Abstract
Background
Kidney disease is prevalent in low-resource settings worldwide, but tests for its diagnosis are often unavailable. The saliva urea nitrogen (SUN) dipstick is a laboratory and electricity independent tool, which may be used for the detection of kidney disease. We investigated the feasibility and performance of its use in diagnosing kidney disease in community settings in Africa.
Methods
Adult patients at increased risk of kidney disease presenting to three community health centres, a rural district hospital and a central hospital in Malawi were recruited between October 2016 and September 2017. Patients underwent concurrent SUN and creatinine testing at enrolment, and at 1 week, 1 month, 3 months and 6 months thereafter.
Results
Of 710 patients who presented at increased risk of kidney disease, 655 (92.3%) underwent SUN testing at enrolment, and were included (aged 38 (29-52) years, 367 (56%) female and 333 (50.8%) with HIV). Kidney disease was present in 482 (73.6%) patients and 1479 SUN measurements were made overall. Estimated glomerular filtration rate (eGFR) correlated with SUN (r=−0.39; p<0.0001). The area under the receiver operating characteristics curve was 0.61 for presenting SUN to detect acute or chronic kidney disease, and 0.87 to detect severe (eGFR <15 mL/min/1.73 m2) kidney disease (p<0.0001; sensitivity 82.3%, specificity 81.8%, test accuracy 81.8%). In-hospital mortality was greater if enrolment SUN was elevated (>test pad #1) compared with patients with non-elevated SUN (p<0.0001; HR 3.3 (95% CI 1.7 to 6.1).
Conclusions
SUN, measured by dipstick, is feasible and may be used to screen for kidney disease in low resource settings where creatinine tests are unavailable.
Keywords: other diagnostic or tool, health systems, prevention strategies, screening, HIV
Key questions.
What is already known?
The diagnosis of kidney disease is a major challenge in low and middle-income countries due to the unavailability of serum creatinine tests. The saliva urea nitrogen dipstick performs well in detecting kidney disease in high-income settings, and in tertiary centres in low-income settings.
What are the new findings?
The use of a saliva urea nitrogen dipstick was feasible in rural and community settings in sub-Sahara Africa, and performed well in diagnosing severe kidney disease.
What do the new findings imply?
The saliva urea nitrogen dipstick may be used to detect kidney disease in locations where serum creatinine is unavailable.
Introduction
Kidney disease, both acute and chronic, is highly prevalent in low-resource settings (LRS), and leads to preventable deaths often in young populations.1 2 In Malawi, a low-income country in sub-Sahara Africa, 21% of general medical admissions have kidney disease, resulting in an in-hospital mortality of 44%.3 One of the biggest barriers to the management of kidney disease in LRS is its timely detection, which predominantly relies on reliable and rapid access to serum creatinine, on which the diagnosis of acute and chronic kidney disease is currently made.4 5 In many LRS, including Malawi, serum creatinine is unavailable in health centres and district hospitals, where the majority of acutely unwell patients at risk of kidney disease present. Even in central hospitals, availability of laboratory measurement of serum creatinine may be intermittent. This is a result of the requirement of relatively expensive reagents, a functioning biochemical analyser and the relevant laboratory expertise, all of which are frequently lacking. Point-of-care (POC) devices obviate the need for a biochemistry laboratory in diagnosing kidney disease.6 Such devices predominantly measure creatinine, but frequently require refrigerated storage of reagents and power, and this precludes their use in the poorest and most rural areas. Diagnosis of kidney disease in these settings, therefore, remains a significant challenge.
Urea nitrogen diffuses from the blood to saliva through the salivary glands and saliva urea nitrogen (SUN) parallels blood urea nitrogen (BUN), which may be measured and used to diagnose kidney disease.7–13 SUN measurement may be by standard laboratory techniques, but also by a simple dipstick method, using an SUN dipstick.14–18 SUN, measured by dipstick, has been previously shown to be effective in diagnosing acute and chronic kidney disease in high-income settings,14 15 and moreover in monitoring response to treatment.16 In LRS, we have demonstrated that the SUN dipstick may be used to screen for kidney disease in general medical admissions to a central hospital in Malawi, and in patients admitted with malaria in a central hospital in Angola, with areas under the receiver operating characteristics (ROC) curve of 0.82 and 0.88, respectively.17 18 The SUN dipstick, therefore, shows promise, as a simple, low technology and non-invasive tool for the detection of kidney disease. However, the feasibility of its use and accuracy to screen for kidney disease in healthcare settings that do not have access to serum creatinine (eg, rural hospitals and community health centres) is unknown. We; therefore, undertook this study to investigate the feasibility and performance of using the SUN dipstick to detect kidney disease across a variety of healthcare settings in sub-Sahara Africa. This was done in association with the International Society of Nephrology 0by25 initiative, which aims to eliminate preventable deaths from Acute Kidney Injury (AKI) worldwide by 2025.19
Methods
Study design and setting
We conducted a prospective multicentre cross-sectional study in five healthcare facilities in Malawi, which were participating in the 0by25 feasibility project.20 These included three health centres (Bangwe Health Centre, Gateway Clinic and Chileka Health Centre), a rural district hospital (Chikwawa district hospital) and a central hospital (Queen Elizabeth Central Hospital, QECH, Blantyre) (online supplementary figure s1). Malawi is ranked as one of the poorest countries worldwide, but despite this government-funded healthcare is provided free at the point of delivery to all.21 This healthcare is resource limited and serum creatinine is unavailable in health centres. In district and central hospitals, creatinine is intermittently available, and if available it often takes 1–3 days for the results to be processed. Patients in Malawi present largely to health centres, which act as primary care facilities and see a large number of patients, frequently more than 200 each day. Health centres have limited diagnostic testing and treatment capability; malaria antigen and HIV testing are largely available, as are a limited range of oral antimicrobial drugs. Further blood tests such as full blood count, any imaging including chest x-ray, or intravenous fluids or antibiotics, requires admission to district hospitals. Specialist renal care, including the provision of renal replacement therapy, requires referral to one of two central hospitals, including QECH.
bmjgh-2020-002312supp001.pdf (1.1MB, pdf)
Participants
All patients presenting to the healthcare facilities between October 2016 and September 2017 were eligible to be screened for risk of kidney disease. A pragmatic approach using this time frame was therefore the determinant of the sample size. Patients were screened for their risk of kidney disease using a risk score, based on comorbidity, nephrotoxic medication use and presenting symptoms and signs (table 1). Those with a risk score of greater than two points were considered at increased risk of kidney disease. We enrolled all patients at increased risk of kidney disease, and these patients underwent POC testing, including measurement of SUN by saliva dipstick, at enrolment. In this analysis, we included adult (aged ≥18 years) participants who had an SUN dipstick measurement recorded at enrolment. Children (aged <18 years) and those without an SUN result at enrolment were excluded.
Table 1.
Scoring system used to assess risk of kidney disease, and prevalence of each variable in the included cohort
| Variable | Points attributed | Prevalence in included cohort (n, %) |
| History of kidney disease | 1 | 6 (0.9) |
| Presence of oliguria (patient reported) | 4 | 217 (33.1) |
| Infection with fever (≥38°C or ≤6°C) | 1 | 348 (53.1) |
| Hypotension (<90/60) or shock (presence of hypotension with signs of decreased organ perfusion, such as altered metal status, oliguria or heart failure) | 2 | 82 (12.5) |
| Pregnancy with hypertension (>140/90 and receiving treatment) or seizures | 2 | 7 (1.1) |
| Swelling (entire body) | 2 | 25 (3.8) |
| Decreased appetite | 1 | 463 (70.7) |
| HIV | 1 | 333 (50.8) |
| Coma or confusion | 2 | 36 (5.5) |
| Anaemia (haemoglobin <90 g/L) or pallor | 1 | 121 (18.5) |
Score 0–2: low risk; 3–6=moderate risk; >6=high risk. Moderate and high risk were deemed at increased risk of kidney disease and enrolled. Maximum points=17.
Data collection
Demographic and clinical data, including comorbidities and presenting symptoms and vital signs, were recorded at enrolment through patient history and review of relevant medical records. Patients underwent SUN and POC creatinine measurement at enrolment, and then again at 1 week, 1 month, 3 months and 6 months thereafter. Patient survival to hospital discharge was recorded.
SUN measurement
Local data collectors, one for each study site, underwent face-to-face training on the methods of SUN measurement by dipstick prior to study initiation. For measurement, unstimulated saliva was collected in a plastic cup and approximately 50 µL of saliva were used to moisten the test pad of a colorimetric SUN dipstick (Integrated Biomedical Technology, IN, USA). The dipstick test pad contains a urease enzyme in a bound form which cleaves SUN when moistened with saliva; this leads to the formation of ammonia and hydroxyl ions resulting in a change in pH which, by a pH indicator substance, consequently changes the colour of the test pad. After 1 min, the colour of the test pad is compared with six reference pads indicating increasing SUN concentrations: 5–14 mg/dL (test pad #1), 15–24 mg/dL (test pad #2), 25–34 mg/dL (test pad #3), 35–54 mg/dL (test pad #4), 55–74 mg/dL (test pad #5) and ≥75 mg/dL (test pad #6) (online supplementary figure s2). Data collectors were unaware of the presenting creatinine result at the time of SUN dipstick interpretation.
bmjgh-2020-002312supp002.pdf (560.5KB, pdf)
Creatinine measurement
Creatinine was measured at the POC using the StatSensor Xpress Creatinine device (Nova Biomedical Cooperation, Waltham, Massachusetts, USA). Prior to study initiation, the reliability of the POC test was confirmed; good correlation and linearity were obtained between simultaneous determination of QECH laboratory and POC creatinine values.22
Definitions
Kidney function at enrolment was defined based on prior history, creatinine and urinalysis. Patients with a serum creatinine obtained within the previous 12 months showing estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD) or with a known history of chronic kidney disease were considered as having CKD. Acute kidney disease (AKD) was defined by the presence of albuminuria and/or eGFR at enrolment lower than 75 mL/min/1.73 m2. Patients not fulfilling these criteria were classified as no-kidney disease (NKD). Based on serum creatinine within 7 days of presentation, patients were further classified as AKI if they underwent an increase or decrease in serum creatinine of ≥0.3 mg/dL within 48 hours or of 1.5 times greater than the reference serum creatinine value at enrolment, in accordance with Kidney Disease Improving Global Outcomes (KDIGO) criteria.4 In patients without a history of CKD, we used the first serum creatinine at enrolment as the reference creatinine on which to detect subsequent changes in kidney function. Kidney status at day 7 is subsequently reported (one of AKI, AKD, stable CKD or NKD).
Outcome measures
We determined the feasibility of using the SUN dipstick to diagnose kidney disease in community and district settings in LRS. We assessed the diagnostic performance of the presenting SUN dipstick result to detect kidney disease, according to KDIGO criteria. We also assessed the diagnostic performance of the presenting SUN dipstick result to detect kidney disease according to various presenting eGFR cut-offs (<60, 45, 30, and 15 mL/min/1.73 m2). Lastly, we assessed patient survival to hospital discharge according to presenting SUN result.
Statistical analysis
Data are presented as number and percentages for categorical variables and mean and SD or median and IQR for continuous variables depending on data distribution. Creatinine and eGFR were plotted according to SUN result for all tests across all time points and compared using a one-way analysis of variance (Kruskal-Wallis and Dunn’s multiple comparison test). SUN was transformed from a categorical variable to a continuous variable using the SUN midpoint corresponding the each test pad result (eg, 9.5 mg/dL for SUN test pad #1, range 5–14 mg/dL), and SUN was then correlated to creatinine, eGFR, and proteinuria using the non-parametric Spearman correlation. For creatinine and eGFR, this was done in all tests, and in tests specifically undertaken at enrolment, at 7 days and at 1 month.
We assessed the diagnostic performance of the presenting SUN dipstick to detect Kidney Disease (AKI, AKD or CKD), and to detect presenting eGFRs of: <60, 45, 30 and 15 mL/min/1.73 m2, respectively. To do this, we calculated the area under the ROC curve, and the sensitivity and specificity of each SUN dipstick result for each of these parameters. We did this in all patients, and specifically in subgroups defined by age, sex, HIV and the presence of dehydration at presentation. We determined the optimal threshold of SUN result for diagnosis according to the maximum Youden’s index (Youden’s index=sensitivity%+specificity% – 100). For the optimal threshold, we determined the positive and negative likelihood ratios and predictive values, as well as the test accuracy, and report these with 95% CIs. We also determined the diagnostic performance of a ‘change in SUN’ (increase or decrease from presentation at day 7) to detect AKD.
We performed a survival analysis, comparing in-hospital survival according to enrolment SUN using the log rank test, and created an HR (95% CI) for mortality comparing patients with elevated enrolment SUN (>test pad #1) to patients with non elevated enrolment SUN (test pad #1). Statistical analysis was performed using GraphPad Prism V.7 (www.graphpad.com). A p<0.05 was considered statistically significant.
Patient and public involvement
The results of this study will be disseminated through the Kidney Patients Association of Malawi.
Results
Cohort description
A total of 244 300 patients presented to the healthcare facilities during the observation period. A total of 1615 patients were screened for risk of kidney disease, and 820 (50.8%) were at increased risk (moderate or high risk score). A total of 710 (86.6%) patients were adults, and 655 (92.3%) had a documented SUN result at presentation and were included in the analysis (figure 1). Three hundred and seventy-one (56.7%) patients were recruited from health centres (Bangwe HC=124, Chileka HC=33, and Gateway Clinic=214), 128 (19.5%) from a rural district hospital (Chikwawa district hospital), and 156 (23.8%) from the emergency department at a central hospital (QECH).
Figure 1.
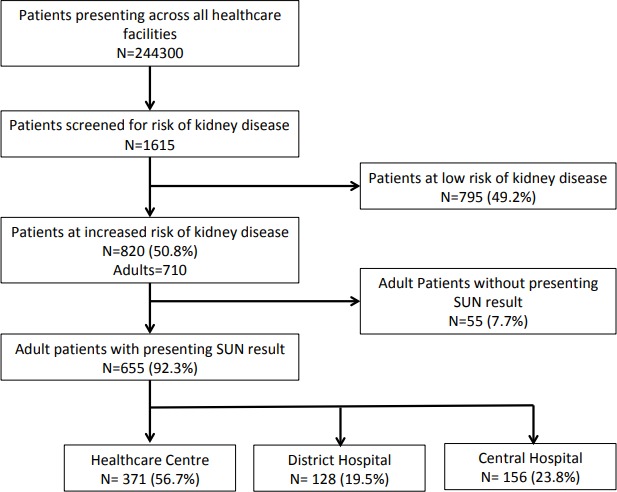
Cohort description. SUN, saliva urea nitrogen.
Median age was 38 years (29–52), and 367 (56%) patients were female (table 2). Median height and weight were 161 cm (157–165) and 54 kg (48–60), respectively. The most common comorbidity was HIV (n=333; 50.8%). Other comorbidities included: hypertension (n=116, 17.7%), anaemia (n=86; 13.1%), diabetes mellitus (n=50, 7.6%), lung disease (n=42, 6.4%), heart disease (n=22, 3.4%), malignancy (n=13, 2.0%) and liver disease (n=12, 1.8%). Six (0.9%) patients had a known history of CKD. The prevalence of variables that contributed to the risk score in included patients is outlined in table 1. The predominant reason for presentation was infectious illness (n=486, 74.2%), most commonly gastrointestinal (n=154; 23.5%), respiratory (n=135, 20.6%) and malaria (n=60, 9.1%).
Table 2.
Baseline characteristics of the study cohort
| Baseline characteristic | Study cohort n=655 |
| Demographic | |
| Age; years (median; IQR) | 38 (29–52) |
| Sex: female (no; %) | 367 (56) |
| Ethnicity: black African (no; %) | 655 (100) |
| Clinical | |
| Height; centimetres (median; IQR) | 161 (157–165) |
| Weight; kilograms (median; IQR) | 54 (48–60) |
| Comorbidities | |
| HIV (no; %) | 333 (50.8) |
| Hypertension (no; %) | 116 (17.7) |
| Anaemia (no; %) | 86 (13.1) |
| Diabetes mellitus (no; %) | 50 (7.6) |
| Lung disease (no; %) | 42 (6.4) |
| Heart disease (no; %) | 22 (3.4) |
| Malignancy (no; %) | 13 (2.0) |
| Liver disease (no; %) | 12 (1.8) |
| Chronic kidney disease (known) (no; %) | 6 (0.9) |
Median presenting creatinine in all patients was 1.3 mg/dL (0.98–1.93), corresponding to a mean presenting eGFR of 68.4 ± 37.9 mL/min/1.73 m2. One hundred and seventy-three (26.4%) patients had normal renal function and 482 (73.6%) patients had kidney disease (subsequent diagnosis at day 7 of AKI=130, 19.9%; AKD=343, 52.2%; and CKD=10, 1.5%). AKI was stage 1 in 71 (54.6%) patients, stage 2 in 25 (19.2%) and stage 3 in 34 (26.2%). Median presenting creatinine in patients with kidney disease was 1.5 mg/dL (1.2–2.5) and mean eGFR was 53.3±33.2 mL/min/1.73 m2.
SUN measurements
A total of 1479 SUN measurements were made across the observation time points (655 at enrolment, 384 at day 7, 246 at 1 month, 124 at 3 months and 70 at 6 months). Creatinine and eGFR according to SUN result are outlined in table 3 and figure 2. There were significant differences in both measures according to SUN result (p<0.0001 for both). SUN, transformed to a continuous variable using the midpoint of each test pad range, correlated with creatinine and eGFR when analysed across all SUN tests undertaken, and in those undertaken specifically at enrolment, at 7 days and at 1 month (table 4). SUN also correlated with proteinuria (Spearman’s r=0.27 (0.18–0.36); p<0.0001), and there were significant differences in SUN according to proteinuria as measured by dipstick (p<0.0001).
Table 3.
Creatinine and EGFR according to sun result
| SUN result |
SUN 1 (5–14 mg/dL) | SUN 2 (15–24 mg/dL) | SUN 3 (25–34 mg/dL) | SUN 4 (35–54 mg/dL) | SUN 5 (55–74 mg/dL) | SUN 6 (>75 mg/dL) | P value |
| No of values | 1239 | 161 | 20 | 18 | 8 | 18 | |
| Creatinine (mg/dL; median, IQR) | 1.03 (0.81–1.35) | 1.67 (1.19–2.76) | 3.61 (1.51–6.37) | 6.01 (2.80–9.63) | 5.87 (4.32–7.00) | 10.5 (6.46–12.00) | <0.0001 |
| eGFR (mL/min/1.73 m2; median, IQR) | 87.1 (63.8–114.9) | 47.5 (24.6–75.6) | 19.1 (10.8–49.3) | 10.2 (7.0–23.3) | 10.1 (8.2–14.7) | 5.9 (1.9–8.6) | <0.0001 |
All SUN tests undertaken at all time points are included. P value represents one-way analysis of variance (Kruskill-Wallis).
SUN, saliva urea nitrogen.
Figure 2.
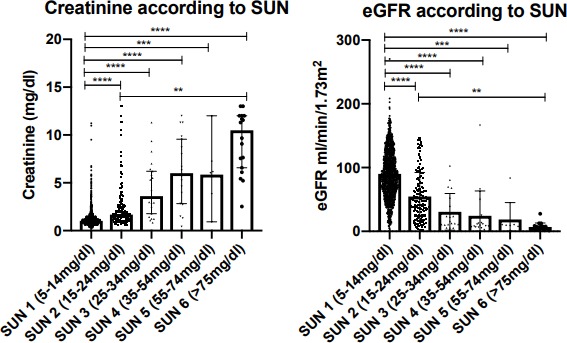
Creatinine and EGFR according to sun result. All SUN tests undertaken at all time points are included. Plots demonstrate median and 95% CIs around the median; statistical significance demonstrated is according to Dunn’s multiple comparison testing (**p<0.01, ***p<0.001, ****p<0.0001). EGFR, estimated glomerular filtration rate; SUN, Saliva Urea Nitrogen.
Table 4.
Correlation between SUN and creatinine, and SUN and EGFR
| All SUN tests | SUN at enrolment | SUN at day 7 | SUN at 1 months | |
| No of pairs | 1464 | 649 | 379 | 244 |
| Spearman r (95% CI) SUN-creatinine | 0.40 (0.35 to 0.44) | 0.48 (0.42 to 0.54) | 0.22 (0.12 to 0.32) | 0.20 (0.07 to 0.32) |
| P value | <0.0001 | <0.0001 | <0.0001 | 0.0022 |
| Spearman r (95% CI) SUN-eGFR | −0.39 (−0.43 to −0.34) | −0.47 (−0.53 to −0.41) | −0.22 (−0.31 to −0.12) | −0.21 (−0.33 to −0.08) |
| P value | <0.0001 | <0.0001 | <0.0001 | 0.0008 |
eGFR, estimated glomerular filtration rate; SUN, saliva urea nitrogen.
Diagnostic performance of SUN
The diagnostic performance of the presenting SUN to detect kidney disease according to a number of different criteria (kidney disease according to KDIGO definitions, and presenting eGFR <60, 45, 30, and 15 mL/min/1.73 m2) is outlined in table 5. The area under the ROC curve ranged from 0.61 (0.56–0.65) for the diagnosis of any Kidney Disease (AKI, AKD or CKD) to 0.87 (0.81–0.93) for the detection of severe kidney disease (presenting eGFR <15 mL/min/1.73 m2) (table 5 and figure 3). The area under the ROC curve to diagnose any kidney disease was similar in patient subgroups defined by age and sex, and in patients with and without HIV (area under ROC curve 0.61 if ≤38 years and 0.60 if >38 years; 0.60 if male and 0.62 if female; 0.62 if HIV and 0.59 if no HIV). The area under the ROC curves in patients with and without symptoms/signs of dehydration at presentation were 0.63 and 0.59, respectively. The optimal threshold according to Youden’s index for diagnosing all parameters was above test pad #1. Specificity was highest, 91.3% (86.1–95.1), when the test was used to detect any kidney disease, with a positive predictive value of 90.5% (85.2–94.0) for this diagnostic criterion. Sensitivity was highest, 82.3% (70.5–90.8), when the test was used to detect a presenting eGFR <15 mL/min/1.73 m2, with a negative predictive value of 97.8% (96.2–98.7) for this diagnostic criterion. Overall, test accuracy improved as the severity of kidney disease to be detected increased, 46% (42.1–49.9) test accuracy for the detection of any kidney disease, compared with 81.8% (78.6–84.7) test accuracy for the detection of an eGFR <15 mL/min/1.73 m2 (sensitivity 82.3%; specificity 81.8%). The sensitivity and specificity for a change in SUN (increase or decrease from enrolment to day 7) to detect AKD or AKI were 81.9% (72.9–88.4) and 25.8% (21.2–31.2), respectively, and therefore, did not provide additional benefit compared with using the SUN at enrolment alone.
Table 5.
Diagnostic performance of presenting SUN (measured by dipstick) to detect any kidney disease, and a presenting EGFR of <60, <45, <30, and <15 mL/min/1.73
| Diagnosis criteria | Any kidney disease (AKI, AKD and CKD) | Presenting eGFR <60 mL/min/1.73 m2 | Presenting eGFR <45 mL/min/1.73 m2 | Presenting eGFR <30 mL/min/1.73 m2 | Presenting eGFR <15 mL/min/1.73 m2 |
| No of SUN tests in analysis | 655 | 649 | 649 | 649 | 649 |
| No of patients with diagnosis (%, prevalence) | 482 (73.6) | 368 (43.3) | 187 (28.8) | 115 (17.7) | 62 (9.6) |
| Area under ROC curve (95% CI) | 0.61 (0.56 to 0.65) | 0.67 (0.63 to 0.71) | 0.71 (0.67 to 0.76) | 0.78 (0.72 to 0.83) | 0.87 (0.81 to 0.93) |
| P value for area under ROC | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Optimal SUN threhold for diagnosis | Above testpad #1 | Above testpad #1 | Above testpad #1 | Above testpad #1 | Above testpad #1 |
| Younden's Index at this threshold | 21 | 33 | 38 | 51 | 64 |
| True positives | 143 | 121 | 100 | 76 | 51 |
| False negatives | 339 | 160 | 87 | 39 | 11 |
| False positives | 15 | 37 | 58 | 82 | 107 |
| True negatives | 158 | 331 | 404 | 452 | 480 |
| Sensitivity (%, 95% CI) | 29.7 (25.6 to 34.0) | 43.1 (37.2 to 49.1) | 53.5 (46.1 to 60.8) | 66.1 (56.7 to 74.7) | 82.3 (70.5 to 90.8) |
| Specificity (%, 95% CI) | 91.3 (86.1 to 95.1) | 90.0 (86.4 to 92.8) | 87.5 (84.1 to 90.3) | 84.6 (81.3 to 87.6) | 81.8 (78.4 to 84.8) |
| Positive likelihod ratio (95% CI) | 3.42 (2.07 to 5.66) | 4.28 (3.07 to 5.98) | 4.26 (3.23 to 5.61) | 4.3 (3.39 to 5.46) | 4.51 (3.67 to 5.55) |
| Negative likelihood ratio (95% CI) | 0.77 (0.72 to 0.83) | 0.63 (0.57 to 0.70) | 0.53 (0.45 to 0.62) | 0.4 (0.31 to 0.52) | 0.22 (0.13 to 0.37) |
| Positive predictive value (%, 95% CI) | 90.5 (85.2 to 94.0) | 76.6 (70.1 to 82.0) | 63.3 (56.7 to 69.4) | 48.1 (42.2 to 54.1) | 32.3 (27.9 to 37.0) |
| Negative predictive value (%, 95% CI) | 31.8 (30.2 to 33.4) | 67.4 (65.0 to 69.7) | 82.3 (79.9 to 84.5) | 92.1 (90.0 to 93.8) | 97.8 (96.2 to 98.7) |
| Test accuracy (%) | 46.0 (42.1 to 49.9) | 69.7 (66.0 to 73.2) | 77.7 (74.3 to 80.8) | 81.4 (78.1 to 84.3) | 81.8 (78.6 to 84.7) |
AKI, acute kidney injury; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ROC, receiver operating characteristics; SUN, saliva urea nitrogen.
Figure 3.
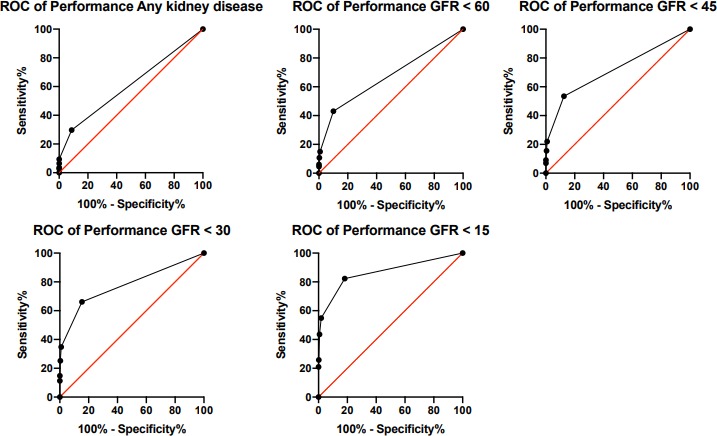
Receiver operating characteristic curves (ROC) for the performance of SUN (measured by dipstick) to detect any kidney disease (AKI, AKD, or CKD), and presenting eGFRs of <60, 45, 30 and 15 mL/min/1.73 m2. AKI, acute kidney injury; AKD, acute kidney disease; CKD, chronic kidney disease; eGFRs, estimated glomerular filtration rates; SUN, saliva urea nitrogen.
Outcome according to SUN
Health facility outcome data were recorded in 436 patients; in-facility death occurred in 46 (10.6%) patients overall. Mortality was higher in patients with elevated SUN (test pad > #1) at enrolment compared with patients with non-elevated enrolment SUN (test pad #1) (log rank p<0.0001; HR 3.25 (1.7–6.1); figure 4).
Figure 4.
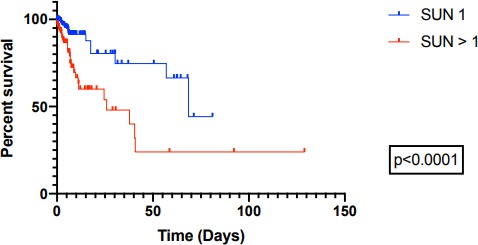
In-hospital survival according to presenting SUN (measured by dipstick). SUN, saliva urea nitrogen.
Discussion
Lack of availability of diagnostic tests is a major obstacle to the management of kidney disease in LRS worldwide. The SUN dipstick is a simple and inexpensive tool that has shown promise in the detection of kidney disease. In this study, we tested the feasibility and performance of its use to detect kidney disease in multiple healthcare settings in sub-Sahara Africa, where serum creatinine is not routinely available.
Main findings
We enrolled 720 adult patients at increased risk of kidney disease presenting to five healthcare facilities in Malawi. SUN testing was feasible with 655 (92.3%) patients undergoing measurement at enrolment. Further SUN testing over a maximum follow-up period of 6 months resulted in 1479 SUN measurements being undertaken overall. Creatinine and eGFR were significantly different between SUN groups, and SUN correlated with both parameters at multiple observation time points. SUN measured at enrolment was specific (91.3%) but not sensitive (29.7%) when used to detect any kidney disease. The diagnostic performance of SUN was best when used to detect severe kidney disease, with an area under the ROC curve and test accuracy of 0.87% and 81.8%, respectively, when used to detect an eGFR <15 mL/min/1.73 m2. In-hospital mortality increased with increasing enrolment SUN.
Interpretation
The majority of acutely unwell patients in LRS present to health centres and district hospitals. In Malawi, like most LRS settings worldwide, these healthcare facilities do not have access to measurement of creatinine making the diagnosis of kidney disease almost impossible until its late stage when oligoanuria has developed. As demonstrated in this and other studies, kidney disease is prevalent in patients presenting to such facilities, particularly in those with additional risk factors for kidney disease.1 23 24 Many facilities are in rural areas, with unreliable power, hence the development of laboratory and electricity independent diagnostic tools to detect kidney disease is important.
In this study, we recruited patients from five healthcare facilities in Malawi that do not routinely have creatinine available. Given the large number of patients that present to these facilities each day, we used a risk score to screen patients for risk of kidney disease, and enrolled patients at increased risk. A high proportion of the enrolled cohort (73.6%) had kidney disease, predominantly AKD and AKI. The cohort was young (median age 38 years), with a high prevalence of HIV (50.8%), presenting predominantly with acute infectious illness. This is representative of populations presenting to such facilities across Malawi, elsewhere in sub-Sahara Africa and in many other LRS around the world.24 25
Undertaking SUN measurement using the dipstick is a simple procedure. It does not require high levels of technical skill, electricity or refrigerated storage of reagents. The result is available within 1 min, meaning initiation of treatment in those identified with kidney disease can be almost immediate unlike waiting for laboratory measurement of serum creatinine. In this study, 92.3% of patients at increased risk of kidney disease underwent successful SUN measurement across all healthcare facilities, demonstrating its use is feasible in community and rural district settings in this part of the world. We did not record the reasons why specific patients did not have SUN recorded, but it is our experience that this is largely due to the inability of the patient to provide sufficient saliva, sometimes due to acute confusion or reduced conscious level.17 26
We assessed the performance of the presenting SUN, measured by dipstick, to detect kidney disease according to a number of different criteria. These included kidney disease according to KDIGO criteria (any of AKI, AKD or CKD), and kidney disease according to a number of different eGFR thresholds. When used to detect any kidney disease, elevated SUN (>test pad #1) had a high specificity of 91.3%, but a low sensitivity of 29.7%. The positive predictive value was high (90.5%), but the negative predictive value was low (31.8%). We can, therefore, say that those patients without kidney disease are unlikely to have an elevated SUN at presentation, and if the presenting SUN is elevated, the patient is likely to have kidney disease. However, a low proportion of patients with kidney disease (according to these criteria) have elevated SUN on admission, and we cannot confidently exclude kidney disease in those with a negative test (SUN=test pad #1). These findings are due to a high number of false negatives (ie, the patient has the disease but the presenting SUN measured by dipstick is not elevated). This may be due to inappropriate test methods being employed, or due to appropriate methods and SUN not being elevated despite the patient having kidney disease, or because the SUN was elevated, but this was not detected appropriately by the dipstick. To determine this, SUN would need to be measured by an alternative method.
The performance of SUN improved as the severity of kidney disease to be detected increased, supporting previous findings.17 18 The strip’s current ability to accurately detect more severe kidney disease and its greater specificity over sensitivity when detecting milder stages of kidney disease is suited to its use in rural and community low-income settings in areas without access to creatinine. Here, it is primarily important to identify those patients with severe kidney disease who may benefit from transfer to tertiary renal care. The low false positive rate of SUN, especially when used to detect milder kidney disease, mean that sparse resources for patient management (eg, patient transfer) are not be wasted through the incorrect labelling of patients with kidney disease. We do not propose the SUN dipstick would replace the use of creatinine. However, in healthcare facilities, including the ones included in this study, that do not have access to creatinine testing, we feel the SUN dipstick provides an option for the detection of kidney disease and its subsequent management. In this study, 143 patients with kidney disease were identified using the strip (true positives), that otherwise would have been left undiagnosed under standard clinical practice conditions. We anticipate this is likely to lead to improved patient outcomes, but this requires further study.
Strengths and limitations
We have previously assessed the utility of SUN in the management of kidney disease in high-income settings, and in admissions to tertiary hospitals in sub-Sahara Africa.14–18 26 In this study, we tested its feasibility and performance in the management of patients at risk of kidney disease presenting to low-resource healthcare facilities where serum creatinine is largely unavailable. Hence, the cohort in which the strip was tested, and the settings where this was done, are highly representative of those where we envisage this strip to be of greatest utility, and is the major strength of this study. We did this in a large number of patients, across multiple centres, in community, rural and urban settings, across all seasons of the year. In addition to assessing the strip’s performance, we also demonstrated that in almost 1500 tests, SUN as a continuous variable correlated with creatinine and eGFR, across multiple observation time points (ie, as renal function changed with treatment). This supports previous findings that SUN may not only be able to be used for diagnosis of kidney disease, but also to monitor response to treatment.16 17 Moreover, we demonstrated that in-hospital mortality increased according to presenting SUN, supporting previous work, which highlighted SUN can identify patients at increased risk, and be used to predict their outcome.17
The high proportion of patients at increased risk of kidney disease undergoing successful SUN testing supports its feasibility, but we would have liked to collect reasons for inability to undertake the test. The study was undertaken in a pragmatic manner and resource and logistical restrictions meant BUN was not measured, and we were, therefore, not able to make a direct correlation between SUN and BUN. We included only patients at increased risk of kidney disease according to one risk score, and therefore, the strips performance in those not at increased risk, or those at increased risk according to alternative risk scores, in this study’s settings is unknown. The cohort is this study was small with low muscle mass, and creatinine measurements may have overestimated true glomerular filtration rate. Similarly, BUN (and hence SUN) may have been disproportionately raised compared with creatinine due to the high proportion of patients presenting with acute infectious illness and dehydration. While we feel the cohort in this study is representative of populations presenting to community and district settings in other regions, to understand the strip’s performance more comprehensively and make our results truly generalisable globally, an international multicentre study would have to be performed.
Conclusion
In adult patients presenting at increased risk of kidney disease to low-resource healthcare facilities in sub-Sahara Africa, the use of an SUN dipstick was a feasible screening test. SUN was able to detect kidney disease according to a number of criteria; its performance was optimal when detecting severe kidney disease. SUN, measured by dipstick, therefore provides an alternative to serum creatinine in the screening of patients for kidney disease in LRS where standard biochemical tests are unavailable.
Acknowledgments
We acknowledge the district and community healthcare workers, and the data collectors who facilitated the undertaking of the study. We also acknowledge the entire renal department at Queen Elizabeth Central Hospital for their ongoing care of patients with kidney disease in Southern Malawi in challenging working conditions.
Footnotes
Handling editor: Sanne Peters
Twitter: @rhysdrevans
Contributors: RDRE, UH, GD, JGR, VC-S, NL, RP-F, RM and EM designed the study; RDRE, UH, HM, MM, EB, NS, ZK, CP, JGR, VC-S, GD, RM and EM analysed the data; RDRE, UH, JGR, VC-S, RP-F, RM and EM drafted and revised the manuscript; all authors approved the final version of the manuscript.
Funding: The International Society of Nephrology.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Patient consent for publication: Not required.
Ethics approval: The University of Malawi, College of Medicine Research Ethics Committee, approved the study (ref: P.06/16/1968).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on request.
References
- 1.Susantitaphong P, Cruz DN, Cerda J, et al. . World incidence of AKI: a meta-analysis. CJASN 2013;8:1482–93. 10.2215/CJN.00710113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewington AJP, Cerdá J, Mehta RL. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int 2013;84:457–67. 10.1038/ki.2013.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans RDR, Hemmilä U, Craik A, et al. . Incidence, aetiology and outcome of community-acquired acute kidney injury in medical admissions in Malawi. BMC Nephrol 2017;18:21. 10.1186/s12882-017-0446-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acute kidney injury (AKI) – KDIGO. Available: http://kdigo.org/guidelines/acute-kidney-injury/ [Accessed 2017 Aug 17].
- 5.Ckd evaluation and management – KDIGO. Available: http://kdigo.org/guidelines/ckd-evaluation-and-management/ [Accessed 2017 Aug 17].
- 6.Raimann JG, Riella MC, Levin NW. International Society of nephrology's 0by25 initiative (zero preventable deaths from acute kidney injury by 2025): focus on diagnosis of acute kidney injury in low-income countries. Clin Kidney J 2018;11:12-19. 10.1093/ckj/sfw134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sein KT, Arumainayagam G. Correlation between serum urea and salivary urea. Clin Chem 1987;33:2303–4. 10.1093/clinchem/33.12.2303 [DOI] [PubMed] [Google Scholar]
- 8.Cardoso EML, Arregger AL, Tumilasci OR, et al. . Assessment of salivary urea as a less invasive alternative to serum determinations. Scand J Clin Lab Invest 2009;69:330–4. 10.1080/00365510802588076 [DOI] [PubMed] [Google Scholar]
- 9.Zúñiga ME, Estremadoyro LO, León CP, et al. . Validation of the salivary urea test as a method to diagnose chronic kidney disease. J Nephrol 2012;25:431–6. 10.5301/jn.5000022 [DOI] [PubMed] [Google Scholar]
- 10.G S, A RK, Y S, et al. . Analysis of blood and salivary urea levels in patients undergoing haemodialysis and kidney transplant. J Clin Diagn Res 2014;8:ZC18–20. 10.7860/JCDR/2014/8081.4553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seethalakshmi C, Koteeswaran D, Chiranjeevi V. Correlation of serum and salivary biochemical parameters in end stage renal disease patients undergoing hemodialysis in pre and post-dialysis state. J Clin Diagn Res 2014;8:CC12–14. 10.7860/JCDR/2014/10404.5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lasisi TJ, Raji YR, Salako BL. Salivary creatinine and urea analysis in patients with chronic kidney disease: a case control study. BMC Nephrol 2016;17:10. 10.1186/s12882-016-0222-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yajamanam N, Vinapamula KS, Sivakumar V, et al. . Utility of saliva as a sample to assess renal function and estimated glomerular filtration rate. Saudi J Kidney Dis Transpl 2016;27:312–9. 10.4103/1319-2442.178549 [DOI] [PubMed] [Google Scholar]
- 14.Raimann JG, Kirisits W, Gebetsroither E, et al. . Saliva urea dipstick test: application in chronic kidney disease. Clin Nephrol 2011;76:23–8. 10.5414/CN106826 [DOI] [PubMed] [Google Scholar]
- 15.Calice-Silva V, Vieira MA, Raimann JG, et al. . Saliva urea nitrogen dipstick - a novel bedside diagnostic tool for acute kidney injury. Clin Nephrol 2014;82:358–66. 10.5414/CN108370 [DOI] [PubMed] [Google Scholar]
- 16.Raimann JG, Calice-Silva V, Thijssen S, et al. . Saliva urea nitrogen continuously reflects blood urea nitrogen after acute kidney injury diagnosis and management: longitudinal observational data from a collaborative, international, prospective, multicenter study. Blood Purif 2016;42:64–72. 10.1159/000445041 [DOI] [PubMed] [Google Scholar]
- 17.Evans R, Calice-Silva V, Raimann JG, et al. . Diagnostic performance of a saliva urea nitrogen dipstick to detect kidney disease in Malawi. Kidney Int Rep 2017;2:219–27. 10.1016/j.ekir.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calice-Silva V, Sacomboio E, Raimann JG, et al. . Diagnostic performance of salivary urea nitrogen dipstick to detect and monitor acute kidney disease in patients with malaria. Malar J 2018;17:477. 10.1186/s12936-018-2627-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta RL, Cerdá J, Burdmann EA, et al. . International Society of nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. The Lancet 2015;385:2616–43. 10.1016/S0140-6736(15)60126-X [DOI] [PubMed] [Google Scholar]
- 20.Macedo E, Garcia-Garcia G, Mehta RL, et al. . International Society of nephrology 0 by 25 project: lessons learned. Ann Nutr Metab 2019;74 Suppl 3:45–50. 10.1159/000500345 [DOI] [PubMed] [Google Scholar]
- 21.World Development Indicators | DataBank. Available: http://databank.worldbank.org/data/reports.aspx?source=2&series=NY.GDP.PCAP.CD&country=MWI# [Accessed 2017 Jun 12].
- 22.American Society of Nephrology | Kidney Week - Abstract Details. Available: https://www.asn-online.org/education/kidneyweek/2017/program-abstract.aspx?controlId=2787644 [Accessed 2019 Oct 22].
- 23.Mehta RL, Burdmann EA, Cerdá J, et al. . Recognition and management of acute kidney injury in the International Society of nephrology 0by25 global snapshot: a multinational cross-sectional study. Lancet 2016;387:2017–25. 10.1016/S0140-6736(16)30240-9 [DOI] [PubMed] [Google Scholar]
- 24.Cerdá J, Mohan S, Garcia-Garcia G, et al. . Acute kidney injury recognition in low- and middle-income countries. Kidney Int Rep 2017;2:530–43. 10.1016/j.ekir.2017.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olowu WA, Niang A, Osafo C, et al. . Outcomes of acute kidney injury in children and adults in sub-Saharan Africa: a systematic review. Lancet Glob Health 2016;4:e242–50. 10.1016/S2214-109X(15)00322-8 [DOI] [PubMed] [Google Scholar]
- 26.Evans RDR, Cooke W, Hemmila U, et al. . A Salivary Urea Nitrogen Dipstick to Detect Obstetric-Related Acute Kidney Disease in Malawi. Kidney Int Rep 2018;3:178–84. 10.1016/j.ekir.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2020-002312supp001.pdf (1.1MB, pdf)
bmjgh-2020-002312supp002.pdf (560.5KB, pdf)


