We read with great interest the report by Jucaud et al1 describing the use of monoclonal antibodies for determining the relative proportions of HLA-I conformational variants displayed by Luminex Single Antigen Beads (LSAB). They confirmed previous results showing that (1) LSAB display significant amounts of HLA-I that are not associated with beta-2-microglobulin (β2m) and/or peptide, as opposed with native and functional peptide-associated β2m-associated heavy-chains (pepA-β2aHC),2 (2) iBeads are largely devoid of β2m-free heavy-chains (β2fHC) but have lower pepA-β2aHC levels,2,3 (3) resting cells only express pepA-β2aHC,4 explaining why antidenatured HLA antibodies (anti-dHLA) rarely provide positive crossmatches and do not impair graft survival.2,3,5 Their findings are interesting but several issues could be discussed.
First, the TFL-006 monoclonal antibody generated by the authors does not seem very efficient at staining the β2fHC that are present on LSAB. Indeed, TFL-006 provided lower mean fluorescence intensities (MFI) than HC-10 on acid-treated beads (that display only β2fHC, letter Figure 1), this later showing, as expected, comparable MFI with W6/32 on nontreated LSAB. In line with this, in patients’ sera, we described anti-dHLA directed against all the beads which are not recognized by TFL-006.3 Therefore, assessing the fraction of β2fHC on HLA-I beads by comparing TFL-006 and W6/32 MFI and stating that HC-10 reactivity on iBeads is mainly due to peptide-free β2m-associated heavy-chains (pepF-β2aHC) solely on the basis that TFL-006 displayed lower MFI than HC-10 should be considered with caution.
FIGURE 1.
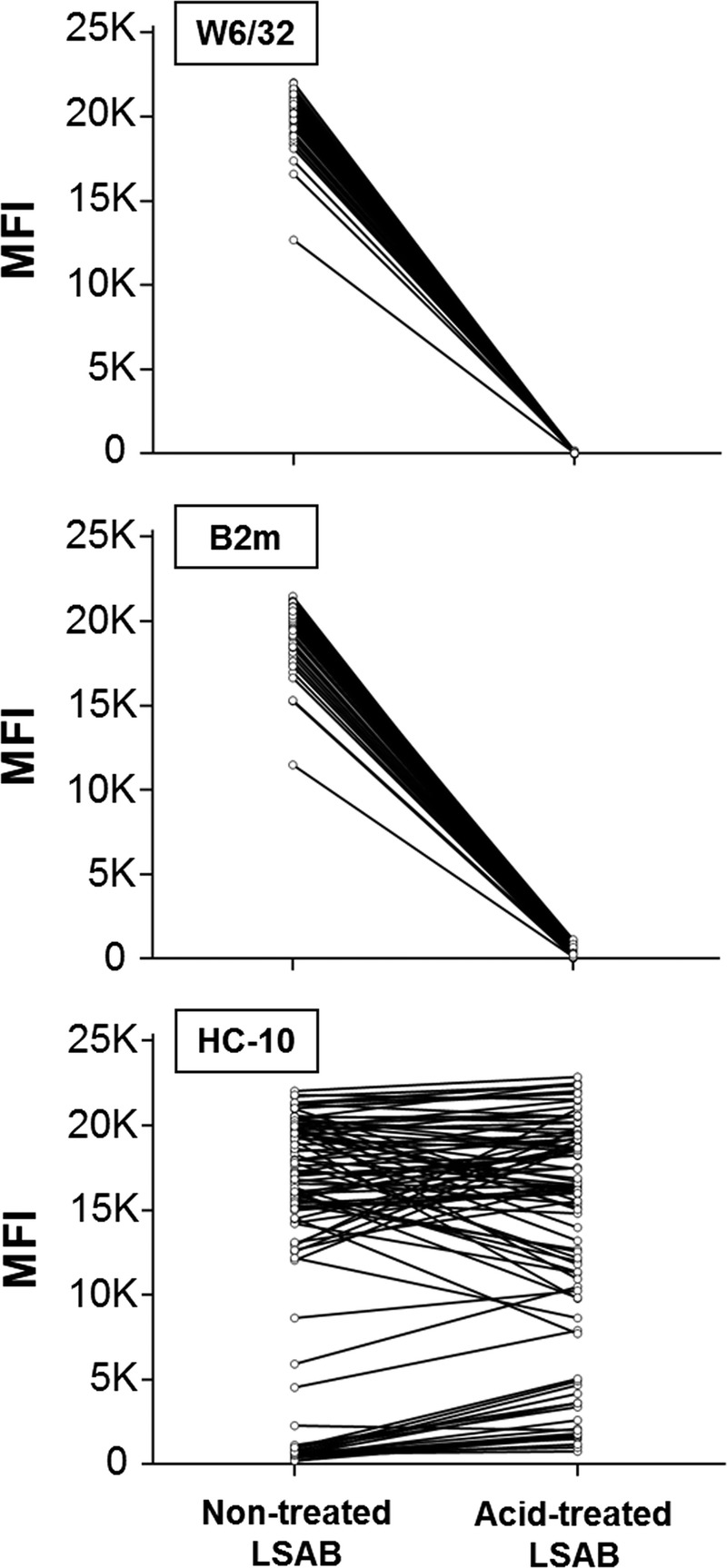
Reactivity of 3 monoclonal antibodies on nontreated and acid-treated HLA-I LSAB. Nontreated and acid-treated LSAB were incubated with W6/32, anti-β2m (both from Thermo Fisher Scientific, Rockford, IL) and HC-10 (kindly provided by One Lambda) at 10 μg/mL, then were washed and incubated with 200-fold diluted PE-labeled Goat F(ab’)2 fragment anti-mouse IgG (H + L) (Beckman Coulter, Marseille, France). After acid treatment, all beads reached MFI < 1000 with W6/32 and 3 beads still had MFI ≥ 1000 with anti-β2m.
Second, the “alkali-treatment” of LSAB described by the authors is performed with concentrated guanidinium chloride, a chaotropic agent, and is much harsher than a denaturation induced by a mild pH change. After this treatment, the MFI of TFL-006 became higher than with acid-treated LSAB, but remained lower than HC-10 MFI that did not reach the same level observed on acid-treated beads. These results indicate that TFL-006 could recognize an isoform of β2fHC that is not denatured the same way as the one present on LSAB, and underline that the kind of LSAB denaturing treatment could greatly impact the denaturation process in ways that are not understood yet.
Finally, the authors proposed that our previous results showing that anti-dHLA can still stain iBeads corresponded to antibodies directed against pepF-β2aHC.3,5 To us, this is highly unlikely because acid-induced denaturation is known to release β2m from HLA molecules, leading to an almost complete loss of β2aHC. Moreover, we clearly demonstrated that reactivities resembling those of anti-dHLA can correspond to anti-native HLA (anti-nHLA) antibodies recognizing peculiar epitopes displayed by pepA-β2aHC that are conserved on β2fHC, and/or mixtures of anti-nHLA and anti-dHLA antibodies recognizing the same antigens.4
In conclusion, Jucaud et al1 brought additional data about denatured HLA-I molecules borne by LSAB. Their results underline the difficulty of correctly interpreting anti-dHLA patterns using strategies relying on bead-specific cutoffs based on monoclonal antibodies reactivity or chemical treatments of LSAB. As suggested by the authors, the transplant community could plead in favor of the resurgence of iBeads production because this assay proved efficient in eliminating the anti-dHLA detection and showed promising results.2,3,5
Footnotes
The authors declare no funding or conflicts of interest.
J.V., G.G. and J.T. contributed to the design of the study. J.V. participated in the performance of the research. J.V. and J.T. participated in data analysis. J.T. and J.V. participated in the writing of the paper. G.G. was involved in critical revision of the manuscript.
Correspondence: Jonathan VISENTIN, Pharm D, PhD, Laboratoire d’Immunologie et Immunogénétique, Hôpital Pellegrin, CHU de Bordeaux, Place Amélie Raba Léon, 33076 Bordeaux Cedex, France. (jonathan.visentin@chu-bordeaux.fr).
REFERENCES
- 1.Jucaud V, Ravindranath MH, Terasaki PI. Conformational variants of the individual HLA-I antigens on Luminex single antigen beads used in monitoring HLA antibodies: problems and solutions. Transplantation. 2016. 10.1097/TP.0000000000001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otten HG, Verhaar MC, Borst HP. The significance of pretransplant donor-specific antibodies reactive with intact or denatured human leucocyte antigen in kidney transplantation Clin Exp Immunol 2013. 173536–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visentin J, Guidicelli G, Nong T. Evaluation of the iBeads assay as a tool for identifying class I HLA antibodies Hum Immunol 2015. 76651–656 [DOI] [PubMed] [Google Scholar]
- 4.Visentin J, Guidicelli G, Moreau JF. Deciphering allogeneic antibody response against native and denatured HLA epitopes in organ transplantation Eur J Immunol 2015. 452111–2121 [DOI] [PubMed] [Google Scholar]
- 5.Visentin J, Marroc M, Guidicelli G. Clinical impact of preformed donor-specific denatured class I HLA antibodies after kidney transplantation Clin Transplant 2015. 29393–402 [DOI] [PubMed] [Google Scholar]


