Abstract
Objectives
To examine physicians’ perceptions of the uptake of biosimilars.
Design
Systematic review.
Data sources
MedLine Ovid and Scopus databases at the end of 2018.
Eligibility criteria
Original scientific studies written in English that addressed physicians’ perceptions of the uptake of biosimilars.
Data extraction and synthesis
The search resulted in altogether 451 studies and 331 after removing duplicates. Two researchers examined these based on the title, abstract and entire text, resulting in 20 studies. The references in these 20 studies were screened and three further studies were included. The data of these 23 studies were extracted. All the publications were quality assessed by two researchers.
Results
Most of the selected studies were conducted in Europe and commonly used short surveys. Physicians’ familiarity with biosimilars varied: 49%–76% were familiar with biosimilars while 2%–25% did not know what biosimilars were, the percentages varying from study to study. Their measured knowledge was generally more limited compared with their self-assessed knowledge. Physicians’ perceptions of biosimilars also varied: 54%–94% were confident prescribing biosimilars, while 65%–67% had concerns regarding these medicines. Physicians seemed to prefer originator products to biosimilars and prescribed biosimilars mainly for biologic-naive patients. They considered cost savings and the lower price compared with the originator biologic medicine as the main advantages of biosimilars, while their doubts were often related to safety, efficacy and immunogenicity. 64%–95% of physicians had negative perceptions of pharmacist-led substitution of biologic medicines.
Conclusions
Physicians’ knowledge of and attitudes towards biosimilars vary. Although physicians had positive attitudes towards biosimilars, prescribing was limited, especially for patients already being treated with biologic medicines. Perceptions of pharmacist-led substitution of biologic medicines were often negative. Education and national recommendations for switching and substitution of biologic medicines are needed to support the uptake of biosimilars.
Keywords: biosimilar, biologic medicine, physician, perception
Strengths and limitations of this study.
This is the first systematic review conducted solely on physicians’ perceptions regarding the uptake of biosimilars.
The literature search was conducted with the help of an experienced information specialist.
Publications selected for this review were quality evaluated by two researchers independently.
The quality evaluation protocol was compiled from four existing evaluation protocols.
The data in the studies included in this review were mainly collected before 2017.
Introduction
Biologic medicines consist of one or multiple biologic active substances and are often manufactured through biotechnology.1 2 They were first developed mainly for rare diseases, but have also improved the treatment of many common diseases such as diabetes, arthritis and psoriasis.1 The flipside of this transformation are the high costs of biologic medicines, which have contributed to increased medical costs globally.3
Biosimilars are biologic medicines highly similar to the originator biologic medicines and with the same standards of quality, safety and efficacy.2–4 Biosimilars are not clinically meaningfully different from the existing reference product. They are not regarded as generic medicines due to the complex manufacturing process and the natural variability between manufacturing batches of biologic medicines. The comparability of the product with the reference product has to be demonstrated, but clinical trials are not required. As a result, biosimilars can be brought to the market at a lower cost in comparison with the originator biologic product. The uptake of biosimilars could lead to healthcare cost savings and better patient access to costly biologic therapies.5 By the end of 2018, 50 biosimilars had received marketing authorisation in Europe and 15 in the USA.6 7
Despite their demonstrated comparability and cost-saving potential, biosimilars have not fully penetrated the market of biologic medicines. The European Union accounts for 80% of the global biosimilar market, but biosimilars constitute only 1% of the total sales of biologic medicines.8 9 It has been stated that decisions to select biologic medicines may be either policy driven or made by individual physicians, which has raised the need to assess the prescribing of biosimilars in a critical manner.10 11 Physicians’ reluctance to prescribe biosimilars may restrict potential savings in medical costs that could enable biologic treatment of larger patient populations and provide more cost-effective treatment, as similar benefits could be gained by using less expensive treatments. Therefore, it is vital to study physicians’ attitudes towards and perceptions of the uptake of biosimilars. The published information on the topic is somewhat contradictory. A previous systematic review focused on healthcare providers’ knowledge, perceptions and prescribing behaviours of biosimilar medicines.12 As the role of physicians is critical in the uptake of biosimilars and gaining the cost-saving potential, a wider understanding of physicians’ perceptions of the uptake of biosimilars with a critical quality evaluation of the published literature was needed. Thus, the aim of this systematic review was to examine physicians’ perceptions of the uptake of biosimilars.
Materials and methods
Literature search
A systematic literature search was conducted in the MedLine Ovid and Scopus databases at the end of 2018. These databases provide a comprehensive selection of scientific publications from the disciplines of pharmacy and medicine. The systematic search strategy (online supplementary appendix 1) was constructed by the research group, and the search was conducted by an experienced information specialist.
bmjopen-2019-034183supp001.pdf (196.1KB, pdf)
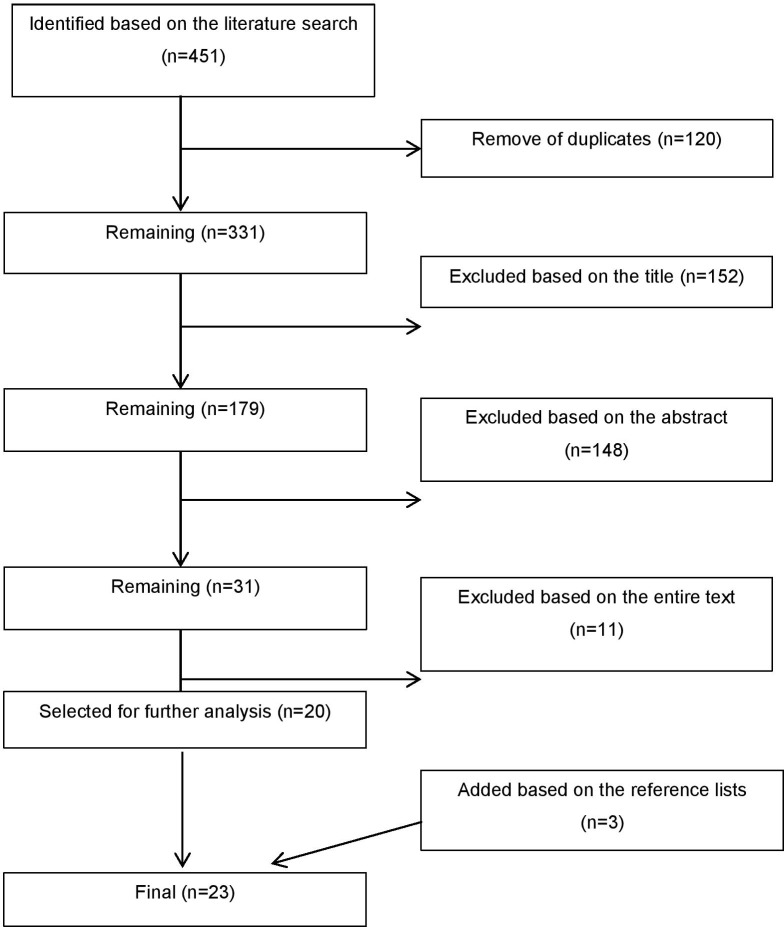
The initial search resulted in 451 studies. After removal of duplicates (n=120), 331 studies remained. These studies were examined based on the title, abstract and entire text by two researchers independently (KS and MM). Of the 331 studies, 152 were excluded based on the title, 148 based on the abstract and 11 based on the entire text. At each stage, the researchers shared their views of the studies, discussed possible differences of opinion and reached a consensus opinion based on the discussion. The inclusion and exclusion criteria of this systematic review are presented in table 1. A total of 20 publications were selected for further analysis. Furthermore, the reference lists of these 20 articles were screened and three further articles that met the inclusion criteria were selected and included in this review, bringing the final number of included studies to 23. The PRISMA flow chart explaining the study inclusion process is presented in figure 1.
Table 1.
Inclusion and exclusion criteria of studies of this systematic review
| Inclusion criteria | Exclusion criteria |
|
|
|
|
|
|
|
|
Figure 1.
PRISMA flow chart explaining the study inclusion process.
Quality assessment
Each of the 23 selected studies was concisely reviewed. Quality assessment was conducted according to a protocol adapted from the protocols of Åkesson et al, Tong et al, the Joanna Briggs Institute and the Swedish Agency for Health Technology Assessment and Assessment of Social Services13–16 (online supplementary appendix 2). The adapted protocol was developed and used in the quality evaluation because the study designs of the included studies varied and there was no single protocol that was suitable for evaluating the studies in a concise manner. Two researchers conducted quality assessments individually and then compared their reviews. Differences in opinions (n=6) were discussed and final evaluation was reached by consensus. In the Results section of this systematic review, the studies assessed as having high quality are emphasised more than those with moderate or low assessed quality.
Data extraction and analysis
A meta-analysis was not conducted due to the various methods and inclusion of both qualitative and quantitative approaches in the studies included in this review. The following information was extracted from the included studies: general information (authors, year of publication and country of publication), aims, methods and results. In regard to results, seven topics for data extraction were identified based on the topics discussed in the publications and discussions within the research group. These topics concerned the physicians’ (1) self-rated knowledge of biosimilars, (2) measured knowledge of biosimilars, (3) information sources about biologic medicines, (4) attitudes towards and experienced advantages and disadvantages of biosimilars, (5) actions in the initiation of biosimilars for biologic-naive patients, (6) actions in switching between originators and biosimilars for patients already being treated with biologic medicines, and (7) thoughts on pharmacist-led substitution of biologic medicines. In the Results section of this systematic review, these seven topics are presented within four broader themes: physicians’ (1) self-rated and measured knowledge of biosimilars and information sources on biologic medicines, (2) attitudes towards and experienced advantages and disadvantages of biosimilars, (3) perceptions of the treatment initiations with biosimilars and on the switches between originator biologic medicines and biosimilars, and (4) attitudes towards pharmacist-led substitution of biologic medicines. All percentages presented in this article refer to the percentages shown in the included studies of physicians with a certain opinion. If more than one study investigated the topic, the ranges of percentages in these studies are shown.
Results
Study characteristics
Physicians’ perceptions of biosimilars have been studied mainly in Europe (n=16)10 17–30 and North America (n = 4).31–34 Single studies have been conducted in Australia (n=1),35 New Zealand (n=1),36 Central and South America (n=1)37 and with participants from multiple African, European and Middle Eastern countries (n=1)38 (table 2). All the studies were published between 2014 and 2019, most of them (n=20)10 17 20–22 24–38 in 2017 or earlier. The data presented in the studies were collected between 2013 and 2017. Most of the 23 selected publications used surveys, typically web-based questionnaires with 11–22 questions, or fully structured short interviews (n=17).10 17 18 22–27 30–32 34–38 In addition, there were one qualitative interview study19 and two real-world cross-sectional studies (n=2)28 29 in which physicians filled a survey form and reported their prescribing, then recruited patients who also filled a questionnaire form to provide information on how the reported prescribing was actualised in practice. There were also discrete choice method surveys (n=2)20 21 in which prescribers were given a hypothetical scenario and possible treatment options, and had to choose their preferred alternative.39 Furthermore, there was one literature review with a survey of the market uptake of biosimilars.33
Table 2.
Characteristics of the 23 studies included in this systematic review
| Reference (country or region) | Aims and methods | Results | ||||||
| Self-rated knowledge | Measured knowledge | Information sources | Attitudes towards and experienced advantages and disadvantages of biosimilars | Initiation of biosimilars (biologic-naive patients) | Switches between originators and biosimilars (patients already treated with biologics) | Pharmacist-led substitution of biologic medicines | ||
| Akhmetov et al 2015 (Ukraine)17 |
Endocrinologists’, oncologists’, nephrologists’, immunologists’ and rheumatologists’ awareness of biosimilars Short interviews with eight close-ended questions, including 6 Likert-type items (n=82), time of the study not reported |
Low to medium levels (not reported more specifically) of biosimilar awareness on a 1–5 scale, where 1=low and 5=high Endocrinologists and nephrologists had higher levels of awareness than other respondents |
N/A | Peer-reviewed journal articles (n=35), internet (n=31), medical conferences (n=20), popular press (n=9), key-opinion leaders (n=3), drug manufacturers (n=2) | On a 1–5 scale, likelihood of prescribing biosimilars: 68% average (specific numbers not reported), 23% below average and 9% above average Majority (n not reported) are likely to try biosimilars in small batches, and then gradually move to larger groups of patients, endocrinologists and nephrologists showing the greatest interest Facilitators of prescribing: 39% cost advantage, 22% certification of safety by EMA or FDA, 22% certification of efficacy by EMA or FDA, 10% propitiousness of the Cabinet of Ministers and 7% trust towards European, American and Japanese biotech companies as importers Majority (n not reported) required 40%–50% lower price for biosimilars than original biologics, endocrinologists typically accepting 20%–30% discount in comparison with rheumatologists and oncologists who anticipated over 50% discount |
N/A | N/A | N/A |
| Aladul et al 2019 (UK)18 |
Knowledge and attitudes of healthcare professionals (n=150 dermatologists, diabetologists, gastroenterologists and rheumatologists) towards infliximab and insulin glargine biosimilars Web-based survey via selected medical associations between August 2016 and January 2017 |
80% were aware that biosimilars were available on their local formulary | 76% correctly considered biosimilars as copies of originators | N/A | 91% considered robust pharmacovigilance studies and 84% the costs as the most important influencer of their prescribing of biosimilars | 22% had major concerns on the efficacy and 14% on the safety of biosimilars that prevented them of starting a biosimilar | 50% had major concerns on the efficacy and 34% on the safety of biosimilars in the switches | N/A |
| Aladul et al 2018 (UK)19 |
Perceptions of consultants with specialties of diabetes mellitus, ulcerative colitis, Crohn’s disease, rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis (n=10) towards biosimilar infliximab, etanercept and insulin glargine and potential barriers and facilitators to their prescribing Semi-structured interviews of purposive convenience sample of West Midlands hospital staff between June and November 2017 |
N/A | All interviewees expressed an understanding of the concept of biosimilars and believed biosimilars were copies of originators | Conferences, pharmaceutical industry representatives, scientific journals and colleagues | Majority of rheumatologists and diabetologists (n not reported) would prescribe the reference product if the prices of the reference product and the biosimilar are equal Gastroenterologists expressed more confidence and fewer concerns than other specialists, stating that indication extrapolation had previously been the major obstacle in the biosimilar uptake, but that it had been overcome Majority of rheumatologists (n not reported) had concerns on indication extrapolation, considering their patients are very sensitive with higher multimorbid risks. Some rheumatologists (n not reported) openly declared being mistrustful on biosimilars Facilitators of prescribing were information from societies, authorities and national registries. Barriers of prescribing were unexpected adverse effects or increase in side effects, patients’ reluctance on using biosimilars, complicated, unsuitable or non–user-friendly administration device, unavailability of dose strengths in comparison with originators |
Majority (n not reported) were content to initiate biosimilars Minority of rheumatologists and diabetologists (n not reported) felt under pressure to initiate new patients with biosimilars by their organisation Two rheumatologists were happier to initiate biosimilars rather than switching |
All gastroenterologists (n=7) and a minority of rheumatologists (n not reported) were content to switch patients from reference products to a biosimilar All those who were content with switching considered that patients should be given the choice between the products Majority of all physicians (n not reported) felt multiple switching based on cost reasons irrational |
Majority (n not reported) has negative view on the pharmacist-led substitution of biologic medicines Minority (n not reported) considered that automatic substitution would be accepted in the next few years |
| Baji et al 2016a (Hungary)20 | Gastroenterologists’ treatment preferences in ulcerative colitis Discrete choice experiment survey in which prescribers are given a hypothetical scenario and possible treatment options, and they are asked to choose the alternative they prefer39 in a Hungarian professional society meeting in 2014 (n=51) |
N/A | N/A | N/A | 20% had no concerns on biosimilars, 67% had some concerns on efficacy and/or safety and 12% did not support the use of biosimilars due to the lack of RCT evidence | 84% of all physicians and 80% of those who had some concerns (67%) chose biosimilar in at least one choice set The most important attribute driving the choice: stopping rule (whether treatment after 12 months is reimbursed) Estimated probability of choosing the originator over biosimilar in the present reimbursement situation: 48%. Probability of choosing the biosimilars with all the benefits offered over the originator in the present situation: 85% vs 15% |
61% of all and 53% of those who were concerned chose biosimilar in at least one of the choice sets The most important attribute driving the choice: stopping rule Estimated probability of choosing the originator over biosimilar in the present reimbursement situation: 71%. Probability of choosing the biosimilars with all the benefits offered over the originator in the present situation: 63% vs 37% |
N/A |
| Baji et al 2016b (Hungary)21 | Gastroenterologists’ treatment preferences in Crohn’s disease Discrete choice experiment survey (in which prescribers are given hypothetical scenario and possible treatment options, and they are asked to choose the alternative they prefer39 in a Hungarian professional society meeting in 2014 (n=51) |
N/A | N/A | N/A | 20% had no concerns on biosimilars, 65% had some concerns on efficacy and/or safety and 12% did not support the use of biosimilars due to the lack of RCT evidence Four clinicians were classified to ‘No biosimilar’ attitude group, 19 to the ‘Biosimilar to new patients only’ group and 27 to the ‘Biosimilar’ group (one clinician was excluded from the analysis) |
Men, senior consultants, working in inflammatory bowel disease centre and treating more patients were more likely to consider biosimilars for biologic-naive patients only Estimated probability of choosing the originator over biosimilar, when no benefits are offered for using the biosimilar: 60%. Probability of choosing the biosimilar with all kinds of benefits over the originator: 89% vs 11% The most important attribute driving the choice: continuity of medicine supply |
Estimated probability of choosing the originator over biosimilar, when no benefits are offered for using the biosimilar: 74%. Probability of choosing the biosimilar with all kinds of benefits over the originator: 44% vs 56% The most important determinant of choice: type of the treatment |
N/A |
| Barsell et al 2017 (USA)31 |
Dermatologists’ knowledge and perceptions of biosimilars, whether a practice gap exists and to study misconception and barriers to biosimilar usage Web survey of 14 multiple-choice questions for the members of five state dermatologic societies and National Psoriasis Foundation in 2015 (n=97) |
62% responded having basic understanding of biosimilars, 27% complete understanding and 11% that they have never heard of biosimilars | 37% were aware that biosimilars are highly similar to the reference product, 26% described biosimilar as ‘generic’, 27% described them as same bio-drug with equal bioequivalence and 10% said they did not know the definition. Those with complete understanding (27%), 21% incorrectly described biosimilar as ‘generic’ | 35% self-study, 25% scientific publications, 17% conferences and seminars, 3% biosimilar company-sponsored events and 20% other | Advantages: 71% low price to patients, 68% easier access to treatment and 65% low price to payers. Disadvantages: 71% efficacy, 66% potential switch to biosimilar without physicians’ knowledge, 66% safety and 63% immunogenicity. 8% believed there were no advantages Convincing physicians of interchangeability: 44% extensive phase I, II and III studies, 37% valid longitudinal data from patient registries, 37% same level of testing (not specified more thoroughly) than generic medicines, 36% evidence of pharmacokinetic and pharmacodynamic equivalence |
25% definitely or highly likely to prescribe a biosimilar 38% will try it on very selected patients |
N/A | 88% believed that pharmacist-led substitution without consulting physicians will be allowed in the future 76% very important and 18% somewhat important to have control over whether patients receive originator or biosimilar |
| Beck et al 2016 (France)22 |
Knowledge, experience and opinions related to biosimilars and to identify expectations, barriers and possible options to promote prescription Web survey of 22 questions for nearly 500 rheumatologists in 2015 (n=116) |
55%/3% considered they had little/no knowledge of biosimilars 5% felt very well informed Hospital-based rheumatologists were likely to be more familiar with biosimilars compared with office-based rheumatologists 98% had at least one question about biosimilars |
85% thought biosimilars are similar to reference products that had gone off-patent; 85% considered biosimilars have no meaningful differences in quality, 80% in safety and 90% in efficacy; 65% thought that the assessment of biosimilarity requires more comprehensive data than generic drugs; and 46% believed that biosimilar marketing authorisation is granted on the sole investigation of pharmacokinetic bioequivalence | 84% self-study and scientific publications, 76% pharmaceutical companies, 72% continuous training, 54% physician colleagues and 19% pharmacist colleagues | 44% agree and 10% strongly agree being in favour of implementation of biosimilars Positive factors: 90% healthcare cost savings, 61% releasing of resources allowing treating additional patients, 49% positive impact on patients’ access to innovative medicines and 46% health policy-makers’ incentives. Barriers: 67% indication extrapolation of efficacy and safety, 66% lack of information about tolerability, 59% risk of increasing patients’ concerns, 57% lack of clinical trials and 55% patients’ wishes to be treated with the originator |
7% had already prescribed biosimilars mentioned in the survey 89% considered it was conceivable to start a treatment for biologic-naive patients |
25% could envision a switch | 58% strongly disagree and 23% disagree of approving substitution by a pharmacist |
| Chapman et al 2018 (UK)23 |
Healthcare professionals’ knowledge and attitudes towards infliximab and insulin glargine biosimilars and factors influencing their prescribing and compare healthcare professionals’ attitudes with the utilisation of these biosimilars in hospitals Web-based survey of 11 questions for societies of dermatology, diabetology, gastroenterology and rheumatology in 2016–2017 and drug utilisation analysis from DEFINE database in 2015–2016 (n=234). Other stakeholders apart from physicians are not addressed in this review |
N/A | 72% correctly thought biosimilars are similar copies of biologic medicines, 18% thought biosimilars are generic biologic medicines, 3% counterfeit medicines, 3% had heard of them but did not know what they were, 3% had never heard of them and 1% new biological medicines 75% knew biosimilars were available on their local formulary |
N/A | Gastroenterologists were most frequent prescribers of biosimilars (prescribing every day or week), followed by rheumatologists, diabetologists and dermatologists The dominant consideration: cost saving Increasing the use of biosimilars: regulatory guidance and robust pharmacovigilance studies, local policy, potential cost saving to organisation (whether or not savings were invested in the prescribers’ department) and robust cost-effectiveness data of biosimilar vs originator |
95% and 90% of gastroenterologists, 92% of rheumatologists, 79% of dermatologists and 75% of diabetologists had no or minor concerns on safety 90% of gastroenterologists, 88% of rheumatologists, 74% of dermatologists and 68% of diabetologists had no or minor concerns on efficacy |
95% of gastroenterologists, 53% of rheumatologists, 78% of dermatologists and 69% of diabetologists had no or minor concerns on safety 93% of gastroenterologists, 55% of rheumatologists, 79% of dermatologists and 65% of diabetologists had no or minor concerns on efficacy |
N/A |
| Cohen et al 2016 (USA)32 |
Dermatologists’, gastroenterologists’, haematologist-oncologists’, medical oncologists’, nephrologists’ and rheumatologists’ awareness, knowledge and perceptions of biosimilars over time (survey will be repeated in 2–3 years) Survey of 19 questions in 2015–2016 (n=1201) |
N/A | 92% of dermatologists, 90% of gastroenterologists, 83% of rheumatologists, 74% of nephrologists, 69% of haematologist-oncologists and 63% of medical oncologists were aware which of the listed medicines in their specialty were biologic 56% of rheumatologists, 33% of gastroenterologists, 31% of dermatologists, 15% of nephrologists, 9% of medical- oncologists and 3% of haematologist- oncologists incorrectly reported there are no biosimilars available |
88% scientific journals, 73% FDA and 64% physician peers. Trust to media was less than 5% | Generally positive attitudes towards biosimilars. Dermatologists and rheumatologists appear less enthusiastic 62% considered the biosimilar will have equivalent efficacy as its originator and 57% that the biosimilar will be at least as safe as the originator 58% had concerns on patient compliance and access to treatments options with originators Positive factors: increased access and utilisation of biologic medicines, expanded treatment options and provided savings for the healthcare system |
N/A | 91% open to switching patients to a biosimilar | N/A |
| Danese et al 2016 (Europe, countries not reported)24 |
Evolution on thinking about biosimilars 1 year after they had become available in the EU. Comparison with the survey published by Danese et al 201425 Web survey with 14 multiple-choice questions for members of European Crohn’s and Colitis Organization in 2015 (n=118) |
56% judged that educational activities that they were exposed to was fair and adequate, while 16% found it unnecessary | N/A | More information was hoped from 75% medical societies, 52% multispecialty safety registries, 47% health institutions and 26% guidelines | 29% totally confident, 18% very confident and 34% confident enough (5%, 8% and 26% in 2013) to prescribe a biosimilar Main advantage: 92% (90% in 2013) cost-sparing. Main issue: 42% the lack of data from clinical trials for all indications 27% (67% in 2013) consider biosimilars have higher immunogenicity compared with the originator and 17% (43% in 2013) different action than the originator 51% (24% in 2013) thought biosimilar should be approved for all the indications of the originator |
N/A | 44% (6% in 2013) would switch a patient with remission | 89% (85% in 2013) disagreed with automatic substitution by a pharmacist 13% support substitution for new prescriptions and 13% for all patients |
| Danese et al 2014 (Europe, countries not reported)25 |
Awareness of and readiness to use biosimilars Web survey of 15 questions for 1000 randomly selected European Crohn’s and Colitis Organization members in 2013 (n=307) |
N/A | 70% were aware that biosimilar is a similar copy, but not equal to the originator, 19% responded that it is a copy of biological agent, identical to the originator, like a generic | Preferred information: 81% multi-specialty international safety registries to monitor safety and effectiveness, 78% health institutions on the development of rules on the use of biosimilars, 66% medical societies, 61% data regarding the registration process for biosimilars and 57% multispecialty practice guidelines | 6% thought that the originator and biosimilar were interchangeable The main advantage: cost-sparing (89%). The main issue: different immunogenicity pattern than the originator (67%) 50% agreed biosimilars can significantly reduce healthcare costs, 27% expected them only having a marginal impact, 6% expected additional costs of introduction, regulation and pharmacovigilance to offset any potential savings 24% agreed that the tested biosimilar could be approved for all indications of the originator in terms of safety and efficacy, 19% for all rheumatological indications, 14% for the specific indication only, 3% stated that all biosimilars could be approved for all indications of the originator and 39% disagreed with all of the above |
61% felt little or no confidence in using biosimilars in their everyday clinical practice, 26% confident enough, 8% very confident, and 5% totally confident | 28% would consider replacing originator with a biosimilar | 64% against the substitution by pharmacist 18% would agree only for new patients |
| Farhat et al 2016 (Algeria, Belgium, Egypt, Iran, Iraq, Italy, Jordan, Lebanon, Sudan and Syria)38 |
Parameters on the acceptance and future prescription of biosimilars and worldwide situation focusing mainly on the EU and US laws, regulations and legislative pathways, pricing and challenging market access Survey for over 150 healthcare professionals in the conference meeting in 2015 (n=117 healthcare professionals responded, of which most were physicians; exact number of physicians who responded not reported). Other stakeholders apart from physicians are not addressed in this review |
N/A | 66% knew what biosimilars were, 12% did not know and 22% had not answered the question. Of those who knew (66%), 62% considered biosimilars bioequivalent to originator and have all preclinical and clinical trials equal to the originator 63% agreed that biosimilars are already marketed in the Arab and Middle Eastern markets, while 45% agreed that they are manufactured in the same region |
N/A | Drivers for prescribing: 69% FDA or EMA approval, 65% lower price of bioequivalence in comparison with the originator, 48% bio-efficacy, 42% safety and 31% good manufacturing practices and high reputation of the manufacturer. 5% think biosimilars do not have advantages 35% considered the cost of treatment should not overcome its effectiveness or safety/tolerance 26% thought lower prices were good news as patients will be treated with biologics 27% consider biosimilars would bring cost savings 49% trust companies highly experienced in manufacturing small-molecule generic drugs and 55% companies with prior experience in manufacturing biologics as biosimilar producers |
41% prescribe biosimilars while 33% do not (note that respondents were also other than physicians) | N/A | N/A |
| Felix et al 2014 (USA)33 |
Challenges and opportunities of market uptake of biosimilars from the perspectives of physicians and payers Survey for physicians that had written about or were familiar with biosimilars based on literature review of Medline-indexed publications (n=14). Other stakeholders apart from physicians are not addressed in this review |
N/A | N/A | N/A | Almost all physicians (n not reported) believed that if biosimilar was approved by FDA it will perform similarly to the originator with regard to safety and efficacy Influences of decision-making: efficacy and safety, out-of-pocket costs to the patient, price of treatment and immunogenicity 50% (n not reported) consider it is very important that there are proven chemical and pharmacokinetic similarities between originators and biosimilars Roughly half (n not reported) consider payer and cost considerations very important |
Four physicians are somewhat likely, six very likely and three not likely to prescribe a biosimilar to a new patient | 31%/61% (n not reported) say they are somewhat likely/very likely to switch an existing patient from originator to biosimilar | N/A |
| Reilly and Gewanter 2015 (Argentina, Brazil, Colombia and Mexico)37 | Understanding of biosimilars, how they use them and their concerns for the future Web-based survey for 6650 prescribers from global market research panel (n=399) |
35% did not consider themselves familiar with biosimilars, meaning they could not define them or had never heard of them | 49% were aware of differences between biologics, biosimilars and non-comparable biologics. 30% were unaware that clinical trials for single indication lead to approval for multiple indications | 71% seminars and conferences, 55% self-study, 32% education from biosimilar companies, 18% clinical trial participation and 4% other means 37% would like to learn from pharmaceutical companies |
88% prescribe biologics | 50% said they believed if two biologic medicines had the same non-proprietary scientific name, patient could receive either product and have the same result | 44% said they believed if two biologic medicines had the same non-proprietary scientific name, patient could be safely switched during a course of treatment, and the patient would have the same result 64% would not be comfortable switching for cost reasons rather medical reasons |
N/A |
| Grabowski et al 2015 (Canada)34 |
Gaps in knowledge and attitudes towards biosimilars of rheumatologists Web-based survey of 29 questions for 369 members of Canadian Rheumatology Association in February 2014 (n=81) |
31% indicated themselves being familiar or very familiar with biosimilars Those with greater than 20 years of practice were significantly more likely to indicate themselves familiar or very familiar than those with 20 or less years of practice |
66% considered biosimilars essentially same as generic drugs 38% were aware of Health Canada’s guidance on clinical requirement for biosimilar approval |
N/A | 94% generally comfortable prescribing biologic medicines to their patients 31% comfortable prescribing biosimilars to their patients if biosimilar was currently available 29% declined until their colleagues recommend it 42% indicated a 30% price reduction, and a third a ≥50% price reduction being reasonable before payers mandated the use of biosimilars over brand name biologics 54% disagreed or strongly disagreed, 32% agreed or strongly agreed and 14% were neutral using biosimilars with extrapolated indications 49% not confident, 19% confident or very confident, and a third neutral on the long-term sustainability profile of the biosimilar with 30 weeks of head-to-head clinical trial |
59% consider offering biosimilars, if biosimilar demonstrates that it is comparable with the brand name drug 72% unlikely or very unlikely, 11% likely or very likely and 16% neutral to offer a biosimilar, when biologic-naive patient is an ideal candidate, where cost is not an issue Greater familiarity with established brand name drugs and uncertainty over the long-term safety of biosimilars were often cited among those unlikely or very unlikely offering biosimilars 54% did not typically prescribe a biosimilar, were likely or very likely to offer a biosimilar, when the provincial payer or insurance company mandated using a biosimilar |
7.5% consider switching, if biosimilar demonstrates that it is comparable with the brand name drug | 88% concerned or very concerned if a pharmacist had the ability to substitute a biologic drug for a biosimilar without the physician’s approval |
| Hallersten et al 2016 (France, Germany, Italy, Poland, Spain, Sweden, UK)30 |
Preferences on type and detail of biosimilar information in Summaries of Product Characteristics (SmPC) and the use of information sources when prescribing biologics including biosimilars by dermatologists, endocrinologists, gastroenterologists, haematologists, nephrologists, oncologists and rheumatologists Web-survey with approximately 30 multiple-choice questions for 210 physicians (30 from each of the country) who were members of panels of physicians (approximately 250–800 physicians per country) who agreed earlier to participate in such survey studies, in 2015 |
N/A | N/A | Frequently used information sources: 63% professional guidelines, 55% SmPCs, 51% peer-reviewed journals, 42% national or hospital formularies The physicians preferred modified SmPC (modified for the purpose of the study) where additional information in the biosimilar label had been added, specifically: (1) clarifying which product (the biosimilar or the reference product) generated which clinical data, (2) inclusion of additional statements indicating that the product is a biosimilar, and (3) that similarity has been evaluated in preclinical and clinical studies |
N/A | N/A | N/A | N/A |
| Hemmington et al 2017 (New Zealand)36 |
Perceptions and attitudes towards efficacy, safety and manufacturing of biosimilars, factors associated with positive attitudes, indication extrapolation and switching, and circumstances in which physicians would be reluctant to prescribe biosimilars Email survey for 327 physicians in medical specialist society (n=110) |
76% reported being familiar and having basic understanding and 13% very familiar and complete understanding of biosimilars, 9% had heard of biosimilars, but could not define them, and 2% had never heard of biosimilars | N/A | N/A | 70% very or somewhat confident of the efficacy of biosimilars Less than 20% had negative views Situations when biosimilars were not prescribed: 32% lack of clinical data, 17% evidence of adverse effects or lack of efficacy, 15% patients do well with current treatment and 6% patients have complex medical history 47% very confident or somewhat confident, 32% not confident and the remainder undecided in indication extrapolation |
71% would prescribe biosimilars for all or some clinical conditions meeting the relevant criteria, 10% would do this for only few or no clinical situations | 51% confident and 28% not very confident or not at all confident to switch patients | N/A |
| O’Callaghan et al. 2017 (Ireland)10 | Medical specialists’, general practitioners’ and community pharmacists’ awareness of and attitudes to biosimilars Email survey of 14–20 questions for 2917 physicians in national professional societies in 2016 (n=253 analysed answers from general practitioners and n=102 from medical specialists). Other stakeholders apart from physicians are not addressed in this review |
44% of medical specialists and 5% of general practitioners very familiar with biosimilars, 41% and 35% familiar, and 6% and 25% had never heard the term ‘biosimilar’ | 25% of medical specialists and 18% of general practitioners considered biosimilars the same as generic medicines 31% of medical specialists incorrectly agreed that biological medicines sharing the same international non-proprietary name were ‘structurally identical’ |
Medical specialists (n=101, not all answered this question): 72% guidelines from professional societies, 68% published literature and 63% educational events GPs (n=247, not all answered this question): 58% national or hospital formularies |
59% of those aware of biosimilars in their therapeutic area (n=73) prescribed biosimilars, while 40% did not Concerns: 81% efficacy in extrapolated indications, 81% immunogenicity, 79% efficacy, 78% safety, 73% quality and 62% traceability |
67% of medical specialists that prescribed biosimilars (n=43) would most likely prescribe a biosimilar for treatment initiation | 28% of medical specialists that prescribed biosimilars (n=43) would be likely to switch from originator to biosimilar | <5% of medical specialists would consider pharmacist-led substitution appropriate 49% consider decisions should be taken by the prescriber on treatment initiation and 61% during treatment course. 43% consider decisions should be agreed with clinician in advance on treatment initiation and 35% during treatment course 84% think notifications for physician very important or critical in treatment initiation and 90% during treatment course |
| O’Dolinar and Reilly 2014 (France, Germany, Italy, Spain and the UK)26 | Nephrologists’, rheumatologists’, dermatologists’, neurologists’, endocrinologists’ and oncologists’ attitudes on biosimilar naming, substitution, and knowledge, sources of information and need for further education on biosimilars Web-based 15 min short survey for 4324 global physician market research panel of at the last quarter of 2013. 470 prescribers (20% of each five countries) completed the survey |
46% responded having basic understanding, 43% complete understanding, 11% could not define biosimilars and 1% had never heard of biosimilars 53% incorrectly thought biosimilar and originator were structurally identical and 37% incorrectly believed biosimilars are clinically tested for all indications |
N/A | 47% conferences and seminars, 35% self-study, 11% studies sponsored by biosimilar companies and 6% equally studies sponsored by innovator companies, clinical trial participation and other routes | 48% said it was very important, 24% critically important, 23% somewhat important, 4% slightly important and 1% not important to have a sole authority to select the medicine | 47% considered that products with the same non-proprietary name could be safely given to a patient with same results, 40% did not think that way | 45% think patients cannot be switched between the products with same non-proprietary names, 39% believed patients could be switched safely and effectively | 62% not acceptable, 35% acceptable and 3% totally acceptable on pharmacist-led substitution 47% very important, 30% critical, 6% slightly important and 1% not important to receive a notification if the pharmacist had dispensed other than prescribed biologic medicine during a repeated treatment |
| van Overbeeke et al 2017 (Belgium)27 | Knowledge and perceptions of patients and physicians with regard to originators and biosimilars and differences in perceptions and the factors influencing their preferences Web survey of multiple-choice and open-ended questions for all 232 Belgian rheumatologists in 2016 (n=41 responded). Other stakeholders apart from physicians are not addressed in this review |
95% considered biosimilars are similar, but not identical | 90% were able to share the most complete definition of a biosimilar | N/A | 7% had prescribed biosimilars. 73% preferred the originator when the prices were equal and 38% when originator was more expensive. When prices were equal, none preferred biosimilar. 93% considered price, 63% safety, 61% quality and 61% efficacy as sources of differences between originators and biosimilars 33% considered biosimilars and originators interchangeable if biosimilarity is proven in the same indication and 38% in indications where the medicine works via the same biological mechanism. 28% considered that biosimilars and originators were never interchangeable 56% think extrapolation could only be performed if efficacy and safety is proven to be similar in one of the indications and if the medicine works via the same mechanism in the other indications. 39% stated the indications should never be extrapolated Positive influencers: clinical trials with positive results and clinical data in the respective indication. Negative influencers: less studied than the originator and no clinical trials in the respective indication |
8% would not prescribe a biosimilar and 60% would only prescribe a biosimilar to biologic-naive patients | N/A | N/A |
| Reilly and Murby 2017 (Australia)35 | Opinions on the naming of biologicals and biosimilars, how the use of these medicines is recorded and their views on substitution of, familiarity with, knowledge of, attitudes to and beliefs in biosimilars Web-based survey for prescribers recruited from a global, commercial database of healthcare professional in 2016 (n=451, of which 160 completed the survey) |
21% considered themselves very familiar and having complete understanding of biosimilars, 73% basic understanding and 6% could not define them | 50% thought biosimilars go through the same regulatory process as original biologics 70% knew biosimilars could be approved for all or for some indications of the originator |
46% published literature, 28% colleagues, 27% information from Pharmaceutical Benefits Advisory Committee, 24% product information label, 19% information from Therapeutic Goods Administration, 18% sales presentative, 13% hospital formulary 43% never used published literature |
N/A | 16% would be comfortable prescribing a biosimilar that was approved for several indications based on clinical trials in only one indication, 11% would not feel comfortable and 73% had some concerns on this | N/A | 54% very and 36% critically important to have sole authority to decide of which biological was dispensed Evidence required for pharmacist-led substitution: 53% clinical trial data of no safety of efficacy risks in switching, 53% clinical trial data of no safety of efficacy risks after multiple switches, 27% in-market experience, 24% observational data and 6% no evidence would be sufficient |
| Sullivan et al 2017 (Germany)28 | Motivators of prescribing biosimilars, preferences matching actual prescribing behaviour and patient acceptance, satisfaction and concerns on biosimilars and how these relate to the treatment with originators or biosimilars Real world, cross-sectional study (in which physicians filled a survey form and reported their prescribing, and recruited patients that also filled a questionnaire form to provide information on how reported prescribing has occurred in practice) in 2015–2016 (n=25). Other stakeholders apart from physicians are not addressed in this review Based on their response, 11 physicians were assigned to investigative (primarily concerned with symptom improvement and disease modification), 7 to conservative (primarily concerned with safety) and 7 to other (influenced primarily by other factors) |
N/A | N/A | N/A | Biosimilars account for 12%–13% of all biologic therapies the respondents prescribe Reasons to prescribe: desire to get experience with the new product (89% of investigative, 100% of conservative and 57% of other), being convinced of equivalent efficacy compared with originators (44%, 67% and 43%), lower cost (44%, 83% and 71%), believing that is economic prescribing (44%, 83% and 57%) and believing that using biosimilars makes savings which can be used elsewhere (22%, 67% and 29%) |
88% would prefer to prescribe originator to biosimilar as first line therapy | N/A | N/A |
| Waller et al 2017 (Germany)29 | Motivators of prescribing biosimilars, preferences matching actual prescribing behaviour, and patient acceptance, satisfaction and concerns on biosimilars and how these relate to the treatment with originators or biosimilars Real world, cross-sectional study (in which physicians filled a survey form and reported their prescribing, and recruited patients who also filled a questionnaire form to provide information on how reported prescribing has occurred in practice) in 2015–2016 (n=50). Other stakeholders apart from physicians are not addressed in this review Based on their response, 23 physicians were assigned to investigative (primarily concerned with symptom improvement and disease modification), 17 to conservative (primarily concerned with safety) and 10 to other (influenced primarily by other factors) |
N/A | N/A | N/A | Biosimilars constitute less than 10% of the biologic therapies the respondents prescribed Reasons to prescribe: desire to get experience with the new product (86% of investigative, 65% of conservative and 50% of other), being convinced of equivalent efficacy compared with originators (64%, 65% and 50%) and lower costs (64%, 71% and 88%) |
>95% would prefer to prescribe originator to biosimilar as first-line therapy | N/A | N/A |
EMA, European Medicines Agency; FDA, Food and Drug Administration; GP, general practitioner; N/A, not available; RCT, randomised controlled trial.
Quality assessment
Of the 23 included studies, seven10 18 20 21 23 27 34 were evaluated as high, six19 22 28–31 as moderate and nine17 24–26 32 33 35 37 38 as low in quality based on the criteria used in this review (table 3). Publications evaluated as high in quality often included well-described and logically presented methods and results sections together with a critical discussion section, which those evaluated as moderate or low quality typically lacked. In general, the quality assessment revealed that there is a lack of valid instruments and studies using qualitative research methods.
Table 3.
Summary of the quality evaluation of the 23 included studies of this systematic review
| Reference | Main strengths | Main limitations | Quality according to the quality assessment protocol |
| Aladul et al 201918 | Results logically and clearly displayed | Details of the questionnaire form were not available, discussion on methodology partly lacking | High |
| Baji et al 2016a20 | Well-described and logically presented methodology, results and discussion | Ethical discussion lacking | High |
| Baji et al 2016b21 | Well-described and logically presented methodology, results and discussion | Critical and ethical discussion partly lacking | High |
| Chapman et al 201823 | Mainly well-described and logically presented methodology, results and discussion | More in-depth information could have been collected by a qualitative study | High |
| Grabowski et al 201534 | Well-described and logically presented methodology, results and discussion | More in-depth information could have been collected by a qualitative study | High* |
| Hemmington et al 201736 | Well-described and logically presented methodology, results and discussion | Details of the questionnaire form were not available, more in-depth information could have been collected by a qualitative study | High |
| O’Callaghan et al 201710 | Well-described and logically presented methodology, results and discussion | More in-depth information could have been collected by a qualitative study | High |
| van Overbeeke et al 201727 | Well-described and logically presented methodology, results and discussion | More in-depth information could have been collected by a qualitative study | High |
| Aladul et al 201819 | Semi-structured interviews provide a more in-depth view on the perceptions of healthcare professional in comparison with short surveys | Exact numbers of respondents which certain opinion (n) not always reported, low number of representatives per each professional group | Moderate* |
| Barsell et al 201731 | Well-presented results and discussion | Details of the questionnaire form were not available, description of methodology lacking, eg, dropout not described, ethical discussion lacking | Moderate |
| Beck et al 201622 | Well-presented results and discussion | Details of the questionnaire form were not available, validity of the instrument unclear, as more in-depth information could have been collected by a qualitative study, dropout not described accurately | Moderate* |
| Hallersten et al 201630 | Results clearly presented | Details of the panel of physicians in different European countries where the respondents were reqruited were not shown. Critical discussion on the method partly lacking | Moderate |
| Sullivan et al 201728 | Results clearly presented | Dropout not described accurately, some inconsistencies in the presentation of methodology and discussion | Moderate* |
| Waller et al 201729 | Well-presented results and discussion | Some inconsistencies in the presentation of methodology, eg, sample selection and dropout | Moderate* |
| Akhmetov et al 201517 | Explicit aims | Clear presentation of results lacking, critical and ethical discussion lacking | Low |
| Cohen et al 201732 | Mainly well-presented results and discussion | Details of the questionnaire form were not available, description of methodology lacking, ethical discussion lacking | Low |
| Danese et al 201624 | Results clearly presented | Details of the questionnaire form were not available, critical and ethical discussion partly lacking, description of methodology partly lacking | Low |
| Danese et al 201425 | Results clearly presented | Statistical analyses lacking, critical and ethical discussion lacking, description of methodology partly lacking, eg, the number of invited members not mentioned | Low |
| Farhat et al 201638 | Mainly logically presented methodology | Aim is not explicitly presented, number of physicians who responded not reported, results presented in table format only, critical discussion lacking | Low* |
| Felix et al 201433 | Explicit aims | Strategic sample selection, details of the questionnaire form were not available, exact numbers of respondents which certain opinion (n) not always reported, description of used statistical methods and data analysis lacking, inconsistency in the description of results | Low |
| Reilly and Gewanter 201537 | Explicit aims | Respondents from market research panel resulting that respondents work in disciplines in which do not necessarily involve biosimilars, such as psychiatry, description of used statistical methods and data analysis lacking, critical and ethical discussion lacking | Low |
| O’Dolinar and Reilly 201426 | Explicit aims | Intentional sample selection, clear presentation of results lacking, critical and ethical discussion lacking | Low |
| Reilly and Murby 201735 | Explicit aims | Description of data collection partly lacking, description of used statistical methods and data analysis lacking | Low |
*Differences in opinions of which quality grade each publication was given, set in consensus.
Self-rated and measured knowledge on biosimilars and sources of information (n = 18)
There was wide variation in physicians’ self-rated knowledge of biosimilars (table 2). Most physicians reported having at least a basic understanding of the topic: 5%–44% reported that they were very familiar and 49%–76% that they were familiar with biosimilars.10 24 26 27 31 34–36 However, 2%–25% of the physicians reported that they did not know what biosimilars are. Physicians with more years of practice and those with specialisation consider themselves more familiar with biosimilars in comparison with less experienced colleagues and general practitioners.10 22 34
Although according to their self-rating the physicians generally were familiar with biosimilars, their actual measured knowledge of the topic appeared to be weaker (table 2). From 18% to 66% of the physicians incorrectly described biosimilars as generic medicines, whereas 31%–72% thought they are structurally identical to originator medicines.10 22 23 25 32 34 35 37 38 However, in three studies, 76%–100% were able to state the complete definition of a biosimilar correctly.18 19 27
The physicians used several sources of information about biologic medicines, such as scientific publications (25%–84%), self-study (35%–84%), pharmaceutical companies (32%–76%), guidelines from professional societies (26%–75%), educational events and conferences (17%–71%), other published literature (46%–68%), physician colleagues (28%–54%), safety registries (52%) and pharmacist colleagues (19%)10 17 19 22 24–26 30–32 35 37 (table 2). One study found that information sources may vary according to the physicians’ educational background, as the most common information source for medical specialists were the guidelines from professional societies, whereas for general practitioners they were national or hospital formularies.10
Attitudes towards and experienced advantages and disadvantages of biosimilars (n=21)
The physicians’ reported attitudes towards biosimilars seem contradictory10 20–25 27–29 31–34 36–38 (table 2). Some (6%–38%) physicians consider biosimilars and originator products interchangeable, while others (28%) never think so.25 27 Some studies show that 65%–67% of physicians have concerns regarding biosimilars,20 21 while others report that 54%–94% of physicians feel somewhat or very confident prescribing biosimilars.10 22 24 34 36 Regardless, a positive attitude towards biosimilars does not automatically translate into prescribing, as physicians seem to prefer originator products to biosimilars.20 27 34 Some studies indicate that there might be differences in attitudes towards biosimilars between specialties: gastroenterologists seem to be frequent prescribers of biosimilars, while dermatologists and rheumatologists seem less enthusiastic.19 23 32
The main experienced advantages of biosimilars are cost savings,18 22–25 31 lower price in comparison with the originator biologic medicine31 38 and the possibility to get experience with the new product28 29(table 2). In addition, single studies reported that robust pharmacovigilance studies,18 easier access to treatment for patients,31 and approval of the European Medicines Agency or the Food and Drug Administration38 were motivators for prescribing biosimilars. The most commonly reported disadvantages were distrust in safety,10 18 22 31 33 efficacy,10 18 22 31 33 immunogenicity10 25 31 and indication extrapolation of biosimilars10 34 or the lack of clinical data on biosimilars.24 26 Single studies also suggested that the quality,10 traceability10 or tolerability22 of biosimilars and patients’ concerns towards biosimilars22 were disadvantages.
Initiation of biosimilars and switches between original biologic medicines and biosimilars (n=21)
The physicians (39%–89%) seemed more willing to prescribe a biosimilar for biologic-naive patients rather than for patients already being treated with biologic medicines10 20 23 25 27–29 31 33–38 (table 2). In discrete choice experiment studies, for example, 61%–84% of gastroenterologists chose biosimilars in at least one of the choice sets for biologic-naive patients.20 21 However, there are also other factors affecting the medicine selection, such as the cost of the medicines. One article reported that if cost were not an issue, only 11% of physicians would choose a biosimilar for treatment initiation.34 In addition, some studies suggest that some personal characteristics may influence the uptake of biosimilars by individual physicians: men, senior consultants and those treating more patients,21 along with those more familiar with brand name medicines and uncertain of the long-term safety of biosimilars34 were often unlikely to choose a biosimilar as initial therapy. Within medical specialties, gastroenterologists (95% with no concerns) appear to be the most confident to use biosimilars in treatment initiations, followed by rheumatologists (92%), dermatologists (79%) and diabetologists (75%).23
The physicians did not seem willing to switch from an originator biologic medicine to a biosimilar10 20–25 32–34 36 37 (table 2). The proportion of physicians willing to switch from an originator to a biosimilar was 51% or less, except in a single study in which the percentage was 91%.10 22 24 25 32 34 36 Similarly, when it comes to treatment initiation, the medical specialty of the physicians affected their willingness to switch biologic medicines.23 Gastroenterologists (95% with no concerns) seemed the most confident concerning switching, followed by dermatologists (78%), diabetologists (69%) and, notably, rheumatologists (53%).
Pharmacist-led substitution of biologic medicines (n=9)
Most physicians (64%–95%) were concerned about or disagreed with pharmacist-led substitution of biologic medicines10 19 22 24–26 31 34 35 (table 2). The studies suggest that having full autonomy in medicine selection and being fully aware of which medicines their patients receive was often crucial for physicians.10 26 31 35 However, according to a single study, 88% of the physicians believed that pharmacist-led substitution without consulting physicians will be allowed in the future.31
Discussion
In this systematic review, physicians’ knowledge of biosimilars varied widely. In general, their measured knowledge was weaker than their self-assessed knowledge. They used multiple sources of information about biologic medicines, most commonly scientific publications, pharmaceutical companies and professional societies. Similarly, their perceptions of biosimilars and the uptake of these medicines also varied. They seemed to prefer originator products to biosimilars, and prescribe biosimilars mainly for biologic-naïve patients. They consider cost savings and the lower price compared with the originator biologic medicines to be the main advantages of biosimilars, while their doubts were related to the safety, efficacy and immunogenicity of biosimilars. Most of the physicians had negative perceptions of pharmacist-led substitution of biologic medicines. The results in this review are in line with an earlier systematic review of healthcare providers’ perceptions of biosimilars.12
Physicians’ knowledge of biosimilars
This study found that physicians’ knowledge of biosimilars in many cases was inadequate, and this may contribute to the low prescribing and uptake of biosimilars.10 12 32 Although this issue has been widely recognised, there is limited evidence of the effectiveness of education interventions on prescribing.40 In contrast, academic detailing has proven to be effective in steering prescribing.41 42 This is a method in which a trained educator meets with a healthcare professional and shares the latest evidence-based information on the topic concerned.43 Besides its effectiveness in steering prescribing patterns, academic detailing has been proven to improve the cost-effectiveness of prescribing and reduce medical costs.44 45 It is vital that in the near future physicians and other healthcare professionals are provided targeted, evidence-based information on biosimilars to support their uptake and to gain the full cost-saving potential of these medicines.46 47 The educational activities of medical societies is also vital in the distribution of appropriate biosimilar information.11
Physicians’ attitudes towards biosimilars and means to enhance the uptake
According to this study, physicians’ attitudes towards biosimilars were contradictory, and the prescribing of biosimilars is more often directed to biologic-naive patients despite the convincing evidence that supports switching.48 Prescribing decisions can either be made by individual physicians or steered by binding policies that vary across countries. Furthermore, besides actual steering policies, there are general differences across health systems in prescribing, dispensing, pricing and reimbursement of biologic medicines that may have effects on the uptake.10 11 In Denmark and Norway, for example, hospital, regional or national tendering is in use, resulting in significant savings in the purchase of biologic medicines.11 49 50 Some countries have implemented incentives for healthcare professionals.11 Prescription quotas defining the ratio of biosimilars of all prescribed biologic medicines are in use in Germany and Sweden,51 while profit-sharing agreements making it possible to use the savings from biosimilar uptake for the benefit of the clinic or the organisation are used in Sweden and the UK.52 53 Pharmacist-led substitution of biologic medicines can also be seen as a potential means to enhance the uptake of biosimilars.11 31 This is legislatively possible in France and in the USA, and for some biological medicines also in Australia.54–56 Furthermore, the implementation of pharmacist-led substitution is currently ongoing in some European countries.46 57 All these initiatives highlight that the weak uptake of biosimilars has been acknowledged globally, and there is a need to discover sustainable means to enhance and stabilise their uptake.11 What complicates the issue is that, for example in Europe, even though the biosimilarity between biologic medicines is stated by the European Medicines Agency, decisions on the interchangeability and substitution are made at the national level. In order to support the uptake of biosimilars, educational measures for both healthcare professionals and patients are needed, although the role of national recommendations, policies and steering in the switching and substitution of biologic medicines should not be understated.31 46 47
Strengths and limitations
The main strengths of our review are that the literature search was conducted with the help of an experienced information specialist, and that the step-by-step review and inclusion of publications as well as the quality evaluation of studies was conducted independently by two researchers in order to avoid bias.58 Compared with the previous systematic review,12 this review included 12 more original publications due to a wider literature search focus. Furthermore, the current study excluded conference papers and Letters to Editors because for the purpose of the quality assessment full information about the methodology of the included studies was needed. One major limitation of this review is that the study-by-study data extraction was done by only one researcher. Furthermore, theses or reports by authorities that could have included research results were excluded from this study. In addition, none of the available protocols for quality assessment covered different types of study settings, so the protocol used in this study was compiled from four separate protocols. Moreover, the included studies were conducted in different countries with unique regulatory laws and policies that undoubtedly affect the uptake and prescribing of biosimilars at the national level. However, it is vital to compile studies from different countries with different systems and policies in order to form a comprehensive view of the current situation concerning the uptake of biosimilars. Another notable point is that the data in the studies included in this review were mainly collected in 2017 or earlier. The topic is very timely and perceptions of the uptake of biosimilars may change in light of new research information, interventions and experience in using these medicines. Thus, there is a need to continue examining physicians’ perceptions, both in general and with different disciplines, particularly with qualitative research methods. Further studies are needed to explore the differences between disciplines in the attitudes towards and prescribing of biosimilars, as the reasons behind these differences could not be explored in detail based on the studies included in this review.
Practical implications
This systematic review provides up-to-date knowledge about physicians’ perceptions of the uptake of biosimilars, and highlights the need for further education and steering on this issue. The knowledge provided by the review may be used in visioning future means to enhance the uptake of biosimilars that could include information sharing and educational interventions by means of, for example, academic detailing. The uptake of biosimilars may also be enhanced by implementing national policies or steering procedures that support the uptake, by means of pharmacist-led substitution of biologic medicines, for example.
Conclusions
This systematic review shows that physicians’ knowledge of and attitudes towards biosimilars vary. Although physicians have positive attitudes towards biosimilars, prescribing is limited, especially for patients already being treated with biologic medicines. Perceptions of the pharmacist-led substitution of biologic medicines are often negative. Education and national recommendations and policies for switching and substitution of biologic medicines are needed to support the uptake of biosimilars.
Supplementary Material
Footnotes
Contributors: KS, MM, JJ and KH-A contributed to the conception or study design. KS and MM acted as principal investigators in the search and evaluation of the literature and in the quality assessment. KS drafted the manuscript. All authors participated in critical revision of the manuscript and approved the final version.
Funding: This study is a part of a larger research project funded by the Social Insurance Institution of Finland (Kela), grant (31/26/2017).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as online supplementary information.
References
- 1.European Commission Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the community code relating to medicinal products for human use, 2001. [Google Scholar]
- 2.United States Food and Drug Administration Biosimilar and interchangeable products, 2018. a. Available: www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/ucm580419.htm [Accessed 27 Jun 2019].
- 3.McCamish M, Woollett G. Worldwide experience with biosimilar development. MAbs 2011;3:209–17. 10.4161/mabs.3.2.15005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Medicines Agency Biosimilar medicines: overview, 2018. a. Available: www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines [Accessed 27 Jun 2019].
- 5.QuintilesIMS 2017 IMS Biosimilar Report—The Impact of Biosimilar Competition in Europe, 2017. Available: http://ec.europa.eu/DocsRoom/documents/23102 [Accessed 27 Jun 2019].
- 6.European Medicines Agency Medicines search, 2018. b. Available: www.ema.europa.eu/en/medicines/field_ema_web_categories%253Aname_field/Human/ema_group_types/ema_medicine/field_ema_med_status/authorised-36/ema_medicine_types/field_ema_med_biosimilar/field_ema_web_categories%253Aname_field/Human/search_api_aggregation_ema_medicine_types/field_ema_med_biosimilar/ema_group_types/ema_medicine/field_ema_med_status/authorised-36?sort=ema_medicine_title&order=asc [Accessed 27 Jun 2019].
- 7.United States Food and Drug Administration Biosimilar Product Information, 2018. b. Available: www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/ucm580432.htm [Accessed 27 Jun 2019].
- 8.Blackstone EA, Joseph PF, Fuhr JP. The economics of biosimilars. Am Health Drug Benefits 2013;6:469–78. [PMC free article] [PubMed] [Google Scholar]
- 9.European Commission What you need to know about biosimilar medicinal products. A consensus information document, 2013. Available: http://ec.europa.eu/DocsRoom/documents/8242/attachments/1/translations [Accessed 27 Jun 2019].
- 10.O'Callaghan J, Bermingham M, Leonard M, et al. . Assessing awareness and attitudes of healthcare professionals on the use of biosimilar medicines: a survey of physicians and pharmacists in Ireland. Regul Toxicol Pharmacol 2017;88:252–61. 10.1016/j.yrtph.2017.06.013 [DOI] [PubMed] [Google Scholar]
- 11.O'Callaghan J, Barry SP, Bermingham M, et al. . Regulation of biosimilar medicines and current perspectives on interchangeability and policy. Eur J Clin Pharmacol 2019;75:1–11. 10.1007/s00228-018-2542-1 [DOI] [PubMed] [Google Scholar]
- 12.Leonard E, Wascovich M, Oskouei S, et al. . Factors affecting health care provider knowledge and acceptance of biosimilar medicines: a systematic review. J Manag Care Spec Pharm 2019;25:102–12. 10.18553/jmcp.2019.25.1.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Åkesson KM, Saveman B-I, Nilsson G. Health care consumers' experiences of information communication technology—a summary of literature. Int J Med Inform 2007;76:633–45. 10.1016/j.ijmedinf.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 14.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349–57. 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- 15.Joanna Briggs Institute Reviewers’ manual, 2014. Available: http://joannabriggs.org/assets/docs/sumari/reviewersmanual-2014.pdf [Accessed 27 Jun 2019].
- 16.Swedish Agency for Health Technology Assessment and Assessment of Social Services Evaluation and synthesis of studies using qualitative methods of analysis, 2016. Available: www.sbu.se/globalassets/ebm/metodbok/sbuhandbook_qualitativemethodsofanalysis.pdf [Accessed 27 Jun 2019].
- 17.Akhmetov I, Farista R, Thimmaraju PK. A study to assess the awareness of the biosimilars among Ukrainian physicians. J Bioanal Biomed 2015;7:66–9. [Google Scholar]
- 18.Aladul MI, Fitzpatrick RW, Chapman SR. Differences in UK healthcare professionals' knowledge, attitude and practice towards infliximab and insulin glargine biosimilars. Int J Pharm Pract 2019;27:214–7. 10.1111/ijpp.12485 [DOI] [PubMed] [Google Scholar]
- 19.Aladul MI, Fitzpatrick RW, Chapman SR. Healthcare professionals' perceptions and perspectives on biosimilar medicines and the barriers and facilitators to their prescribing in UK: a qualitative study. BMJ Open 2018;8:e023603. 10.1136/bmjopen-2018-023603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baji P, Gulácsi L, Golovics PA, et al. . Perceived risks contra benefits of using biosimilar drugs in ulcerative colitis: discrete choice experiment among gastroenterologists. Value Health Reg Issues 2016. a;10:85–90. 10.1016/j.vhri.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 21.Baji P, Gulácsi L, Lovász BD, et al. . Treatment preferences of originator versus biosimilar drugs in Crohn’s disease; discrete choice experiment among gastroenterologists. Scand J Gastroenterol 2016. b;51:22–7. 10.3109/00365521.2015.1054422 [DOI] [PubMed] [Google Scholar]
- 22.Beck M, Michel B, Rybarczyk-Vigouret M-C, et al. . Rheumatologists' perceptions of biosimilar medicines prescription: findings from a French web-based survey. BioDrugs 2016;30:585–92. 10.1007/s40259-016-0202-5 [DOI] [PubMed] [Google Scholar]
- 23.Chapman SR, Fitzpatrick RW, Aladul MI, Knowledge AMI. Knowledge, attitude and practice of healthcare professionals towards infliximab and insulin glargine biosimilars: result of a UK web-based survey. BMJ Open 2017;7:e016730. 10.1136/bmjopen-2017-016730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danese S, Fiorino G, Michetti P. Changes in biosimilar knowledge among European Crohn's Colitis Organization [ECCO] members: an updated survey. J Crohns Colitis 2016;10:1362–5. 10.1093/ecco-jcc/jjw090 [DOI] [PubMed] [Google Scholar]
- 25.Danese S, Fiorino G, Michetti P. Viewpoint: Knowledge and viewpoints on biosimilar monoclonal antibodies among members of the European Crohn's and Colitis Organization. J Crohns Colitis 2014;8:1548–50. 10.1016/j.crohns.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 26.O’Dolinar RO, Reilly MS. Biosimilars naming, label transparency and authority of choice—survey findings among European physicians. GaBI J 2014;3:58–62. [Google Scholar]
- 27.van Overbeeke E, De Beleyr B, de Hoon J, et al. . Perception of originator biologics and biosimilars: a survey among Belgian rheumatoid arthritis patients and rheumatologists. BioDrugs 2017;31:447–59. 10.1007/s40259-017-0244-3 [DOI] [PubMed] [Google Scholar]
- 28.Sullivan E, Piercy J, Waller J, et al. . Assessing gastroenterologist and patient acceptance of biosimilars in ulcerative colitis and Crohn’s disease across Germany. PLoS One 2017;;;12:e0175826. 14. 10.1371/journal.pone.0175826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waller J, Sullivan E, Piercy J, et al. . Assessing physician and patient acceptance of infliximab biosimilars in rheumatoid arthritis, ankylosing spondyloarthritis and psoriatic arthritis across Germany. Patient Prefer Adherence 2017;11:519–30. 10.2147/PPA.S129333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallersten A, Fürst W, Mezzasalma R. Physicians prefer greater detail in the biosimilar label (SmPC)—results of a survey across seven European countries. Regul Toxicol Pharmacol 2016;77:275–81. 10.1016/j.yrtph.2016.03.021 [DOI] [PubMed] [Google Scholar]
- 31.Barsell A, Rengifo-Pardo M, Ehrlich A. A survey assessment of US dermatologists' perception of biosimilars. J Drugs Dermatol 2017;16:612–5. [PubMed] [Google Scholar]
- 32.Cohen H, Beydoun D, Chien D, et al. . Awareness, knowledge, and perceptions of biosimilars among specialty physicians. Adv Ther 2017;33:2160–72. 10.1007/s12325-016-0431-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felix A, Gupta A, Choen JP, et al. . Barriers to market uptake of biosimilars in the US. GaBI J 2014;3:108–15. [Google Scholar]
- 34.Grabowski D, Henderson B, Lam D, et al. . Attitudes towards subsequent entry biologics/biosimilars: a survey of Canadian rheumatologists. Clin Rheumatol 2015;34:1427–33. 10.1007/s10067-014-2835-4 [DOI] [PubMed] [Google Scholar]
- 35.Reilly MS, Murby SP. A survey of Australian prescribers’ views on the naming and substitution of biologicals. GaBI J 2017;8:107–13. [Google Scholar]
- 36.Hemmington A, Dalbeth N, Jarrett P, et al. . Medical specialists' attitudes to prescribing biosimilars. Pharmacoepidemiol Drug Saf 2017;26:570–7. 10.1002/pds.4186 [DOI] [PubMed] [Google Scholar]
- 37.Reilly MS, Gewanter HL. Prescribing practices for biosimilars: questionnaire survey findings from physicians in Argentina, Brazil, Colombia and Mexico. GaBI J 2015;4:161–6. 10.5639/gabij.2015.0404.036 [DOI] [Google Scholar]
- 38.Farhat F, Othman A, el Karak F, et al. . Review and results of a survey about biosimilars prescription and challenges in the Middle East and North Africa region. Springerplus 2016;5:1–9. 10.1186/s40064-016-3779-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ 2012;21:145–72. 10.1002/hec.1697 [DOI] [PubMed] [Google Scholar]
- 40.Sipilä R, Helin-Salmivaara A, Korhonen MJ, et al. . Change in antihypertensive drug prescribing after guideline implementation: a controlled before and after study. BMC Fam Pract 2011;12:1–12. 10.1186/1471-2296-12-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solomon DH, Van Houten L, Glynn RJ, et al. . Academic detailing to improve use of broad-spectrum antibiotics at an academic medical center. Arch Intern Med 2001;161:1897–902. 10.1001/archinte.161.15.1897 [DOI] [PubMed] [Google Scholar]
- 42.O'Brien MA, Rogers S, Jamtvedt G, et al. . Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2007:CD000409. 10.1002/14651858.CD000409.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Resource Center for Academic Detailing Introductory guide to academic detailing, 2017. Available: www.narcad.org/uploads/5/7/9/5/57955981/introductory_guide_to_ad.pdf [Accessed 16 Jul 2019].
- 44.Simon SR, Rodriguez HP, Majumdar SR, et al. . Economic analysis of a randomized trial of academic detailing interventions to improve use of antihypertensive medications. J Clin Hypertens 2007;9:15–20. 10.1111/j.1524-6175.2006.05684.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel B, Afghan S. Effects of an educational outreach campaign (IMPACT) on depression management delivered to general practitioners in one primary care trust. Ment Health Fam Med 2009;6:155–62. [PMC free article] [PubMed] [Google Scholar]
- 46.Moorkens E, Vulto AG, Huys I, et al. . Policies for biosimilar uptake in Europe: an overview. PLoS One 2017;12:e0190147. 10.1371/journal.pone.0190147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cook JW, McGrath MK, Dixon MD, et al. . Academic oncology clinicians' understanding of biosimilars and information needed before prescribing. Ther Adv Med Oncol 2019;11:1758835918818335. 10.1177/1758835918818335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen HP, Blauvelt A, Rifkin RM, et al. . Switching reference medicines to biosimilars: a systematic literature review of clinical outcomes. Drugs 2018;78:463–78. 10.1007/s40265-018-0881-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mack A. Norway, biosimilars in different funding systems. What works? GaBI J 2015;4:90–2. 10.5639/gabij.2015.0402.018 [DOI] [Google Scholar]
- 50.Lunddahl B. Pharmacovigilance on biologicals and biosimilars: a Danish perspective. GaBI J 2016;5:123–4. 10.5639/gabij.2016.0503.030 [DOI] [Google Scholar]
- 51.Rémuzat C, Kapuśniak A, Caban A, et al. . Supply-side and demand-side policies for biosimilars: an overview in 10 European member states. J Mark Access Health Policy 2017;5:1307315. 10.1080/20016689.2017.1307315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Razanskaite V, Bettey M, Downey L, et al. . Biosimilar infliximab in inflammatory bowel disease: outcomes of a managed switching programme. J Crohns Colitis 2017;11:266–70. 10.1093/ecco-jcc/jjw216 [DOI] [PubMed] [Google Scholar]
- 53.Flodmark C-E, Lilja K, Woehling H, et al. . Switching from originator to biosimilar human growth hormone using dialogue teamwork: single-center experience from Sweden. Biol Ther 2013;3:35–43. 10.1007/s13554-013-0011-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thimmaraju PK, Rakshambikai R, Farista R, et al. . Legislations on biosimilar interchangeability in the US and EU—developments far from visibility, 2018. Available: www.gabionline.net/Sponsored-Articles/Legislations-on-biosimilar-interchangeability-in-the-US-and-EU-developments-far-from-visibility [Accessed 16 Jul 2019].
- 55.Cauchi R. State laws and legislation related to biologics medications and substitution of biosimilars, 2018. Available: www.ncsl.org/research/health/state-laws-and-legislation-related-to-biologic-medications-and-substitution-of-biosimilars.aspx [Accessed 16 Jul 2019].
- 56.Australian Government Department of Health Biosimilar awareness initiative, 2018. Available: www.health.gov.au/internet/main/publishing.nsf/Content/biosimilar-awareness-initiative [Accessed 16 Jul 2019].
- 57.Larkin H, Macdonald J, Lumsden R. Pharmacy-mediated substitution of biosimilars—a global survey benchmarking country substitution policies. GaBI J 2017;6:157–64. 10.5639/gabij.2017.0604.034 [DOI] [Google Scholar]
- 58.Singh S. How to conduct and interpret systematic reviews and meta-analyses. Clin Transl Gastroenterol 2017;8:e93. 10.1038/ctg.2017.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-034183supp001.pdf (196.1KB, pdf)