Abstract
Background
We examined if African or Asian ethnicity was associated with lower access to kidney transplantation (KT) in a Canadian setting.
Methods
Patients referred for KT to the Toronto General Hospital from January 1, 2003, to December 31, 2012, who completed social work assessment, were included (n = 1769). The association between ethnicity and the time from referral to completion of KT evaluation or receipt of a KT were examined using Cox proportional hazards models.
Results
About 54% of the sample was white, 13% African, 11% East Asian, and 11% South Asian; 7% had “other” (n = 121) ethnic background. African Canadians (hazard ratio [HR], 0.75; 95% CI: 0.62-0.92]) and patients with “other” ethnicity (HR, 0.71; 95% CI, 0.55-0.92) were less likely to complete the KT evaluation compared with white Canadians, and this association remained statistically significant in multivariable adjusted models. Access to KT was significantly reduced for all ethnic groups assessed compared with white Canadians, and this was primarily driven by differences in access to living donor KT. The adjusted HRs for living donor KT were 0.35 (95% CI, 0.24-0.51), 0.27 (95% CI, 0.17-0.41), 0.43 (95% CI, 0.30-0.61), and 0.34 (95% CI, 0.20-0.56) for African, East or South Asian Canadians and for patients with “other” ethnic background, respectively.
Conclusions
Similar to other jurisdictions, nonwhite patients face barriers to accessing KT in Canada. This inequity is very substantial for living donor KT. Further research is needed to identify if these inequities are due to potentially modifiable barriers.
Kidney transplantation (KT) is associated with reduced morbidity and mortality,1–4 improved quality of life,5,6 and reduced healthcare costs7,8 when compared with dialysis. Living donor KT (LDKT) is the treatment of choice for suitable patients with end-stage kidney disease (ESKD), because of shortage of deceased donors9 and better outcomes.10–12 However, KT, and LDKT in particular, is underused.2,13
The contribution of ethnocultural factors to accessing KT has mainly been documented for African Americans, Native Americans, and Hispanics14–17 living in the U.S. African Americans take longer to complete the KT evaluation (KTE)18–20 and are less likely to get waitlisted and transplanted19,21,22 than whites. A U.S. study showed that transplantation among Asians was even lower than among African Americans.23
There are differences in the social environment and healthcare delivery in Canada versus the United States; therefore, clinical research findings in one country cannot be readily extrapolated to the other. Although individuals with African or Asian background represent almost 10% of the population of Canada24 (close to 20% in Ontario25), only a few studies assessed ethnic disparities in access to KT in Canada.26–28 Two studies analyzing data from before 2000 showed that KT rates were lower for ethnic groups (Aboriginal, African, South Asian, and East Asian Canadians) compared with white Canadians.27,28
To our knowledge, no data have been reported about the association between ethnicity and completing the KTE in Canada. Furthermore, no studies have evaluated access to KT in a more contemporary Canadian cohort. Therefore, we aimed to assess the association between ethnicity and the likelihood of completing the KTE and receiving a KT in a large Canadian transplant center.
MATERIALS AND METHODS
Study Design and Population
This is a single-center, retrospective cohort study of adults (≥18 years) referred to the Toronto General Hospital for KT between January 1, 2003, and December 31, 2012 (n = 2584). Patients were followed up until December 31, 2013. We excluded multiorgan transplant candidates (n = 63) and patients who did not complete the social work assessment (n = 752) (Figure 1). We obtained approval for this study from the University Health Network Research Ethics Board. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.”
FIGURE 1.
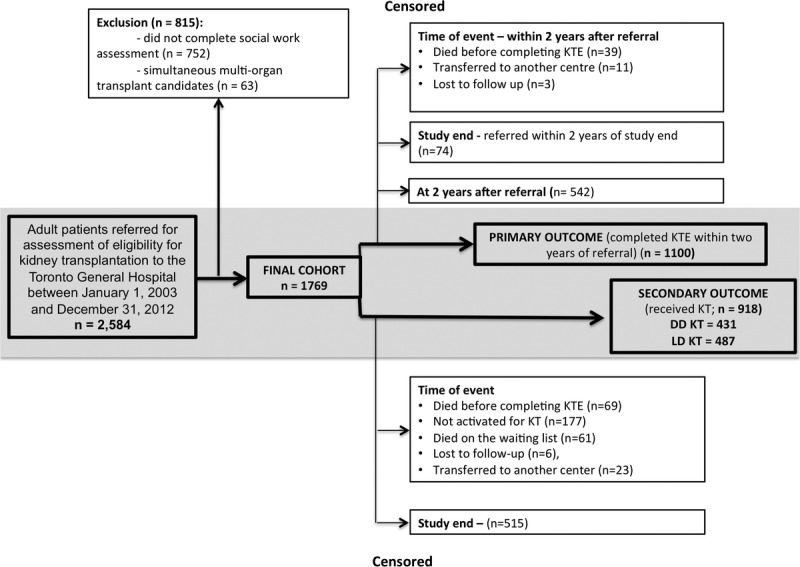
Study flow diagram.
Data Sources and Management
We created a data collection form to abstract and record information about ethnicity as well as about language barrier and marital status from the pretransplant social work assessment notes. These notes have been transcribed into the Organ Transplant Tracking Record (OTTR) software. OTTR has been the main electronic medical record system for patients referred, waitlisted, and transplanted at our center since 2000. If any information was absent or unclear, letters dictated by physicians and progress notes, and so on, were examined.
After quality checks, the data collected for this study were merged with our in-center research database, the Comprehensive Renal Transplant Research Information System (CoReTRIS).29 CoReTRIS contains recipient, donor, transplant, laboratory, pathology, treatment, and follow-up data for all patients who received KT at our center since 2000. Moreover, it includes detailed clinical information on all new referrals since January 1, 2003. These data have been abstracted from patient charts (electronic and paper), entered into the database, and audited for completeness and accuracy.
Exposure Assessment and Classification
The exposure of interest was ethnicity as identified primarily in the social work notes. The notes were usually transcribed or scanned into OTTR. The social work assessment at our institute has been a semistructured detailed interview with the patient. The overall structure and content areas of this evaluation have not changed during the study period. During the interview, the following areas are explored by the social worker: personal and family history; current social situation, living environment; work history, financial situation and drug coverage; support systems (personal support, housing situation, transportation, and so on); substance use and mental health history (smoking, alcohol use, recreational drugs, history of mental health problems, concerns regarding adherence); expectations from and motivation for KT; planning for KT (family discussion, financial and logistic planning). In addition, any indication of country of origin, immigration date, or ethnic background was also considered. The following categories were generated30,31: (1) white, (2) African, (3) East Asian (eg, Chinese, Japanese, Korean), (4) South Asian (eg, Indian, Pakistani, Sri Lankan, Indo-Caribbean), and (5) Other (Canadian First Nations, Pacific-Islander, Middle Eastern, and so on).
Outcome Assessment and Classification
The primary outcome of interest was time from referral to completion of the KTE (ie, activate on the waiting list, not activate on the waiting list, or clear a patient for LDKT) within 2 years of referral. We also conducted sensitivity analyses with the outcome “cleared for transplant” (ie, activate a patient to the waiting list or clear a patient for LDKT). The secondary outcomes of interest were: (1) receipt of any KT; (2) deceased donor KT (DDKT) or (3) LDKT.
Patient Follow-Up
The date of referral to the transplant center, completion of the KTE, transplant or death were recorded in OTTR. The data were abstracted, then stored in CoReTRIS after quality checks and audits.29 The time origin for all analyses was the date of referral to the transplant center. For the primary outcome (ie, completing the KTE) patients were followed until decision about transplant candidacy or study end. Participants who died before completion of the KTE were censored at the time of death. Patients who did not complete the KTE but who had been referred within 2 years of study end (December 31, 2012) were censored at study end. Patients who were still in evaluation at 2 years after referral were censored at that time point (Figure 1). Patients accepted for KT were followed up until transplantation or study end. Participants who died while waiting for KT, were lost to follow-up or transferred to another center were censored at the time of the event (Figure 1).
Potential Confounders
To assess the independent association between the exposure and outcome, we built multivariable Cox proportional hazards models that adjusted for covariates that were chosen based on their association with the exposure and outcome, clinical experience, and data from the literature. Variables entered in the models included recipient age, sex, marital status, employment, ability to communicate in English (as described by the social worker), socioeconomic status, comorbidities, blood group, and peak panel-reactive antibody (PRA) at the time of referral. Sociodemographic, clinical characteristics, and comorbidities were obtained from CoReTRIS. To characterize socioeconomic status, we used the Ontario Marginalization Index (OMI).32 The OMI is a census-based and geographically based index that considers residential instability, material deprivation, ethnic concentration, and dependency. Participants were allocated to a deprivation quintile based on their postal code. Quintiles 1 to 5 of the OMI represent the least to the most deprived, respectively. Comorbid conditions adjusted for in multivariable models included diabetes mellitus, coronary artery disease or myocardial infarction, heart failure, stroke, peripheral vascular disease, chronic lung disease, and/or nonskin cancer.
Statistical Analysis
Categorical variables were described using frequencies and percentages while continuous variables were depicted using the mean (standard deviation, SD) for normally distributed data and the median (interquartile range [IQR]) for skewed variables. We evaluated the distribution of baseline characteristics across ethnic categories using parametric and nonparametric tests as appropriate. We graphically assessed the cumulative probabilities of the study endpoints using the Kaplan-Meier product limit method, and examined differences across survival functions using the log-rank test. We explored univariable and multivariable associations between the exposure and outcome in Cox proportional hazards models. To account for potentially varying baseline hazards over the relatively long time period of study entry, we stratified our Cox models for 2 eras of study entry (cutoff December 31, 2007, the mid-point of our study period). We sequentially fit models that contained an expanding set of covariates. The proportional hazards assumption was tested using scaled Schoenfeld residuals. No important departures from proportionality were detected.
We conducted sensitivity analyses using the outcome “cleared for transplant” (activated to the waiting list or cleared for LDKT) instead of “completing KTE”. In additional sensitivity analyses, we recoded censored cases (other than censored for study end) as ones reaching an endpoint to assess if informative censoring could have biased our results. We also tested our models with recoding censored observations as reaching the 2-year follow-up without an endpoint.
Missingness was less than 5% for all variables, except for peak PRA (38%) and the Ontario Marginalization Index (8%). We used the method of multiple imputation by chained equations to address missingness.33 This method replaces missing values with a set of imputed values in different imputed data sets based on the joint distribution of existing values of variables entered in the imputation model. We performed analyses on 5 complete imputed data sets and combined the results using Rubin’s rules.
We performed sensitivity analyses to test the robustness of our findings with regards the potential impact of missing information about ethnicity on our results after case wise deletion of participants with “unknown” ethnicity.
We performed all statistical analyses using Stata 13.0 (StataCorp, College Station, TX). A 2-sided P value less than 0.05 was considered statistically significant.
RESULTS
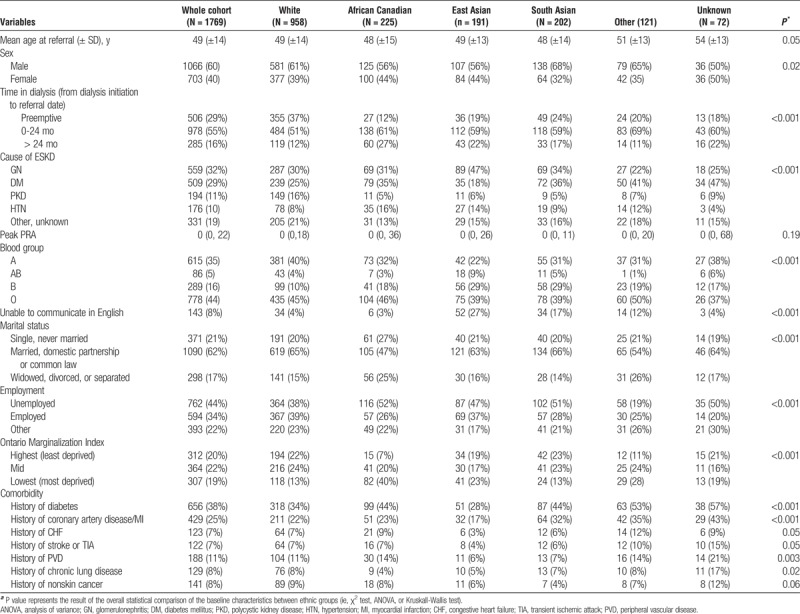
During the study period, 2584 patients were referred for assessment of KT eligibility. After applying the a priori exclusion criteria, we included 1769 potential KT recipients for the analysis (Figure 1). The baseline characteristics of the study cohort are shown in Table 1. About 54% of the sample (958 patients) were white, 13% (225 patients) African Canadian, 11% (191 patients) East Asian, and 11% (202 patients) South Asian Canadians. Seven percent of patients had other (n = 121) and 4% unknown (n = 72) ethnic background. Diabetic kidney disease was the most frequent cause of ESKD among South Asian and African Canadian patients, whereas glomerular diseases were more frequent among East Asian Canadians. AB and B blood group were most prevalent among patients with Asian background (Table 1). Language barrier was mainly present in patients with Asian backgrounds. Socioeconomic deprivation was more prevalent among African Canadians, compared with other groups.
TABLE 1.
Baseline characteristics of the study sample
A total of 1100 patients completed the KTE within 2 years of referral (median follow-up: 307 [IQR: 193-452] days). Of these patients, only 103 were deemed ineligible for transplantation. During the follow-up period, 918 patients received either a deceased or living donor KT over a median of 629 (IQR, 362-1120) days from the time of referral. Four hundred eighty-seven patients received LDKT (Figure 1).
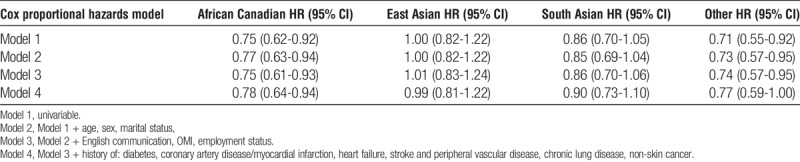
Compared with white Canadians (68.9%; 95% confidence interval [CI], 65.8-71.8), African Canadians (59.1%; 95% CI, 52.5-65.7) and patients with “other” ethnicity (54.8%; 95% CI, 46.1-64.0]), but not South Asian (61.7%; 95% CI, 54.8-68.6]) or East Asian Canadians (67.3%; 95% CI, 60.5-74.0), had a lower cumulative probability of completing the KTE within 2 years of referral (Figure 2). African Canadian and “other” ethnicity were associated with a significantly reduced unadjusted hazard ratio (HR) of completing the KTE in a univariable Cox proportional hazards model (HR, 0.75; 95% CI, 0.62-0.92; P = 0.006 and 0.71; 95% CI, 0.55-0.92; P = 0.013, respectively) (Table 2). Sequential adjustments for an expanding set of covariates did not appreciably alter the point estimate, and the association between ethnicity and completing the KTE remained statistically significant. In the final model (model 4), the adjusted HR for completing the KTE was 0.78 (95% CI, 0.64-0.94; P = 0.012) and 0.77 (95% CI, 0.59-1.00; P = 0.05) for patients with African Canadian and “other” ethnicity, respectively (Table 2). We found qualitatively similar results in analyses using “cleared for transplant” as outcome (Table S1, SDC, http://links.lww.com/TP/B396).
FIGURE 2.
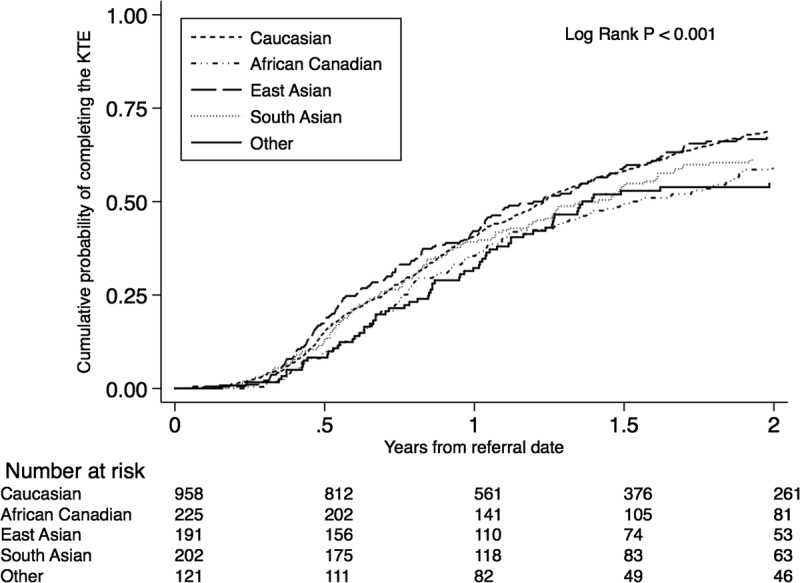
Cumulative probability of completing the KT evaluation by ethnicity.
TABLE 2.
Multivariable adjusted, era stratified likelihood of completing the KTE within 2 years after referral for patients with various ethnic backgrounds
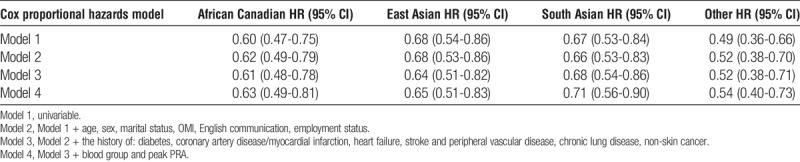
Ethnicity was associated with lower cumulative probability of receiving a KT (either from living or deceased donor) (Figure 3). In multivariable Cox proportional hazards models, all ethnic groups assessed had lower likelihood of receiving a KT (Table 3).
FIGURE 3.
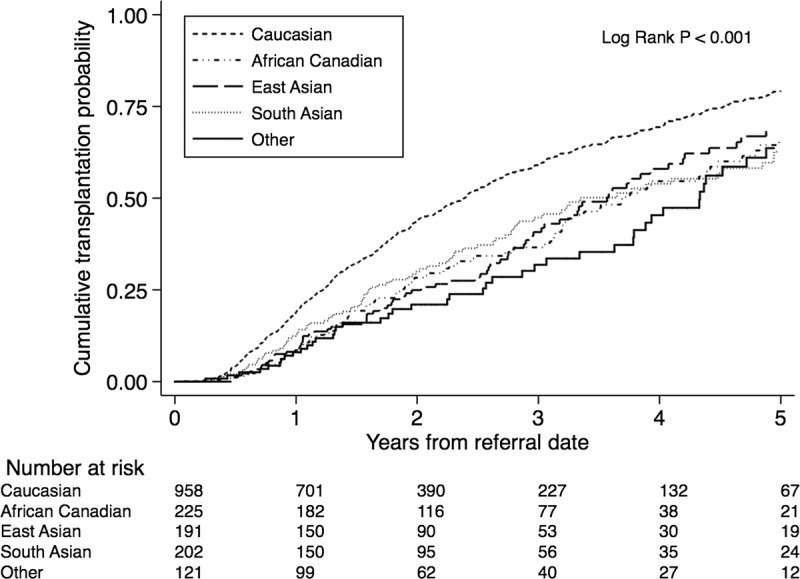
Cumulative probability of receiving a deceased donor KT by ethnicity.
TABLE 3.
Multivariable adjusted, era stratified likelihood of receiving a KT for patients with various ethnic backgrounds
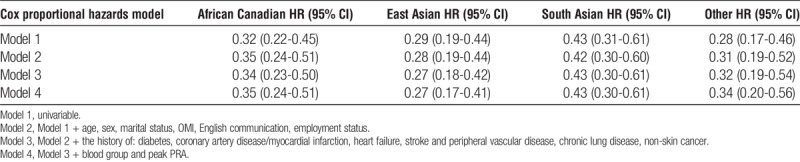
All ethnic groups, except East Asian Canadians, had similar probability of receiving a DDKT (Figure S1, SDC, http://links.lww.com/TP/B396). The association between ethnicity and access to DDKT remained qualitatively unchanged after multivariable adjustment (Table S2, SDC, http://links.lww.com/TP/B396). In contrast, there was a striking difference between white and non-white patients in receiving LDKT (Figure 4). Compared with white Canadians (61.1%; 95% CI, 54.3-68.0), African (31.0%; 95% CI, 19.8-46.4), East Asian (21.4%; 95% CI, 12.9-34.4), South Asian Canadians (38.4%; 95% CI, 26.1-54.0), and patients with “other” ethnicity (20.9%; 95% CI, 11.6-35.9) had significantly lower cumulative probability of receiving LDKT. These significant differences remained qualitatively unchanged after adjusting for sociodemographic and clinical variables (Table 4).
FIGURE 4.
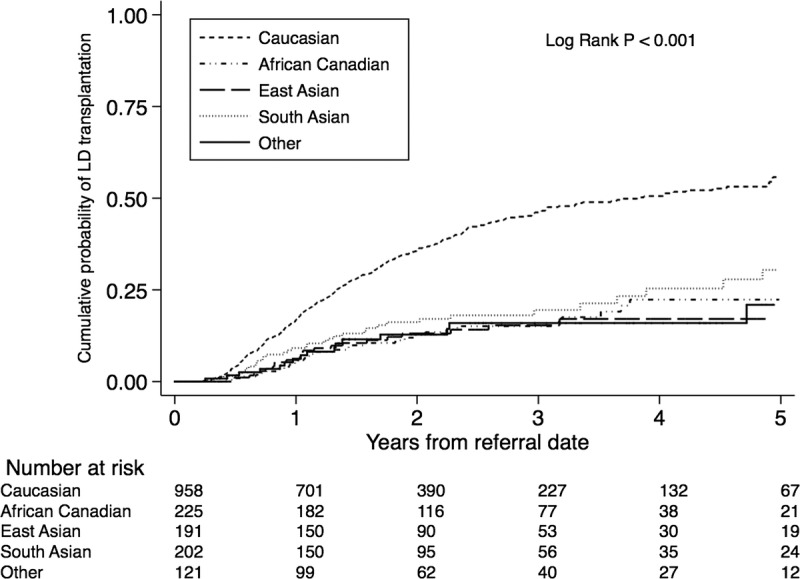
Cumulative probability of receiving a living donor KT by ethnicity.
TABLE 4.
Multivariable adjusted, era stratified likelihood of receiving a living donor KT for patients with various ethnic backgrounds
We tested the robustness of the primary results in several sets of sensitivity analyses (Table S3-S5, SDC, http://links.lww.com/TP/B396). The HR estimates did not materially change for the strategies used to re-analyze the data.
DISCUSSION
In this retrospective study, we showed that African Canadians and patients with “other” ethnicity were less likely to complete the KTE when compared with white Canadians. Furthermore, patients with African, Asian, or “other” ethnicity were significantly less likely than whites to receive a KT or a LDKT.
These results suggest that ethnic background represents a significant barrier to accessing LDKT in a Canadian setting. This is important for at least 2 reasons. First, reduced access to LDKT for African or Asian Canadian patients will likely result in poorer health outcomes. Furthermore, some of the ethnocultural barriers may be modifiable, as demonstrated for African Americans in the United States.16,17,34–36
Similar to our findings, studies in the United States reported that African Americans were substantially less likely to complete the KTE compared to whites. Socioeconomic status, geographical location, insurance status, patient preferences, lack of transplant related knowledge, experiences with discrimination were some of the potential factors that were felt to contribute.14,16,19,22,23,35,37–40 African Canadians in our sample were more likely to be deprived compared with other ethnic groups. The reduced access to waitlisting for African Canadians, however, remained significant even after adjusting for the Ontario Marginalization Index. Patient preferences, behavioural factors, health literacy, lack of understanding of the process, mistrust, or communication barriers may contribute to the observed inequities.
Similarly, patients with “other” ethnicity were also less likely to complete KTE. Most patients in this group were Indigenous, Hispanic, Middle Eastern, or Pacific Islander Canadians. The low number of individuals in each subgroup did not allow for more detailed analysis. Significant disparities in accessing KT have been reported for Indigenous People in Canada41–43 and for the other ethnicities in the United States.23
Important differences between the transplant evaluation process in the United States and at our center need to be considered when interpreting our results. In the United States most of the KTE is organized by the transplant centers after the patient had been referred. At our center, referral for KT is only accepted once the patient completed most of their KTE, which is done at or in close proximity to the dialysis unit. This is preferred by most patients, primarily because the reduced travel time and costs and better coordination with dialysis scheduling. On the other hand, this can contribute to inefficiencies because the priorities of the dialysis staff primarily focus on dialysis related concerns. Among others, this may be a contributing factor to the frequently lengthy evaluation process.
Access to DDKT was similar between the ethnic groups, except East Asian Canadians were more likely to receive a DDKT, primarily several years after referral. The reason for this difference is not clear at this point. It is possible that this may be due, in part, to longer waiting time for Asian patients with blood group “B” and “AB” and a consequent allocation of compatible blood group “O” kidney to those patients.
Patients of African or Asian background had substantially lower likelihood of receiving LDKT. Similar differences had been demonstrated in the United States and also in 2 earlier Canadian papers that used pre-2000 registry data.27,28 In our more contemporary and more granular data set, we demonstrate that the presence and magnitude of ethnic disparities in accessing LDKT have remained largely unchanged over the last decades in Canada.
Only limited information is available about access to KT outside the United States. Dudley et al44 reported that non-white patients in the United Kingdom were more likely to be waitlisted than whites. This could support the assumption that universal access to healthcare may reduce or eliminate ethnic disparities in access to KT. The Canadian experience reported here, however, seems to partially contradict this assumption: access to healthcare is universal in Canada but some of the ethnic disparities seem to be similar to those seen in the U.S. Similar to our findings, a few reports indicated that disparities in access to KT are found even when universal healthcare coverage is provided.45
The lack of transplant related knowledge is a modifiable barrier to KT.16,37,46,47 The differences between ethnic groups observed in our analysis may reflect a lack of awareness about the benefits of LDKT, concerns about safety of the donor or culturally determined negative attitudes towards LDKT.
African American patients may have fewer suitable living donors due to familial clustering of comorbidity48 and this could also be true for Asian Canadians. Differences in the family structure and social networks may also contribute to the observed disparities in LDKT.40,49 African Canadian patients were less likely to be married or having common law relationship, that might have limited the number of potentially available living donors. Finally, recent immigrants may not have family members available to donate and may not be sufficiently integrated into the local community to allow identification of potential living donors. Compatible with the potential role of social networks in accessing LDKT, preliminary results of our study demonstrated that unrelated donor candidates were less frequently identified by African or Asian when compared with white Canadians.50
Little is known about the factors that may contribute to the observed differences in patients of Asian background. Prasad et al speculated that the lack of awareness about the benefits of KT and language barriers may play a role.51 In 2 qualitative studies the lack of communication about death and organ donation within the family or in the community, and the need to preserve an intact body emerged as potential barriers to deceased donation among East Asian52 and South Asian53 Canadians. Type 2 diabetes mellitus is very prevalent among South Asian Canadians and this may limit the availability of suitable living donors. Culture specific beliefs about the potential negative impact of surgery on one’s health, concerns about the safety and subsequent health of the living donor, concerns about the expenses associated with the surgery and subsequent financial stability of the family and also potential fear from stigma associated with chronic illness may limit the willingness of patients with Asian background to consider living donor transplant or may prevent potential living donors to come forward.
Our study is notable for its relatively large sample size, carefully documented clinical and sociodemographic variables and long follow-up. However, limitations of the analysis also deserve note. First, our data set is derived from a single-center, which may limit generalizability. Second, a significant proportion of the referred patients did not complete social work assessment and thus had to be excluded. Importantly, reasons for not completing the social work assessment may be related to ethnocultural background. There are several potential reasons why a patient may not have available record of social worker assessment in our database. Patients who had relatively recently referred or were still in the process of assessment may not have yet completed the social work assessment. On the contrary, for some patients, who had initiated the workup but the process halted for some reason, the social worker visit was not completed. For patients who were referred during the earlier years of the recruitment period, hard copies of the social work note could not have been located or may had been sent off site. For some patients only a brief note about the assessment summary was available in our electronic records that did not have sufficient information for extraction for the purposes of this study. Third, ethnic background was unknown for some participants. However, no systematic biases in reporting and recording ethnic information could be identified and our results are consistent with similar studies from Canada and other countries. Fourth, information about ethnicity was abstracted from patient records and ethnicity was not based on self-report. We are aware that the ethnocultural categories used in our study are not homogenous. At this stage of our understanding and within the limitations of a retrospectively collected data set, however, we felt that more granular geographic, ethnic or religious diversity could not be analyzed appropriately. Finally, although we adjusted for multiple sociodemographic and clinical covariates, the potential for residual confounding cannot be excluded.
We conclude that African Canadians and patients with “other ethnicity” are less likely to complete the KT evaluation compared to whites. Furthermore, our results indicate that non-white (vs white) patients face barriers to accessing LDKT in Canada. Further research is needed to identify if these inequities are due to potentially modifiable barriers.
ACKNOWLEDGMENTS
The authors would like to thank the students of the Multi-Organ Transplant Student Research Training Program for collecting, entering, and auditing data for the Comprehensive Renal Transplant Research Information System (CoReTRIS) at the Toronto General Hospital, University Health Network.
Footnotes
I.M. is the recipient of an unrestricted education grant from Astellas Pharma Canada to support the adaptation of “Explore Transplant” patient education program for Ontario. S.J.K. is the recipient of an unrestricted educational grant from Astellas Pharma Canada to organize an annual conference for Ontario physicians caring for kidney transplant recipients on various clinical topics (eg, skin cancer, bone disease, failing allograft). Other authors of this article have no competing interest to disclose.
I.M., O.F., M.N., and S.J.K. participated in research design. A.B., M.M., O.F., and Y.L. contributed to data abstraction, data entry and data management. A.B., O.F., Y.L., and I.M. contributed to the analysis of the data. I.M., M.N., A.D.W., and S.J.K. contributed to interpretation of the data. All authors participated in drafting and finalizing the article.
Correspondence: Istvan Mucsi, MD, PhD, FRCPC, FASN, Multiorgan Transplant Unit, Toronto General Hospital, University Health Network, PMB 11C-188, 585 University Avenue Toronto, Ontario, Canada M5G 2N2. (istvan.mucsi@utoronto.ca).
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
Access to kidney transplantation (KT) is significantly reduced for all ethnic groups assessed compared to Caucasian Canadians, and this is primarily driven by differences in access to living donor KT. Further research is needed to identify the causes of inequities. Supplemental digital content is available in the text.
REFERENCES
- 1.Wolfe RA, Ashby VB, Milford EL. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant N Engl J Med 1999. 3411725–1730 [DOI] [PubMed] [Google Scholar]
- 2.Tennankore KK, Kim SJ, Baer HJ. Survival and hospitalization for intensive home hemodialysis compared with kidney transplantation J Am Soc Nephrol 2014. 252113–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schold JD, Meier-Kriesche HU. Which renal transplant candidates should accept marginal kidneys in exchange for a shorter waiting time on dialysis? Clin J Am Soc Nephrol 2006. 1532–538 [DOI] [PubMed] [Google Scholar]
- 4.Gill JS, Schaeffner E, Chadban S. Quantification of the early risk of death in elderly kidney transplant recipients Am J Transplant 2012. 13427–432 [DOI] [PubMed] [Google Scholar]
- 5.Kovacs AZ, Molnar MZ, Szeifert L. Sleep disorders, depressive symptoms and health-related quality of life—a cross-sectional comparison between kidney transplant recipients and waitlisted patients on maintenance dialysis Nephrol Dial Transplant 2011. 261058–1065 [DOI] [PubMed] [Google Scholar]
- 6.Laupacis A, Keown P, Pus N. A study of the quality of life and cost-utility of renal transplantation Kidney Int 1996. 50235–242 [DOI] [PubMed] [Google Scholar]
- 7.Haller M, Gutjahr G, Kramar R. Cost-effectiveness analysis of renal replacement therapy in Austria Nephrol Dial Transplant 2011. 262988–2995 [DOI] [PubMed] [Google Scholar]
- 8.Wong G, Howard K, Chapman JR. Comparative survival and economic benefits of deceased donor kidney transplantation and dialysis in people with varying ages and co-morbidities PLoS One 2012. 7e29591–e29599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.System USRD. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD 2013. [Google Scholar]
- 10.Terasaki PI, Cecka JM, Gjertson DW. High survival rates of kidney transplants from spousal and living unrelated donors N Engl J Med 1995. 333333–336 [DOI] [PubMed] [Google Scholar]
- 11.Gjertson DW, Cecka JM. Living unrelated donor kidney transplantation Kidney Int 2000. 58491–499 [DOI] [PubMed] [Google Scholar]
- 12.Kanellis J. The CARI guidelines. Justification for living donor kidney transplantation Nephrology (Carlton 2010. 15S72–S79 [DOI] [PubMed] [Google Scholar]
- 13.Information CIfH. 2014 CORR Report: Treatment of End-Stage Organ Failure in Canada, 2003 to 2012. 2014. [Google Scholar]
- 14.Ayanian JZ, Cleary PD, Weissman JS. The effect of patients’ preferences on racial differences in access to renal transplantation N Engl J Med 1999. 3411661–1669 [DOI] [PubMed] [Google Scholar]
- 15.Boulware LE, Hill-Briggs F, Kraus ES. Effectiveness of educational and social worker interventions to activate patients’ discussion and pursuit of preemptive living donor kidney transplantation: a randomized controlled trial Am J Kidney Dis 2013. 61476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waterman AD, Peipert JD, Hyland SS. Modifiable patient characteristics and racial disparities in evaluation completion and living donor transplant Clin J Am Soc Nephrol 2013. 8995–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purnell TS, Hall YN, Boulware LE. Understanding and overcoming barriers to living kidney donation among racial and ethnic minorities in the United States Adv Chronic Kidney Dis 2012. 19244–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexander GC, Sehgal AR. Barriers to cadaveric renal transplantation among blacks, women, and the poor JAMA 1998. 2801148–1152 [DOI] [PubMed] [Google Scholar]
- 19.Patzer RE, Perryman JP, Schrager JD. The role of race and poverty on steps to kidney transplantation in the Southeastern United States Am J Transplant 2012. 12358–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall YN, Choi AI, Xu P. Racial ethnic differences in rates and determinants of deceased donor kidney transplantation J Am Soc Nephrol 2011. 22743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sequist TD, Narva AS, Stiles SK. Access to renal transplantation among American Indians and Hispanics J Am Soc Nephrol 2004. 44344–352 [DOI] [PubMed] [Google Scholar]
- 22.Patzer RE, Amaral S, Wasse H. Neighborhood poverty and racial disparities in kidney transplant waitlisting J Am Soc Nephrol 2009. 201333–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purnell TS, Xu P, Leca N. Racial Differences in Determinants of Live Donor Kidney Transplantation in the United States Am J Transplant 2013. 131557–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsay C. Profiles of ethnic communities in Canada. <http://www5.statcan.gc.ca/olc-cel/olc.action?objId=89-621-X&objType=2&lang=en&limit=1> Published 2001. Accessed April 4, 2016. [Google Scholar]
- 25.Government of Canada SC. 2011 National Household Survey Profile. < http://www12.statcan.gc.ca/nhs-enm/2011/dp-pd/prof/details/Page.cfm?Lang=E&Geo1=PR&Code1=35&Data=Count&SearchText=Ontario&SearchType=Begins&SearchPR=01&A1=All&B1=All&GeoLevel=PR&GeoCode=35>. Published 2011. 2015. [Google Scholar]
- 26.Yeates K, Yeates K. Health disparities in renal disease in Canada Semin Nephrol 2010. 3012–18 [DOI] [PubMed] [Google Scholar]
- 27.Yeates KE, Schaubel DE, Cass A. Access to renal transplantation for minority patients with ESRD in Canada J Am Soc Nephrol 2004. 441083–1089 [DOI] [PubMed] [Google Scholar]
- 28.Tonelli M, Hemmelgarn B, Gill JS. Patient and allograft survival of Indo Asian and East Asian dialysis patients treated in Canada Kidney Int 2007. 72499–504 [DOI] [PubMed] [Google Scholar]
- 29.Famure O, Phan NA-T, Kim SJ. Health information management for research and quality assurance: the Comprehensive Renal Transplant Research Information System Healthc Manage Forum 2014. 2730–36 [DOI] [PubMed] [Google Scholar]
- 30.Canada S. Classification of population group. <http://www.statcan.gc.ca/eng/concepts/definitions/ethnicity01> Published 2009. Accessed August 5th, 2015. [Google Scholar]
- 31.Report T-HT. We ask because we care- The Tri-Hospital + TPH Health Equity Data Collection Research Project. Report. 2013;51. [Google Scholar]
- 32.Matheson FI, Dunn JR, Smith KL. Development of the Canadian Marginalization Index: a new tool for the study of inequality Can J Public Health 2012. 103S12–S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice Stat Med 2011. 30377–399 [DOI] [PubMed] [Google Scholar]
- 34.Rodrigue JR, Paek MJ, Egbuna O. Making house calls increases living donor inquiries and evaluations for blacks on the kidney transplant waiting list Transplantation 2014. 98979–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patzer RE, Gander J, Sauls L. The RaDIANT community study protocol: community-based participatory research for reducing disparities in access to kidney transplantation. BMC Nephrol. 2014;15:171. doi: 10.1186/1471-2369-15-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patzer RE, Paul S, Plantinga L. A randomized trial to reduce disparities in referral for transplant evaluation J Am Soc Nephrol 2017. 28721–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patzer RE, Perryman JP, Pastan S. Impact of a patient education program on disparities in kidney transplant evaluation Clin J Am Soc Nephrol 2012. 7648–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purnell TS, Powe NR, Troll MU. Measuring and explaining racial and ethnic differences in willingness to donate live kidneys in the United States Clin Transplant 2013. 27673–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boulware LE, Meoni LA, Fink NE. Preferences, knowledge, communication and patient-physician discussion of living kidney transplantation in African American families Am J Transplant 2005. 51503–1512 [DOI] [PubMed] [Google Scholar]
- 40.Ladin K, Hanto DW. Understanding disparities in transplantation: do social networks provide the missing clue? Am J Transplant 2010. 10472–476 [DOI] [PubMed] [Google Scholar]
- 41.Promislow S, Hemmelgarn B, Rigatto C. Young aboriginals are less likely to receive a renal transplant: a Canadian national study. BMC Nephrol. 2013;14:11. doi: 10.1186/1471-2369-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeates KE, Cass A, Sequist TD. Indigenous people in Australia, Canada, New Zealand and the United States are less likely to receive renal transplantation Kidney Int 2009. 76659–664 [DOI] [PubMed] [Google Scholar]
- 43.Yeates KE, Schaubel DE, Cass A. Access to renal transplantation for minority patients with ESRD in Canada Am J Kidney Dis 2004. 441083–1089 [DOI] [PubMed] [Google Scholar]
- 44.Dudley CR, Johnson RJ, Thomas HL. Factors that influence access to the national renal transplant waiting list Transplantation 2009. 8896–102 [DOI] [PubMed] [Google Scholar]
- 45.Schoenmaker NJ, Tromp WF, van der Lee JH. Disparities in dialysis treatment and outcomes for Dutch and Belgian children with immigrant parents Pediatr Nephrol 2012. 271369–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kucirka LM, Grams ME, Balhara KS. Disparities in provision of transplant information affect access to kidney transplantation Am J Transplant 2011. 12351–357 [DOI] [PubMed] [Google Scholar]
- 47.Gupta N, Salter ML, Garonzik-Wang JM. Actual and perceived knowledge of kidney transplantation and the pursuit of a live donor Transplantation 2014. 98969–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bratton C, Chavin K, Baliga P. Racial disparities in organ donation and why Curr Opin Organ Transplant 2011. 16243–249 [DOI] [PubMed] [Google Scholar]
- 49.Clark CR, Hicks LS. Promoting access to renal transplantation: the role of social support networks in completing pre-transplant evaluations J Gen Intern Med 2008. 231187–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yung P, Cremin-Endes C, Bansal A. Asian Canadian patients are less likely to have potential living donors when first presenting for evaluation: a single center experience Abstract. In: Banff-CST Joint Scientific Meeting 2015. Vancouver, BC, Canada: 2015. [Google Scholar]
- 51.Prasad GVR. Renal transplantation for ethnic minorities in Canada: inequity in access and outcomes? Kidney Int 2007. 72390–392 [DOI] [PubMed] [Google Scholar]
- 52.Molzahn AE, Starzomski R, McDonald M. Chinese Canadian beliefs toward organ donation Qual Health Res 2005. 1582–98 [DOI] [PubMed] [Google Scholar]
- 53.Molzahn AE, Starzomski R, McDonald M. Indo-Canadian beliefs regarding organ donation Prog Transplant 2005. 15233–239 [DOI] [PubMed] [Google Scholar]


