments, and Traditional Ambulatory Care Settings in the United States. JAMA Intern Med. 2018;178(9):1267–1269. doi: 10.1001/jamainternmed.2018.1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poon SJ, Schuur JD, Mehrotra A. Trends in Visits to Acute Care Venues for Treatment of Low-Acuity Conditions in the United States From 2008 to 2015. JAMA Intern Med. 2018;178(10):1342–1349. doi: 10.1001/jamainternmed.2018.3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayward GN, Moore A, Mckelvie S, Lasserson DS, Croxson C. Antibiotic prescribing for the older adult: beliefs and practices in primary care. J Antimicrob Chemother. 2019;74(3):791–797. doi: 10.1093/jac/dky504 [DOI] [PubMed] [Google Scholar]
- 23.Yoshikawa TT. Antimicrobial resistance and aging: beginning of the end of the antibiotic era? J Am Geriatr Soc. 2002;50(7 Suppl):S226–229. [DOI] [PubMed] [Google Scholar]
- 24.Gharbi M, Drysdale JH, Lishman H, et al. Antibiotic management of urinary tract infection in elderly patients in primary care and its association with bloodstream infections and all cause mortality: population based cohort study. BMJ. 2019;364:l525. doi: 10.1136/bmj.l525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson W, Tonkin-Crine S, Pavitt SH, et al. Factors associated with antibiotic prescribing for adults with acute conditions: an umbrella review across primary care and a systematic review focusing on primary dental care. J Antimicrob Chemother. 2019;74(8):2139–2152. doi: 10.1093/jac/dkz152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz KL, Brown KA, Etches J, et al. Predictors and variability of antibiotic prescribing amongst family physicians. J Antimicrob Chemother. 2019;74(7):2098–2105. doi: 10.1093/jac/dkz112 [DOI] [PubMed] [Google Scholar]
- 27.King LM, Fleming-Dutra KE, Hicks LA. Advances in optimizing the prescription of antibiotics in outpatient settings. BMJ. 2018;363:k3047. doi: 10.1136/bmj.k3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzales R, Sauaia A, Corbett KK, et al. Antibiotic Treatment of Acute Respiratory Tract Infections in the Elderly: Effect of a Multidimensional Educational Intervention. J Am Geriatr Soc. 2004;52(1):39–45. doi: 10.1111/j.1532-5415.2004.52008.x [DOI] [PubMed] [Google Scholar]
- 29.Jones BE, Sauer B, Jones MM, et al. Variation in Outpatient Antibiotic Prescribing for Acute Respiratory Infections in the Veteran Population: A Cross-sectional Study. Ann Intern Med. 2015;163(2):73–80. doi: 10.7326/m14-1933 [DOI] [PubMed] [Google Scholar]
- 30.Losier M, Ramsey TD, Wilby KJ, Black EK. A Systematic Review of Antimicrobial Stewardship Interventions in the Emergency Department. Ann Pharmacother. 2017;51(9):774–790. doi: 10.1177/1060028017709820 [DOI] [PubMed] [Google Scholar]
- 31.Wallis M, Marsden E, Taylor A, et al. The Geriatric Emergency Department Intervention model of care: a pragmatic trial. BMC Geriatr. 2018;18(1):297. doi: 10.1186/s12877-018-0992-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Link TL, Townsend ML, Leung E, Kommu S, Vega RY, Hendrix CC. Reducing Inappropriate Antibiotic Prescribing for Adults With Acute Bronchitis in an Urgent Care Setting: A Quality Improvement Initiative. Adv Emerg Nurs J. 2016;38(4):327–335. doi: 10.1097/TME.0000000000000122 [DOI] [PubMed] [Google Scholar]
- 33.Fay LN, Wolf LM, Brandt KL, et al. Pharmacist-led antimicrobial stewardship program in an urgent care setting. Am J Health Syst Pharm. 2019;76(3):175–181. doi: 10.1093/ajhp/zxy023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polinski JM, Harmon SL, Henderson KJ, Barker T, Sussman A, Gagliano NJ. Antibiotic stewardship in the retail clinic setting: Implementation in 1100 clinics nationwide. Healthc Amst Neth. 2017;5(3):89–91. doi: 10.1016/j.hjdsi.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 35.Incze MA, Redberg RF, Katz MH. Overprescription in Urgent Care Clinics—The Fast and the Spurious. JAMA Intern Med. 2018;178(9):1269–1270. doi: 10.1001/jamainternmed.2018.1628 [DOI] [PubMed] [Google Scholar]
- 36.Ray KN, Shi Z, Gidengil CA, Poon SJ, Uscher-Pines L, Mehrotra A. Antibiotic Prescribing During Pediatric Direct-to-Consumer Telemedicine Visits. Pediatrics. April 2019:e20182491. doi: 10.1542/peds.2018-2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uscher-Pines L, Mulcahy A, Cowling D, Hunter G, Burns R, Mehrotra A. Antibiotic prescribing for acute respiratory infections in direct-to-consumer telemedicine visits. JAMA Intern Med. 2015;175(7):1234–1235. doi: 10.1001/jamainternmed.2015.2024 [DOI] [PubMed] [Google Scholar]
- 38.Martinez KA, Rood M, Jhangiani N, Kou L, Boissy A, Rothberg MB. Association Between Antibiotic Prescribing for Respiratory Tract Infections and Patient Satisfaction in Direct-to-Consumer Telemedicine. JAMA Intern Med. 2018;178(11):1558–1560. doi: 10.1001/jamainternmed.2018.4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Charani E, Edwards R, Sevdalis N, et al. Behavior change strategies to influence antimicrobial prescribing in acute care: a systematic review. Clin Infect Dis. 2011;53(7):651–662. doi: 10.1093/cid/cir445 [DOI] [PubMed] [Google Scholar]