Abstract
Background
The liver as transplantation site for human pancreatic islets is a harsh microenvironment for islets and it lacks the ability to retrieve the graft. A retrievable, extrahepatic transplantation site that mimics the pancreatic environment is desired. Ideally, this transplantation site should be located subdermal for easy surgical-access but this never resulted in normoglycemia. Here, we describe the design and efficacy of a novel prevascularized, subcutaneously implanted, retrievable poly (D,L-lactide-co-ε-caprolactone) scaffold.
Method
Three dosages of rat islets, that is, 400, 800, and 1200, were implanted in immune compromised mice to test the efficacy (n = 5). Islet transplantation under the kidney capsule served as control (n = 5). The efficacy was determined by nonfasting blood glucose measurements and glucose tolerance tests.
Results
Transplantation of 800 (n = 5) and 1200 islets (n = 5) into the scaffold reversed diabetes in respectively 80 and 100% of the mice within 6.8 to 18.5 days posttransplant. The marginal dose of 400 islets (n = 5) induced normoglycemia in 20%. The glucose tolerance test showed major improvement of the glucose clearance in the scaffold groups compared to diabetic controls. However, the kidney capsule was slightly more efficacious because all 800 (n = 5) and 1200 islets (n = 5) recipients and 40% of the 400 islets (n = 5) recipients became normoglycemic within 8 days. Removal of the scaffolds or kidney grafts resulted in immediate return to hyperglycemia. Normoglycemia was not achieved with 1200 islets in the unmodified skin group.
Conclusions
Our findings demonstrate that the prevascularized poly (D,L-lactide-co-ε-caprolactone) scaffold maintains viability and function of islets in the subcutaneous site.
Pancreatic islet transplantation via infusion of islets into the portal vein is a promising therapy for typ. 1 diabetes mellitus.1 It results in normalization of blood glucose levels and induces insulin independence for more than a year but is still associated with low long-term success rates. Within 5 years after islet transplantation less than 50% of the patients are still normoglycemic.2–4 The reasons for graft failure are considered to be multifactorial,5,6 but the portal vein as infusion route and engraftment in the liver are considered to be major contributors. Liver-specific issues such as high concentrations of immunosuppressive drugs,5,7 large numbers of natural killer T cells,8 and the instant blood-mediated inflammatory reaction9 results in loss of a large number of islets immediately after transplantation. Islets are also exposed to relative low oxygen and nutrient availability caused by lower vascularization degree10 in the liver compared to the native pancreas, resulting in impaired function and cell death.
Several alternative transplantations sites were investigated,11 but the human body may not provide an optimal transplantation site for pancreatic islets. An optimal transplantation site should be readily accessible, have the ability to bear an adequate tissue volume, have a sufficient vascular network, and should provide support to the islets to prevent loss of function and cell death.11 Other desirable characteristics of a transplantation site for islets are the ability to retrieve and monitor the cellular graft. To meet these criteria, we propose a retrievable extrahepatic transplantation site, also referred to as scaffold, which is implanted at a readily accessible site, such as under the skin, and mimics the pancreatic environment.6,12
The proposed subcutaneous site cannot be used in an unmodified form, because it is associated with low oxygen tensions and poor vascularization. As a consequence, transplantation of islets in an unmodified subcutaneous site rarely succeeded.13,14 Several biomaterials were used to modify the subcutaneous site to facilitate islet survival, but resulted in variable outcomes.14,15 Many of the strategies failed due to the foreign-body or other inflammatory responses against the biomaterials.16 Better biomaterials are needed,6,12 which should not interfere with functional islet survival or provoke a severe immune reaction to prevent cell loss.
In previous studies, we identified a polymer suitable for islet transplantation by extensive in vitro and in vivo research.17,18 Poly (D,L-lactide-co-ε-caprolactone) (PDLLCL) was one of the few tested biomaterials that had no toxic effects and supported islet functionality and viability nor did it provoke a severe inflammatory reaction when subcutaneously implanted.17 The in vivo experiments also showed that a minimum preimplantation period of 1 month was needed to stimulate vascularization of the PDLLCL scaffold. This period is also required to dampen the foreign-body response prior to islet transplantation.17
Here, we present a novel PDLLCL-based scaffold containing special channels to facilitate implantation of islets. The scaffold was embedded with fibrin to support angiogenesis and islet survival. The novel scaffold is versatile and can be easily scaled up to bear higher amounts of islets. Because nothing is known about the efficacy of this type of subcutaneous scaffold as transplantation site for islets, we implanted graded loads of islets and compared survival and graft-function with similar sized grafts implanted under the kidney capsule.
MATERIALS AND METHODS
Experimental Design
Streptozotocin-induced diabetic immune compromised athymic nude mice (Foxn1nu) were used as transplant recipients to test the efficacy of a subcutaneously implanted poly(68/32[15/85 D/L-lactide]-co-ε-caprolacton (PDLLCL) scaffold. Scaffolds were implanted 4 weeks before islet transplantation to allow vascularization. Three dosages of 400, 800, and 1200 rat islets were transplanted in either the scaffold or under the kidney capsule. The kidney capsule served as a positive control because the kidney capsule is a well-established standard in mice,19 and islets are easily retrievable for histology. Furthermore, 1200 islets were transplanted in an unmodified subcutaneous pocket. The percentage of animals becoming normoglycemic, the duration to achieve normoglycemia, and the glucose tolerance (at 4 and 8 weeks after transplantation) were used as measure for efficacy. Islet grafts were removed 10 weeks after islet transplantation for histological analysis and to study return to hyperglycemia. Islet grafts were sectioned and stained to study islet engraftment, vascularization, and survival.
Scaffold
The PDLLCL scaffold (10 by 15 mm) was obtained from Polyganics B.V. (Groningen, The Netherlands). More details about the scaffold are provided in the SDC, http://links.lww.com/TP/B399. Several prototypes were evaluated before the final design was tested with rodent islets in diabetic athymic nude mice.
To facilitate ingrowth of blood vessels and mimic the pancreatic environment, fibrin was incorporated around the channels before implantation. Human fibrinogen (2 mg/mL; Sigma-Aldrich, St. Louis, MO) was dissolved in CMRL (Corning Cellgro, Manassas, VA) with 1% Penicillin-Streptomycin (Corning Cellgro). To form a fibrin gel, 100 μL of this fibrinogen solution was mixed with 1 μL of 100 U/mL thrombin IIa (Sigma-Aldrich) and transferred onto the scaffold. The fibrin gel solidified within the pores of the scaffold during a 1-hour incubation at room temperature and subsequently another incubation of 1 hour at 37°C.
Animals
The University of California Institutional Animal Care and Use Committee at the University of Irvine approved all described animal procedures (Institutional Animal Care and Use Committee 2008-2850). Sprague-Dawley rats (Envigo Harlan, Placentia, CA) with a body weight ranging between 250 and 279 g served as islet donors. Male athymic nude mice (Charles River, Wilmington, MA) at 8 weeks old were used as transplant recipients. Rat donors instead of mice donors were used to reduce the number of experimental animals. Animals were housed at the University of California Irvine animal facility and maintained under 12-hour light/dark cycles with ad libitum access to water and standard chow.
Islet Isolation
Islets were isolated as previously described.20 Briefly, pancreata were infused with a Collagenase V (Sigma-Aldrich) solution in Hank balanced salt solution (Corning Cellgro). The distended pancreata were carefully harvested and digestion was completed in an 18-minute incubation at 37°C. A Ficoll (Gradient stock solution; Corning Cellgro) density-gradient was used to purify the islets from the exocrine tissue. To quantify the yield and to estimate the purity, the isolated islets were stained with dithizone (MP Biomedicals, Santa Ana, CA).
Islet Transplantation and Surgery
Nude mice were rendered diabetic by an intraperitoneal streptozotocin (Sigma Aldrich) injection of 180 mg/kg in 0.1 M sodium citrate buffer, pH 4.5. Diabetes was confirmed by 2 consecutive measurements of nonfasting blood glucose above 350 mg/dl. A Bayer Health Care (Whippany, NJ) blood glucose monitor was used to measure blood glucose levels. After diabetes confirmation, the mice received a subcutaneous insulin implant (LinBit; LinShin, Scaraborough, Canada) to reduce the discomfort caused by the diabetic state, and scaffolds were subcutaneously implanted on the back of the mice. To allow the scaffold to vascularize, islet transplantation into this scaffold was carried out 4 weeks after implantation. Briefly, a small incision was made just above the scaffold to remove the tubing from the scaffold, creating 2 channels for islets. The islets were placed in these channels using a 23G Hamilton syringe (SGE Analytical Science, Austin, TX). The mice received either 400, 800, or 1200 islets. As an additional control mice received 1200 islets in an unmodified subcutaneous pocket of 2 cm created with forceps.
Diabetic mice of the control group received islets under the kidney capsule. Briefly, mice were anesthetized and a small incision was made in the skin and the peritoneum just above the kidney to access the kidney capsule. With a 25G needle (Becton Dickinson, Franklin Lakes, NJ) an opening in the kidney capsule was created, subsequently a glass rod was used to obtain a subcapsular pocket for islet transplantation. Control mice were also transplanted with either 400, 800, or 1200 islets. The insulin implant was removed directly after transplantation in all groups, and all mice received ibuprofen (0.2 mg/mL) as an analgesic postsurgery.
During the 10 weeks after transplantation, blood glucose levels of the mice were measured on a weekly basis. Animals were considered to be normoglycemic when nonfasting blood glucose levels collected from the tail vein were below 150 mg/dL. When the blood glucose levels remained above 350 mg/dL or higher, the animal was killed by a blunt incision through the heart after which the graft was removed for histological analysis.
Intraperitoneal Glucose Tolerance Test
At 4 and 8 weeks after islet transplantation, an intraperitoneal glucose tolerance test (IPGTT) was performed to measure the clearance of an intraperitoneally injected glucose load.21 Mice were fasted overnight before the start of the IPGTT. Glucose (3 g/kg; Sigma-Aldrich) was injected intraperitoneally at time 0, blood samples were collected from the tail at 0, 10, 30, 60, 90, and 120 minutes to measure the glucose concentration with a Bayer Health Care blood glucose monitor. The glucose clearance was expressed as the area under the curve (AUC).22 Diabetic and healthy mice served as controls.
Histology
The scaffolds and the kidneys were carefully excised 10 weeks after islet transplantation. To exclude endocrine pancreas regeneration and confirm graft dependency of normoglycemia, blood glucose levels were measured for 1 week after removal of the islet graft. Also, pancreas biopsies were taken to exclude β-cell regeneration. The histology is further described in detail in the SDC, http://links.lww.com/TP/B399.
Statistics
Statistical analysis was carried out in GraphPad Prism (version 5.0b; GraphPad Software, Inc., La Jolla, CA). A Shapiro-Wilk normality test was performed to test the data for normality. For statistical analysis of the duration before achieving normoglycemia, a Mann-Whitney U test was done, while for the IPGTT data a 2-way analysis of variance with a Bonferroni post hoc test was applied, P values less than 0.05 were considered significant. The data are respectively presented in mean ± standard deviation in case of parametric distribution and median ± interquartile range in case of nonparametric data distribution.
RESULTS
Scaffold Design
Several prototypes of our scaffold were tested before the implantation-studies. To expedite readily infusion of the islets, early prototypes were modified with channels. Introduction of channels after implantation was associated with bleeding, lack of control of the length of the channels, and inflammatory responses. The success rates of transplantations were low in the pilot studies. Introduction of the channels with iron rods after casting without keeping them patent during the 4-week implantation period was also associated with low success rates due to the need to open the channels with a needle upon infusion of the islets. Selection of the biomaterial for keeping the channel patent required a detailed study of the potential responses against the biomaterials. Finally, polyethylene tubing with high hydrophobicity was chosen, because this material did not provoke severe responses and was not associated with any cell adhesion, which facilitated the removal upon the introduction of the islets (Figure 1). Fibrin was introduced as it facilitates ingrowth of blood vessels23 and mimics the extracellular matrix environment that islets need for survival. This final concept was used to test the efficacy as transplantation site for pancreatic islets by introducing this small scaffold of 10 × 15 × 5 mm with a volume of 750 mm3 under the skin.
FIGURE 1.
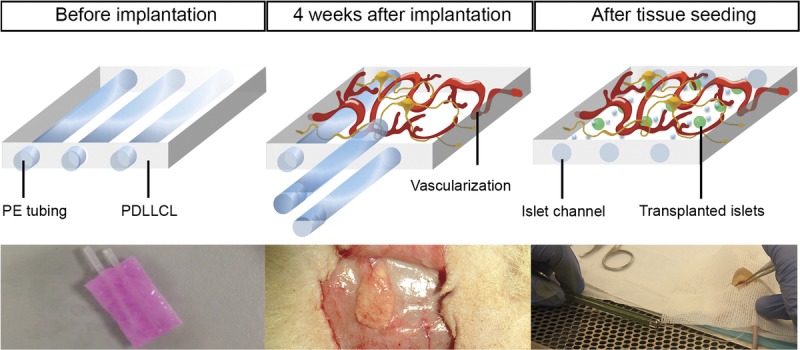
PDLLCL scaffold concept. The PDLLCL scaffold contains PE rods with high hydrophobicity to keep the channels patent during the 4-week implantation period before introduction of islets. Fibrin facilitates vascularization (pink substance in left picture). The scaffold is implanted subcutaneously 4 weeks before introduction of islets during which the foreign body response is dampened and vascularization is completed. Upon removal of the rods, the islets can be easily infused (right picture). PE, polyethylene.
Restoration of Blood Glucose Levels
Complete normoglycemia after rat islet transplantation was defined as blood glucose levels below 150 mg/dL. Three islet doses, that is, 400, 800, and 1200 islets, were transplanted either in the scaffold or under the kidney capsule (n = 5 per dose and site). From the animals that received 400 islets under the kidney capsule 40% became normoglycemic within 8 ± 4.2 days (mean ± standard deviation), whereas from the scaffold group. 20% became normoglycemic within 55 days. This percentage increased up to 80% when the islet dose in the scaffold was increased to 800 islets, the animals of this group became normoglycemic within 18.5 ± 14.2 days (Figure 2). All animals that received 1200 islets in the scaffold became normoglycemic within 6.8 ± 4.0 days. Also, all animals that received 800 or 1200 islets under the kidney capsule became normoglycemic. These animals became normoglycemic within respectively 2.4 ± 1.3 and 1.2 ± 0.4 days. The time to become normoglycemic was statistical significantly longer for scaffold recipients then for animals from the kidney capsule groups (P < 0.05). None of the animals that received 1200 islets in an unmodified subcutaneous pocket became normoglycemic (n = 5). All animals with a successful islet graft remained normoglycemic for the duration of the study, which was a period of 10 weeks. After this period, the islet grafts were removed and all animals returned to their pretransplant hyperglycemic state (Figure 2). In addition, pancreas biopsies were stained with aldehyde fuchsin24 to confirm the absence of β cells in the native pancreas, defined as <5% of healthy controls (HCs), but no regeneration was observed (data not shown).
FIGURE 2.
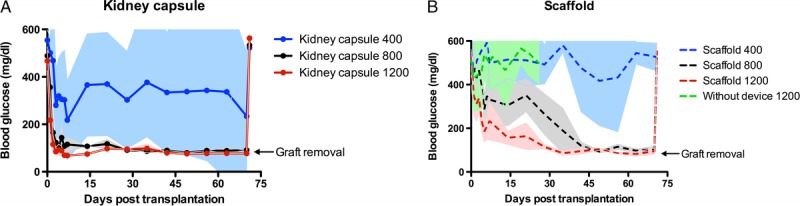
Long-term blood glucose levels after islet transplantation under the kidney capsule and in the scaffold. Nonfasting blood glucose measurements after rat-islet transplantation under the kidney capsule (A) or in a preimplanted subcutaneous scaffold (B) in a diabetic mouse model. In both conditions 3 dosages of islets were transplanted: 400, 800, and 1200 islets. Furthermore, nonfasting blood glucose levels of mice transplanted with 1200 islets in an unmodified subcutaneous pocket were also shown (B). After 70 days the graft was removed (arrow) after which the animals returned to hyperglycemia. Data points represent blood glucose mean with standard deviation (n = 5).
Improved Glucose Tolerance
To test the metabolic function of the islet grafts an IPGTT was performed at 4 and 8 weeks after transplantation. In view of the low percentage of mice becoming normoglycemic in the 400 islets groups and the unmodified subcutaneous site, IPGTT data of these mice were excluded from this analysis (Figure 3). An intraperitoneal glucose load was cleared 6 times faster by HC mice (7066 mg/dL per minute [6705-12 109], n = 4, p < 0.05) compared to the diabetic control (DC) mice (43 210 mg/dL per minute [22 750-52 410], n = 3). After 4 weeks, both islets under the kidney capsule and in the scaffold statistical significantly improved the clearance compared to the DC (P < 0.05). The glucose clearance of 800 islets under the kidney capsule (n = 5) was 13 115 mg/dL per minute (7166-13 893). Transplantation of 1200 islets under the kidney capsule (n = 5) statistical significantly (P < 0.05) reduced the clearance to 5602 mg/dL per minute (4570-8171) compared with the DC. These glucose clearance levels were similar to the HCs. Both the 800 (14672 mg/dL per minute [7294-18 762], n = 4) and 1200 (17 901 mg/dL per minute [11 393-26 559], n = 4) islets in the scaffold reduced the clearance to one third of the DC. After 8 weeks, similar clearance levels were observed. The 800 islets under the kidney capsule resulted in a clearance of 9866 mg/dL per minute (8861-20 140), and the 1200 islets reduced the clearance statistical significantly (P < 0.05) to 7931 mg/dL per minute (5194- 9281) compared with the DC. Both 800 (20454 mg/dL per minute [11 623-26 490]) and 1200 (23 313 mg/dL per minute [16 212-27 860]) islets in the scaffold statistical significantly reduced the clearance to 50% of the DC (P < 0.05), but this was approximately twice as high as the clearance of the both kidney capsule groups.
FIGURE 3.
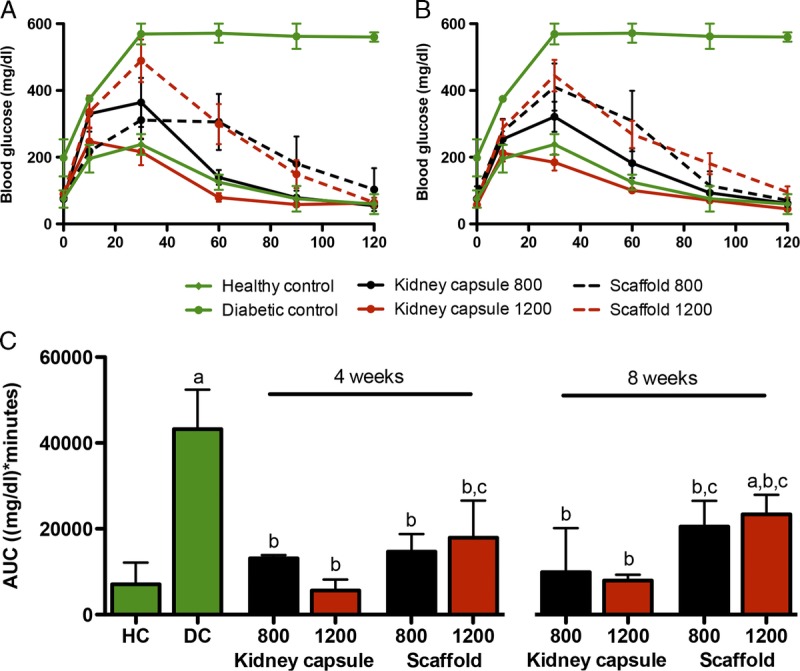
Glucose clearance after IPGTT. The blood glucose levels after a glucose bolus at 4 (A) and 8 (B) weeks after islet transplantation. The glucose clearance from the blood expressed as AUC (mg/dL per minute) for HC mice, DC mice, and the 800 and 1200 islets groups of the kidney capsule and the scaffold. The clearance was measured 4 and 8 weeks after islet transplantation (C). Median and interquartile range are plotted (n = 5), a statistical analysis was carried out using a 2-way ANOVA with a Bonferroni post hoc test (P < 0.05). (a) significantly different compared to the HC, (b) significantly different compared to the DC, (c) significantly different compared to the 1200 islets transplanted under the kidney capsule of the same time point.
Insulin-Positive Cells in Scaffolds
At the end of the experiment, clear differences were found between the histopathology of the different dosages (Figure 4). Scaffolds with 400 islets contained low numbers of insulin positive cells. No intact islets were found in this group (Figure 4D). However, large numbers of insulin positive islets were found in both the 800 and 1200 islets scaffold groups (Figures 4E and F). Islets had normal spherical shapes and were not organized in sheet shape as observed under the kidney capsules (Figures 4A-C). Islets were found in different sizes and were also found in between the PDLLCL illustrating that the islets can spread in the scaffold.
FIGURE 4.
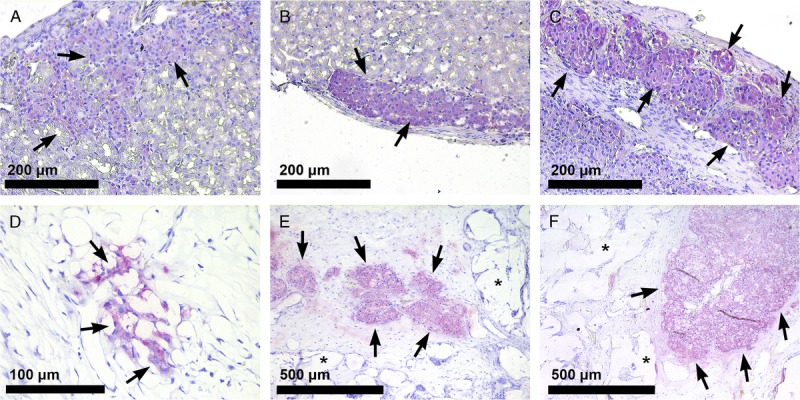
Histological analysis of islets transplanted under the kidney capsule or in the PDLLCL scaffold. Insulin staining (SIGMAFAST Fast Red, arrows) of 400 (A), 800 (B), and 1200 (C) rat islets under the kidney capsule (20×) 10 weeks after transplantation. Insulin positive cells (40×) 10 weeks after transplantation of 400 islets into the subcutaneous PDLLCL scaffold (D) and insulin-positive islets (10×) after transplantation of 800 (E) and 1200 islets (F) in the polymer (*) scaffold.
We found no signs of foreign body responses (Figure 5A). We screened for presence of multinucleated giant cells and granulocyte invasion25,26 as signs of such a response but these were never found. PDLLCL vessels were found intact and in close contact with either islets or with cells that invaded the scaffold in the 4-week pretransplant period (Figures 5B/C). There was a clear separation between the scaffold ingrowth and the surrounding adipose and muscle tissue. As a consequence, the scaffold could be easily removed after which the animals became hyperglycemic.
FIGURE 5.
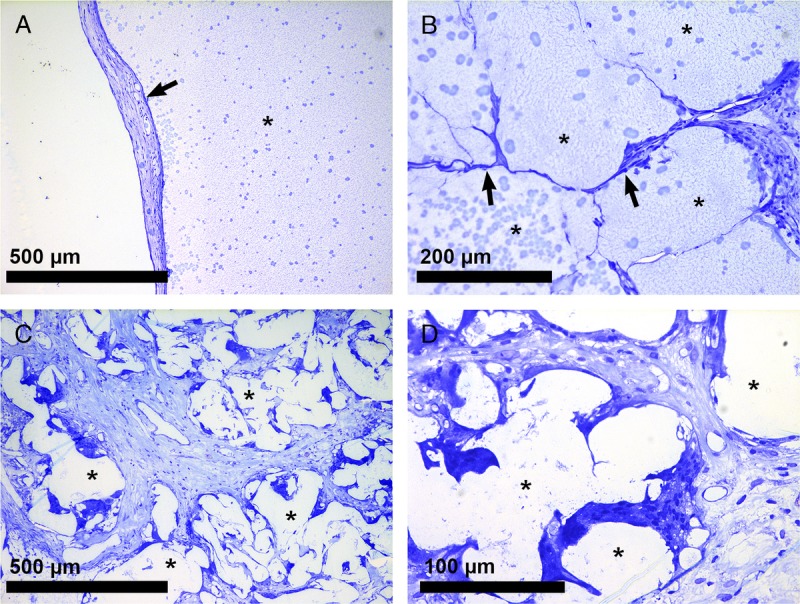
Interaction of PDLLCL with surrounding cells. Minor fibrosis (A; arrow) and no immune cells were observed (10×) in and around the islet scaffold (*) 10 weeks after transplantation. Biomaterial-cell interactions were found between PDLLCL fibers (*) and cells (B/C). These cells were integrated in the scaffold (20×; arrows). Panel D shows a high power field (40×) of these interactions.
DISCUSSION
PDLLCL is a Food and Drug Administration–approved polymer, which has shown to induce minimal tissue responses in animals and humans.27–29 Clinical studies have shown that it supports wound repair, facilitate recovery of nerve defects, and can also function as anti-adhesive sheet.27–29 The present study demonstrates that PDLLCL is also a suitable polymer for creating a subcutaneous islet transplantation site. Transplantation of 1200 islets, that is, the equivalent of the number of islets in a normal mouse pancreas,30 resulted in normoglycemia in 100% of the scaffold mice recipients. Lowering the dosage to 800 islets induced normoglycemia in 80% of the recipients. With the lowest concentration of 400 islets still 20% became normoglycemic. In the unmodified skin we have never been able to induce normoglycemia with these relative low numbers of islets nor were others.31–33
To monitor the engraftment in the scaffolds, IPGTTs were performed at 4 and 8 weeks after transplantation. At both time points, transplantation of 800 or 1200 islets in our scaffold majorly improved the IPGTT compared with the DCs. However, there was no difference in AUC between the 800 and 1200 islet recipients. This might be explained by competition for oxygen and other essential nutrients when islets are transplanted in groups of 1200 in our scaffold instead of 800. This might be associated with higher loss of islet tissue in the period between transplantation and vascularization. There was no statistically significant difference between the 4 and 8 weeks IPGTT in the scaffolds animals, indicating that engraftment was already complete at 4 weeks after implantation.
The present study showed that the subcutaneous scaffold was a less efficacious transplantation site than the kidney capsule. All recipients in the 1200 and 800 islets groups became normoglycemic after transplantation under the kidney capsule while 400 islets induced normoglycemia in 40% of the kidney capsule recipients. This was to be expected because the kidney capsule site is known for its fast islet metabolic engraftment and function.11 However, the advantage of readily accessibility, minor surgery, retrievability, and the absence of the risk of damaging internal organs makes to our opinion the somewhat lesser efficacy of the subcutaneous scaffold compared to the kidney capsule still acceptable. Conceivable approaches to improve the efficacy might be a further enhancement of vascularization in the pretransplant period.
The success of a scaffold in cell transplantation and tissue engineering applications depends on its pore characteristics.34 We therefore applied salt leaching to create an open network of PDLLCL pores with distances in the final concept of 250 to 425 μm. The porous structure was also flexible and embedded in an extracellular matrix of fibrin to facilitate ingrowth of endothelial cells due to the pro-angiogenic properties of fibrin.35 The success of this approach was illustrated in histology demonstrating many cells that were integrated into the scaffold and having cell-biomaterial interactions in the absence of inflammatory cells. However, it is known that there are differences between the skin of rodents and humans.36 For example, the epithelium of the epidermis in colonized by different immune cell population, which could play a role in different cell-biomaterial interactions and inflammation. Therefore, further experiments in animal models with a skin similar to that of humans are needed.
Several attempts to modify the subcutaneous site for islet transplantation were not very successful due to foreign body and inflammatory responses.16 Except for selecting biocompatible polymers also other approaches have shown success. Pepper et al3,31 temporarily implanted a biomaterial to create a vascularized site and a microenvironment favorable for islet tissue and removed this biomaterial before islet transplantation.3,31 This strategy favored engraftment but the islet graft is not easily retrievable, as the graft is not enclosed by a material. Our approach allows retrieval of the graft, which might be advantageous when replenishable cell-sources, such as stem cells that still suffer from issues such as teratoma formation, are becoming a realistic option for application.37,38 Ludwig et al39,40 modified the subcutaneous space by implanting an oxygenated chamber system composed of an immune isolating membrane not allowing entry of any inflammatory cells. In this way no immunosuppressive drugs are needed and the immune system cannot directly attack the islets.
We investigated the foreign body response against PDLLCL by implanting the material subcutaneously in Albino Oxford rats, which are known to be highly immune responsive to tissues and biomaterials.18 We compared the responses against 2 other Food and Drug Administration–approved biomaterials, that is, polysulfone and poly(ethylene oxide terephthalate)/polybutylene terephthalate block copolymer. Compared with the other 2 materials PDLLCL only induced minor foreign body responses. These responses were completed 4 weeks after implantation, which was the reason to choose the 4-week implantation and prevascularization period in this study.
The current design is the result of many years of testing prototypes and adaptations. The scaffold had a volume of 750 mm3 and could easily accommodate 1200 islets per channel. For this experiment, 2 channels contained enough volume for the islet dosages, but it is possible to prepare scaffolds of this size with 3 islet channels containing 2000 islets per channel, so it can carry a total of 6000 islets. For human application usually 5000 islets/kg is needed.41 This implies for example for a person weighing 70 kilogram that a scaffold should carry 350 000 islets. This is a 58-fold increase in islet mass implying during scale up at least a scaffold volume of 43 500 mm3. This is about the size of a credit card (approximately 46750 mm3; 85 × 55 × 10 mm) that can easily be implanted under the skin of a human recipient.
The current scaffold concept is not only applicable for pancreatic islets but may also serve as scaffold for alternative insulin producing cell sources. These alternative cell sources are needed, as the availability of donor pancreata can never meet the demand for islets when cell transplantation in typ. 1 diabetes mellitus is becoming a feasible option. During the past few years, research showed the applicability of adult and juvenile porcine islets,42,43 human embryonic stem cells,37 or human induced pluripotent stem cell derived pancreatic progenitor cells.38 For the use of these alternative cell sources, it is important that the cell graft can be monitored and retrieved, as there is a realistic risk of teratoma formation when using stem cells. Our subcutaneous PDLLCL transplantation site provides easy monitoring and removal, and we therefore foresee application of our device in this field of research.
Our findings demonstrate that the current design of our PDLLCL scaffold exerted protective effects on the viability and function of islets and improved the engraftment of islets in the subcutaneous site. This study shows that the scaffold is a potential alternative for intraportal islet transplantation.
ACKNOWLEDGMENTS
The authors thank Margot Beukers (DCTI), Michael Alexander (University of California, Irvine, USA), and Antonio Flores (University of California) for their support during this study.
Footnotes
This research is part of the Diabetes Cell Therapy Initiative (DCTI) (FES 2009 program, Dutch ministry of welfare and sports, and the Dutch diabetes research foundation). It is also supported by a JDRF short-term fellowship for discovery consortia grant (March 2015) and a JDRF research grant (May 2016).
The authors declare no conflicts of interest.
A.M.S., S.L., and B.d.H. conducted the data collection and analysis. They and the other academical authors (A.A.v.A., E.d.K., M.M.F., J.L., P.d.V.) are responsible for the study design, decision to publish, and preparation of the article. The private partners have contributed to the project through regular discussion (D.T.H., L.S.).
Correspondence: Alexandra M. Smink, PhD, Pathology and Medical Biology, Section Immunoendocrinology, University of Groningen, University Medical Center Groningen, Hanzeplein 1, EA11, 9713 GZ, Groningen, The Netherlands. (a.m.smink@umcg.nl).
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
Rat pancreatic islets were implanted in diabetic nude mice either in a scaffold that had been vascularized after subcutaneous implantation or under the kidney capsule. Although the kidney capsule was superior to revert diabetes, the scaffold also allowed control of diabetes using higher numbers of islets. Supplemental digital content is available in the text.
REFERENCES
- 1.Shapiro AM, Ricordi C, Hering BJ. International trial of the edmonton protocol for islet transplantation N Engl J Med 2006. 3551318–1330 [DOI] [PubMed] [Google Scholar]
- 2.Schuetz C, Markmann JF. Islet cell transplantion: update on current clinical trials Current Transplantation Reports 2016. 3254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pepper AR, Gala-Lopez B, Ziff O. Current status of clinical islet transplantation World J Transplant 2013. 348–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro AM, Ricordi C. Islet cell transplantation procedure and surgical technique. In: Textbook of organ transplantation. John Wiley & Sons, Ltd; 2014:682–690. [Google Scholar]
- 5.Harlan DM, Kenyon NS, Korsgren O. Current advances and travails in islet transplantation Diabetes 2009. 582175–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smink AM, Faas MM, de Vos P. Toward engineering a novel transplantation site for human pancreatic islets Diabetes 2013. 621357–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacotte S, Berney T, Shapiro AJ. Immune monitoring of pancreatic islet graft: towards a better understanding, detection and treatment of harmful events Expert Opin Biol Ther 2011. 1155–66 [DOI] [PubMed] [Google Scholar]
- 8.Toyofuku A, Yasunami Y, Nabeyama K. Natural killer T-cells participate in rejection of islet allografts in the liver of mice Diabetes 2006. 5534–39 [PubMed] [Google Scholar]
- 9.Naziruddin B, Iwahashi S, Kanak MA. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation Am J Transplant 2014. 14428–437 [DOI] [PubMed] [Google Scholar]
- 10.Carlsson PO, Palm F, Andersson A. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site Diabetes 2001. 50489–495 [DOI] [PubMed] [Google Scholar]
- 11.Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts Curr Diab Rep 2011. 11364–374 [DOI] [PubMed] [Google Scholar]
- 12.Dufour JM, Rajotte RV, Zimmerman M. Development of an ectopic site for islet transplantation, using biodegradable scaffolds Tissue Eng 2005. 111323–1331 [DOI] [PubMed] [Google Scholar]
- 13.Simeonovic CJ, Dhall DP, Wilson JD. A comparative study of transplant sites for endocrine tissue transplantation in the pig Aust J Exp Biol Med Sci 1986. 6437–41 [DOI] [PubMed] [Google Scholar]
- 14.Sakata N, Aoki T, Yoshimatsu G. Strategy for clinical setting in intramuscular and subcutaneous islet transplantation Diabetes Metab Res Rev 2014. 301–10 [DOI] [PubMed] [Google Scholar]
- 15.Saito T, Ohashi K, Utoh R. Reversal of diabetes by the creation of neo-islet tissues into a subcutaneous site using islet cell sheets Transplantation 2011. 921231–1236 [DOI] [PubMed] [Google Scholar]
- 16.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials Semin Immunol 2008. 2086–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smink AM, de Haan BJ, Paredes-Juarez GA. Selection of polymers for application in scaffolds applicable for human pancreatic islet transplantation. Biomed Mater. 2016;11:035006. doi: 10.1088/1748-6041/11/3/035006. [DOI] [PubMed] [Google Scholar]
- 18.Smink AM, Hertsig DT, Schwab L, et al. A retrievable, efficacious polymeric scaffold for subcutaneous transplantation of rat pancreatic islets. Ann Surg. 2016. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan R, Buder B, Alexander M. Juvenile porcine islets can restore euglycemia in diabetic athymic nude mice after xenotransplantation Transplantation 2015. 99710–716 [DOI] [PubMed] [Google Scholar]
- 20.Lamb M, Storrs R, Li S. Function and viability of human islets encapsulated in alginate sheets: In vitro and in vivo culture Transplant Proc 2011. 433265–3266 [DOI] [PubMed] [Google Scholar]
- 21.Ayala JE, Samuel VT, Morton GJ. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice Dis Model Mech 2010. 3525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin D, Bellanne-Chantelot C, Deschamps I. Long-term follow-up of oral glucose tolerance test-derived glucose tolerance and insulin secretion and insulin sensitivity indexes in subjects with glucokinase mutations (MODY2) Diabetes Care 2008. 311321–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riopel M, Trinder M, Wang R. Fibrin, a scaffold material for islet transplantation and pancreatic endocrine tissue engineering Tissue Eng Part B Rev 2015. 2134–44 [DOI] [PubMed] [Google Scholar]
- 24.Smelt MJ, de Haan BJ, Faas MM. Effects of acute cytomegalovirus infection on rat islet allograft survival Cell Transplant 2011. 201271–1283 [DOI] [PubMed] [Google Scholar]
- 25.de Vos P, Spasojevic M, de Haan BJ. The association between in vivo physicochemical changes and inflammatory responses against alginate based microcapsules Biomaterials 2012. 335552–5559 [DOI] [PubMed] [Google Scholar]
- 26.Smelt MJ, Faas MM, de Haan BJ. The role of alloresponsive Ly49+ NK cells in rat islet allograft failure in the presence and absence of cytomegalovirus Cell Transplant 2014. 231381–1394 [DOI] [PubMed] [Google Scholar]
- 27.Meek MF, Coert JH. US Food and Drug Administration/conformit europe- approved absorbable nerve conduits for clinical repair of peripheral and cranial nerves Ann Plast Surg 2008. 60466–472 [PubMed] [Google Scholar]
- 28.Meek MF, Den Dunnen WF. Porosity of the wall of a neurolac nerve conduit hampers nerve regeneration Microsurgery 2009. 29473–478 [DOI] [PubMed] [Google Scholar]
- 29.Bertleff MJ, Meek MF, Nicolai JP. A prospective clinical evaluation of biodegradable neurolac nerve guides for sensory nerve repair in the hand J Hand Surg [Am 2005. 30513–518 [DOI] [PubMed] [Google Scholar]
- 30.Desgraz R, Herrera PL. Pancreatic neurogenin 3-expressing cells are unipotent islet precursors Development 2009. 1363567–3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pepper AR, Gala-Lopez B, Pawlick R. A prevascularized subcutaneous device-less site for islet and cellular transplantation Nat Biotechnol 2015. 33518–523 [DOI] [PubMed] [Google Scholar]
- 32.Kerby A, Bohman S, Westberg H. Immunoisolation of islets in high guluronic acid barium-alginate microcapsules does not improve graft outcome at the subcutaneous site Artif Organs 2012. 36564–570 [DOI] [PubMed] [Google Scholar]
- 33.Kim JS, Lim JH, Nam HY. In situ application of hydrogel-type fibrin-islet composite optimized for rapid glycemic control by subcutaneous xenogeneic porcine islet transplantation J Control Release 2012. 162382–390 [DOI] [PubMed] [Google Scholar]
- 34.O’Brien FJ. Biomaterials & scaffolds for tissue engineering Mater Today 2011. 1488–95 [Google Scholar]
- 35.Jiang B, Waller TM, Larson JC. Fibrin-loaded porous poly(ethylene glycol) hydrogels as scaffold materials for vascularized tissue formation Tissue Eng Part A 2013. 19224–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation Nat Rev Immunol 2014. 14289–301 [DOI] [PubMed] [Google Scholar]
- 37.Calafiore R, Montanucci P, Basta G. Stem cells for pancreatic beta-cell replacement in diabetes mellitus: actual perspectives Curr Opin Organ Transplant 2014. 19162–168 [DOI] [PubMed] [Google Scholar]
- 38.Shahjalal HM, Shiraki N, Sakano D. Generation of insulin-producing β-like cells from human iPS cells in a defined and completely xeno-free culture system J Mol Cell Biol 2014. 6394–408 [DOI] [PubMed] [Google Scholar]
- 39.Ludwig B, Rotem A, Schmid J. Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist Proc Natl Acad Sci U S A 2012. 1095022–5027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ludwig B, Reichel A, Steffen A. Transplantation of human islets without immunosuppression Proc Natl Acad Sci U S A 2013. 11019054–19058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCall M, Shapiro AM. Update on islet transplantation. Cold Spring Harb Perspect Med. 2012;2:a007823. doi: 10.1101/cshperspect.a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bottino R, Trucco M. Use of genetically-engineered pig donors in islet transplantation World J Transplant 2015. 5243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamb M, Laugenour K, Liang O. In vitro maturation of viable islets from partially digested young pig pancreas Cell Transplant 2014. 23263–272 [DOI] [PubMed] [Google Scholar]


