Abstract
Background
Extracellular histones are cytotoxic molecules that are related to cell stress and death. They have been shown to play a crucial role in multiple pathophysiologic processes like sepsis, inflammation, vascular dysfunction, and thrombosis. Their role in organ donation and graft function and survival is still unknown. The aim of this study was to assess whether an association exists between the presence of extracellular histones in machine perfusates and deceased donor kidney viability.
Methods
Machine perfusates of 390 donations after circulatory death kidneys were analyzed for histone concentration, and corresponding graft function and survival were assessed.
Results
Extracellular histone concentrations were significantly higher in perfusates of kidneys with posttransplant graft dysfunction (primary nonfunction and delayed graft function) and were an independent risk factor for delayed graft function (odds ratio, 2.152; 95% confidence interval [95% CI], 1.199-3.863) and 1 year graft failure (hazard ratio, 1.386; 95% CI, 1.037-1.853), but not for primary nonfunction (odds ratio, 1.342; 95% CI, 0.900-2.002). One year graft survival was 12% higher in the group with low histone concentrations (P = 0.008) as compared with the group that contained higher histone concentrations.
Conclusions
This study warrants future studies to probe for a possible role of cytotoxic extracellular histones in organ viability and suggests that quantitation of extracellular histones might contribute to assessment of posttransplant graft function and survival.
The use of hypothermic machine perfusion (HMP) to preserve deceased donor kidneys continues to increase,1–3 likely due to the general consideration that HMP limits the effects of ischemia to the tissue.4,5 HMP performs superior to static cold storage in terms of graft survival and posttransplant graft function.6–8 It is assumed that especially high-risk donor kidneys, that is, kidneys from old donors, donors with increased morbidity and donors after circulatory death (DCD), may benefit from this preservation technique.1,3,9,10 In addition to improved transplant outcome, HMP provides an opportunity to easily perform viability tests on donor kidneys by measurement of pump parameters or analysis of machine perfusate.11–13
Machine perfusate metabolites and other components have been studied extensively over the last decades, but controversy exists regarding the value of such analyses to assess kidney quality. Several studies have shown the increased presence of a number of different metabolites in machine perfusates and associations with higher rates of delayed graft function (DGF) or primary nonfunction (PNF) were made.1,11–14 However, none of these individual molecules at present provides conclusive predictive value for short-term graft function.
Recent insights into cellular mechanisms underlying microcirculatory stress and, consequently, organ failure point toward a critical role of (extracellular) histones.15,16 Because extracellular histones, H2B, H3, and H4, are cytotoxic and cause endothelial damage,17–23 we hypothesized that extracellular histones in the machine perfusate reflect cell stress and damage (Figure 1) and the activation of an amplifying cascade of histone release and endothelial damage which together ultimately contribute to transplant failure. The aim of this study was to assess whether an association exists between the presence of extracellular histones in machine perfusates and kidney viability. We measured histone H3 levels in a large group (n = 390) of unselected machine perfused DCD kidneys and revealed that histone H3 levels correlated positively with DGF and 1 year graft failure.
FIGURE 1.
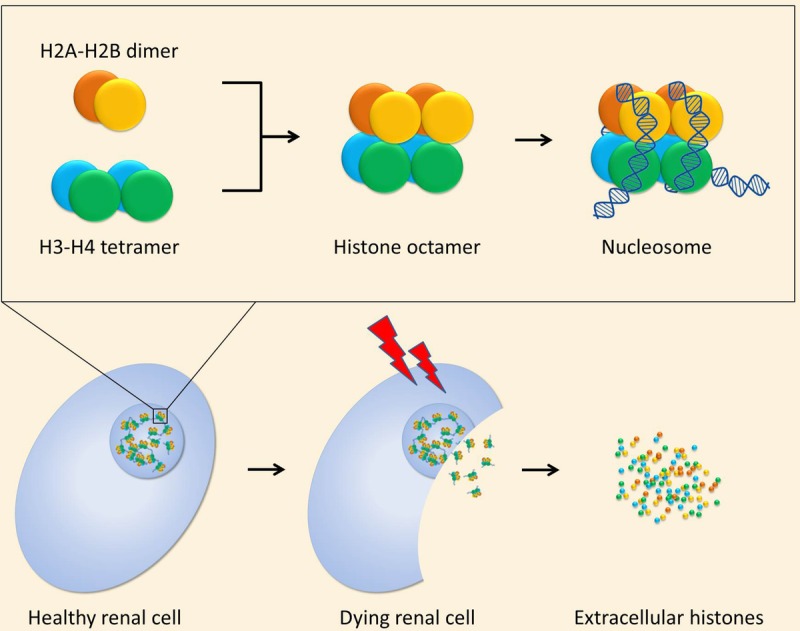
Top half: Histones 2A (orange) and 2B (yellow) form a dimer. Histones 3 (blue) and 4 (green) also form a dimer, which associates with another H3-H4 dimer to form a H3-H4 tetramer. All combined, a histone octamer is formed, which serves as the core structure of a nucleosome. DNA is wrapped around these histone cores to package the genome inside the nucleus. Lower half: A healthy renal cell contains a nucleus with nucleosomes. After ischemic injury, the renal cell may die and release histones into the extracellular space.
MATERIALS AND METHODS
Donor, Graft, and Recipient Data
Between January 1997 and March 2013, all DCD kidneys that were preserved using hypothermic pulsatile machine perfusion in our medical center, for at least 4 hours, were used for analysis. This included kidneys procured and transplanted in our center or elsewhere, within the Eurotransplant region.
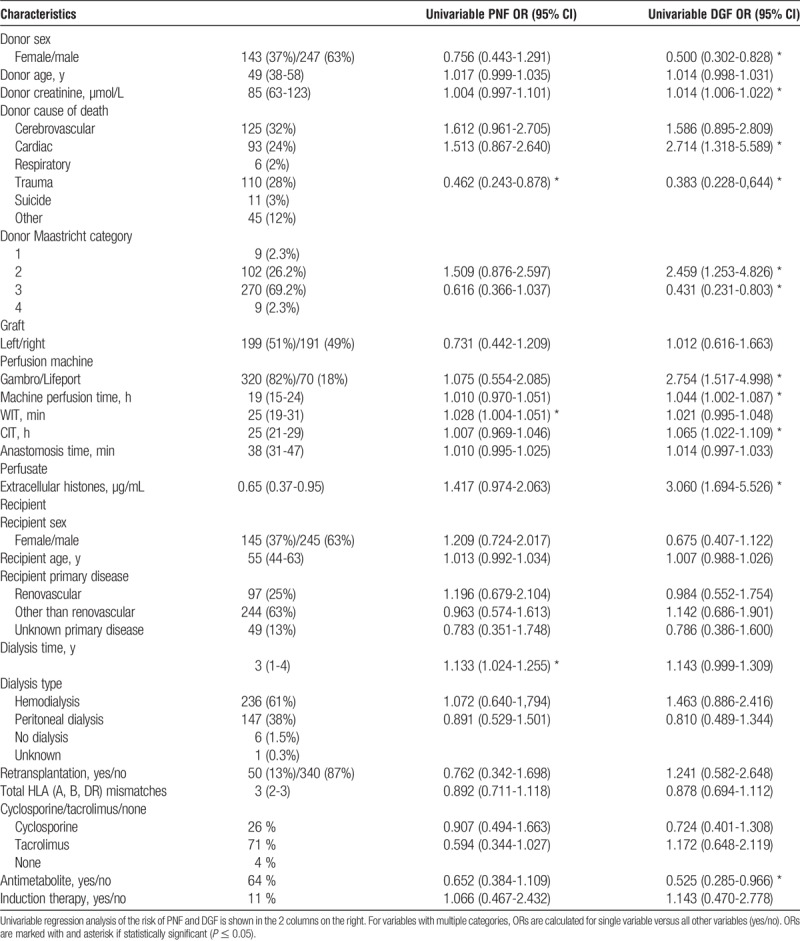
Donor data were routinely registered and analyzed for donor sex, age (years), most recent serum creatinine (μmol/L), cause of death (cerebrovascular, cardiac, respiratory, trauma, suicide, other), and DCD Maastricht category (1, 2, 3, or 4).
The following graft characteristics were recorded: left or right kidney, type of machine perfusion (Gambro (Lund, Sweden) or Lifeport (Lifeport Kidney Transporter, model no. LKT-100-P; Organ Recovery Systems, Des Plaines, IL), duration of machine perfusion time, warm ischemia time (WIT) (defined as time of death, as witnessed by an absent circulation, until time of cold perfusion), cold ischemia time (CIT) (defined as the period between start of cold perfusion until start of first anastomosis), and anastomosis time.
Recipient characteristics and follow-up data were recorded and retrieved: recipient sex, age (years), primary kidney disease (renovascular or other), dialysis type (no, hemo dialysis or peritoneal dialysis), years of dialysis, number of previous transplants, total number of HLA mismatches (including HLA-A, HLA-B, and HLA-DR; scoring 0-6), and immunosuppressive regimen. The immunosuppressive regimen changed during the study period, but mainly included use of a calcineurin inhibitor (cyclosporine or tacrolimus) and prednisolone. This was combined with sirolimus, mycophenolate mofetil, or azathioprine, depending on the protocol at the time. Dacluzimab was given to patients with increased immunologic risk if needed.
The collection and storage of patient data was performed in agreement with the “Code of Conduct for Health Research” by the Dutch Federation of Biomedical Scientific Societies (http://www.federa.org/).
Kidney Procurement, Preservation, and Machine Perfusion
All kidneys were procured from DCD donors who included all types of DCD donors according to the Maastricht criteria (DCD 1-4).24 Perfusion was either performed by using a double-balloon triple-lumen (DBTL) catheter or by direct cannulation of the aorta, as previously described.25 After nephrectomy, kidneys were placed on pulsatile perfusion machines. Before May 2007, the Gambro PF-3B perfusion machines (Gambro) were used with 500-mL UW-MPS (Belzer MPS; Trans-Med, Elk River, MN) as perfusion solution. This machine was set with a systolic pressure of 55 mm Hg for the first hour, after which flow was kept constant. As of June 2007, kidneys were placed on the Lifeport Kidney Transporter (model no. LKT-100-P; Organ Recovery Systems) with 1 L of Kidney Perfusion Solution 1 (Organ Recovery Systems) and perfused at 30 to 40 mm Hg pressure. All kidneys were perfused at a mean temperature of 4°C and pH was adjusted when lower than 7.10. Perfusion characteristics, that is, temperature, pressure, flow, renal resistance were recorded during perfusion and any abnormalities were registered.
Sample Collection and Histone H3 Analysis
During machine perfusion, perfusate samples were taken at 1, 2, and 4 hours after start of perfusion, centrifuged, cooled, and stored at −80°C. To ensure a balanced state of perfusion, the samples of 4 hours of perfusion were used for biochemical analysis.
Histone H3 levels were determined semiquantitatively as previously described.18 In brief, kidney perfusate samples were centrifuged for 4 minutes at 3000g, subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene diflouride membranes (Bio-Rad Laboratories, Hemel Hempstead, UK) by semidry blotting. After blocking for 1 hour in triethanolamine-buffered saline with Tween + 5% (w/v) nonfat dry milk (Bio-Rad Laboratories), membranes were incubated with a rabbit polyclonal H3 antibody overnight at 4°C (1:1000, sc-8654-R; Santa Cruz Biotechnology, Heidelberg, Germany), followed by incubation with a biotin-conjugated goat antirabbit IgG antibody for 30 minutes at room temperature (1:1000, Vector Laboratories Burlingame, CA) and incubation with StreptABComplex/AP solution (1:3000, Dako Cytonation, Glostrup, Denmark) for 30 minutes at room temperature. Protein bands were detected by BCIP/NBT (Sigma-Aldrich, St. Louis, MO) and band densities were quantified by ImageQuant TL software (GE Healthcare, Little Chalfont, UK). Purified H3 (Roche, Basel, Switzerland) served as a reference to calculate unknown H3 concentrations (μg/mL) in the perfusate by comparing the densities of all H3 specific bands in these samples with the density of known concentrations of purified H3.
Study Outcome Parameters
Immediate graft function (IGF), DGF, and PNF were scored as outcome measures for short-term graft function. DGF was scored if dialysis was needed in the first week after transplantation. PNF was scored if the graft never showed adequate function and dialysis was needed all through the follow-up period of the patient. Serum creatinine levels were analyzed for all recipients at 3 and 12 months and up to 5 years each year after transplantation if possible.
Data Analysis
Numerical variables are presented as mean ± standard deviations if approximately normally distributed, and as median (interquartile range [IQR]) otherwise. Categorical variables are presented as number (percentage). Analyses were performed on all perfusates collected, irrespective of any preanalytical variable. A P value of 0.05 or less was considered to represent statistical significance. To allow comparison of determined concentrations between the Gambro and Lifeport perfusion systems, the relative twofold concentration in the former was taken into account and corrected for.
Comparison of histone H3 concentrations between groups based on graft function (PNF vs DGF vs IGF) or based on type of donor (uncontrolled vs controlled) was performed using the Mann-Whitney test. Univariable and multivariable binary logistic regression analyses were used for assessment of the risk of PNF (vs DGF or IGF) or of DGF (vs IGF), where the effects are presented as odds ratio (OR); 95% confidence interval (CI). Multivariable analysis included clinically important risk factors (ie, WIT, CIT, anastomosis time, donor age, and type of donor (uncontrolled vs controlled), the latter was excluded in subgroup analysis for uncontrolled and controlled DCD kidneys). The discriminative capacity of histone H3 concentrations on graft function was assessed by presenting our data as receiver operating curves from which the area under the receiver operating curve (AURC) (95% CI) was calculated. For multivariable predictive analysis, predictive probabilities were calculated with and without Histone H3 concentrations and the added value of extracellular histones to the model was assessed. Correlations between histone H3 concentrations and WIT, donor creatinine, donor age and posttransplant serum creatinine were tested with a t-test for Spearman’s rho (rs). Categorical comparison between groups (1- and 5-year graft survival, histone H3 concentrations below or above the median) was performed using Pearson χ2 test. Graft survival was censored for death for all patients who died with a functioning graft. A Kaplan-Meier analysis was used to analyze graft survival, and differences between curves were assessed using log-rank tests. A Cox proportional hazards model was applied to calculate the hazard ratio (HR) of histone H3 concentrations at 1- and 5-year graft failure. The multivariable Cox proportional hazard model included aforementioned clinical factors.
RESULTS
Patients
From January 1997 until March 2013, 439 transplanted DCD kidneys were machine perfused by our center. After careful assessment of machine perfusion records, 10 kidneys were excluded from analysis because of significant leakage, obstruction or documented malperfusion. Next, 10 kidneys were transplanted outside of the Eurotransplant region, and for 6 kidney transplantations, follow-up data could not be obtained; of another 6, machine perfusion records were missing or incomplete. For 17 kidneys, machine perfusate was not available, because samples were used in previous studies, or sample collection was wrongly performed at the time. A remaining total of 390 perfusate samples were included.
Donor, graft, and patient characteristics are shown in Table 1. Of the 390 kidneys entering analysis, a majority of 270 (69%) were procured from DCD-3 donors. Median donor age was 49 (IQR, 38-58) years. After death, the median WIT was 25 (IQR, 19-31) minutes, median CIT was 25 (IQR, 21-29) hours and median anastomosis time 38 (IQR, 31-47) minutes.
TABLE 1.
Donor, graft, and recipient characteristics are shown as median (IQR) if the variable is numerical and as number (percentage) if categorical
Graft Function
A majority of transplantations resulted in DGF: 228 of the analyzed 390 (58.5%). Kidneys developed PNF in 76 (19.5%) cases and IGF in 86 (22.1%) cases after transplantation. Median overall machine perfusate extracellular histone concentration was 0.65 (IQR, 0.37-0.95) μg/mL. Extracellular histone concentration (in μg/mL) was significantly higher in kidneys which developed PNF (median, 0.73; IQR, 0.44-1.00; P < 0.001) and DGF (median, 0.70; IQR, 0.43-0.98; P < 0.001) compared with IGF (median, 0.42; IQR, 0.07-0.78) (Figure 2). The difference between the histone H3 concentration in the PNF, as compared with the DGF group, did not reach significance (P = 0.437).
FIGURE 2.

Histone concentration are significantly higher in PNF (median, 0.73; IQR, 0.44-1.00 μg/mL; P < 0.001) and DGF (median, 0.70; IQR, 0.43–0.98 μg/mL; P < 0.001) groups compared with IGF (median, 0.42; IQR, 0.07-0.78 μg/mL). Boxplots are presented as median and quartiles, and whiskers include full range of values.
The risk of PNF was not significantly increased by elevation of histone H3 levels as assessed by univariable and multivariable regression analyses (OR, 1.417; 95% CI, 0.974-2.063; OR, 1.342; 95% CI, 0.900-2.002, respectively). Furthermore, the discriminative power of histone H3 concentrations on development of PNF was weak in univariable analysis (AURC, 0.573; 95% CI, 0.502-0.645). This significance increased however after correction of the data for confounding clinical factors in the multivariable model (AURC, 0.647; 95% CI, 0.577-0.717), with a minor added value of extracellular histones to the predictive model (AURC without histones, 0.640; 95% CI, 0.571-0.708) (Figure 3).
FIGURE 3.
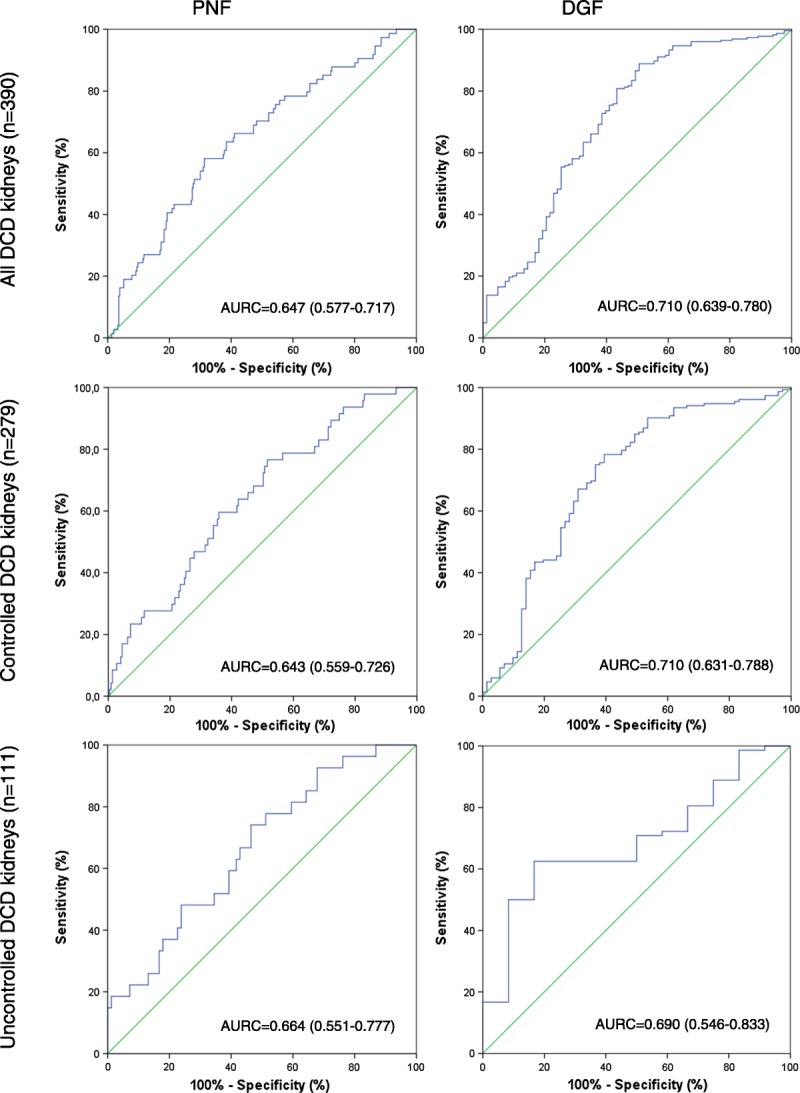
The predictive value of extracellular histones on PNF and DGF, for all DCD kidneys and after subgroup analysis for controlled and uncontrolled DCD kidneys. Receiving operator curves are shown and AURC (95% CI) calculated by multivariable analysis, including clinically important risk factors.
We observed that, for development of DGF, histone H3 concentration was an independent risk factor in both univariable (OR, 3.060; 95% CI, 1.694-5.526) and multivariable analysis (OR, 2.152; 95% CI, 1.199-3.863). However, the predictive value of extracellular histone concentrations in perfusates on DGF was poor (AURC, 0.674; 95% CI, 0.604-0.743), but increased to fair after correction for confounding clinical factors (AURC, 0.710; 95% CI, 0.639-0.780), in which histone H3 concentration added minor value to the predictive model (AURC without histones, 0.689; 95% CI, 0.621-0.757) (Figure 3).
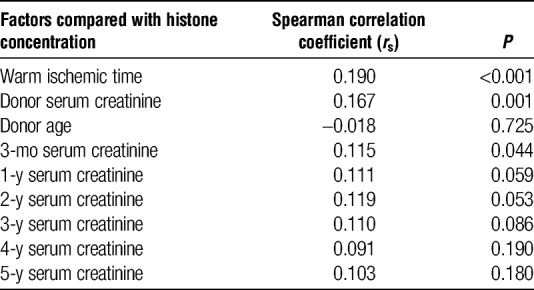
Extracellular histone concentrations correlated with serum creatinine at 3 months after kidney transplantation (rs = 0.115, P = 0.044). Thereafter, at 1, 2, 3, 4, and 5 years follow-up, this correlation was no longer significant (rs = 0.111, P = 0.059; 0.119, P = 0.053; 0.110, P = 0.086; 0.091, P = 0.190; 0.103, P = 0.180, respectively) (Table 2).
TABLE 2.
Correlation of histone concentration to donor, graft and outcome factors
Graft Survival
Median follow-up was 6.1 (IQR, 3.8-8.7) years. Overall, 1 year death-censored graft survival was 77%, and at 5 years, this was 71%. Figure 4 shows Kaplan-Meier curves with a significantly better survival of kidneys with histone H3 concentrations below the median of 0.65 μg/mL than those that have levels of extracellular histones that are above the median (log-rank, P = 0.026). Analysis excluding kidneys with PNF did not result in a significant difference (log-rank, P = 0.443). One year graft survival was significantly better for the group with a histone concentration below the median concentration than above (graft survival, 83% vs 71%; P = 0.008, respectively). This difference persisted up to 5 years (graft survival, 76% vs 65%; P = 0.014, respectively).
FIGURE 4.
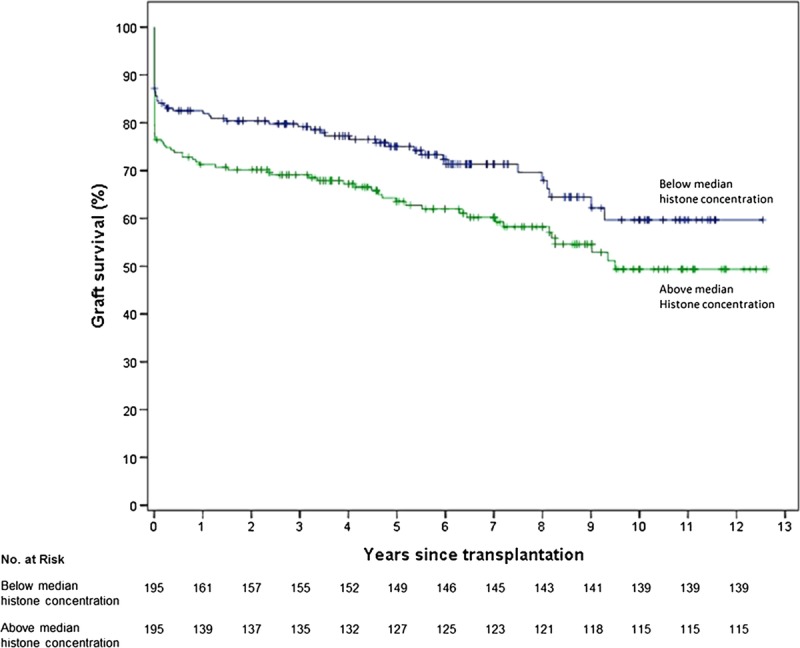
Kaplan-Meier graph of graft survival after transplantation. After 1 year, the group with histone H3 concentrations below the median had a 12% higher graft survival compared to group with histones H3 concentrations above the median (P = 0.008). Data were censored at the time of death for patients who died with a functioning graft.
The risk of graft failure within 1 year significantly increased with increasing histone concentration (HR, 1.411; 95% CI, 1.071-1.858) in a univariable Cox regression model. Multivariable analysis also resulted in a significantly increase (HR, 1.386; 95% CI, 1.037-1.853). The risk of 5-year graft failure was significantly increased with increasing histone concentration in the univariable analysis (HR, 1.335; 95% CI, 1.026-1.738), but not in the multivariable analysis (HR, 1.270; 95% CI, 0.960-1.680). Predictive value on graft failure within 1 and 5 years was weak after univariable analyses (AURC, 0.547; 95% CI, 0.479-0.614; AURC, 0.537; 95% CI, 0.477-0.597, respectively). Although the predictive value increased after correction for confounding clinical factors (AURC, 0.637; 95% CI, 0.573-0.702; AURC, 0.648; 95% CI, 0.590-0.707, respectively), there was no added value of extracellular histones to the model for 1- and 5-year graft failure (AURC without histones, 0.641; 95% CI, 0.577-0.703; AURC without histones, 0.649; 95% CI, 0.591-0.707, respectively.)
Factors Associated With Increased Extracellular Histone Concentrations
Extracellular histone concentrations weakly correlated to WIT (rs = 0.190, P < 0.001) and donor creatinine (rs = 0.167, P < 0.001). Histone concentrations did not correlate with donor age. The median histone H3 concentration in uncontrolled donors was significantly higher than that in controlled donors (median, 0.73; IQR, 0.52-1.1 μg/mL; n = 111 vs median, 0.59; IQR, 0.28-0.92 μg/mL; n = 279; P < 0.001, respectively) (Table 2).
Controlled Versus Uncontrolled DCD
Because there is a difference in histone concentration between uncontrolled (Maastricht categories 1 and 2) and controlled (Maastricht categories 3 and 4) DCD donors, we performed subgroup analyses.
Perfusates from uncontrolled DCD donor kidneys (n = 111) showed no significant difference in histone H3 concentrations between the groups with PNF, DGF, or IGF (PNF vs DGF, P = 0.884; PNF vs IGF, P = 0.988; DGF vs IGF, P = 0.929). Furthermore, histone H3 concentration was not associated with an increased risk of graft dysfunction (PNF or DGF). Only in multivariable predictive analyses, histone H3 concentration was predictive for PNF (AURC, 0.664; 95% CI, 0.551-0.777) and DGF (AURC, 0.690; 95% CI, 0.546-0.833) (Figure 3).
Controlled DCD perfusates (n = 279) contained a significantly higher median (IQR) concentration of histone H3 in kidney with PNF (0.74 (0.42-0.96) μg/mL, P < 0.001) and DGF (0.63 (0.39-0.97) μg/mL, P < 0.001) compared to IGF perfusates (0.32 (0.02-0.67) μg/mL). For controlled DCD, we found that histone H3 concentration is an independent risk factor for DGF (univariable OR, 3.071; 95% CI, 1.588-5.941; multivariable OR, 2.433; 95% CI, 1.253-4.723); for PNF, however, such significance could not be shown. Furthermore, the discriminative power for development of PNF and DGF of extracellular histone levels in this subgroup is comparable to the entire group of perfusates (PNF univariable AURC, 0.597; 95% CI, 0.512-0.683; PNF multivariable AURC, 0.643; 95% CI, 0.559-0.726; DGF univariable AURC, 0.683; 95% CI, 0.607-0.759; and DGF multivariable AURC, 0.710; 95% CI, 0.631-0.788) (Figure 3).
One-year death-censored graft survival was 78%, and at 5 years, this was 72%. Comparing the groups with histone H3 concentrations above and below the median, Kaplan-Meier analysis showed a significant (log rank, P = 0.012) difference in graft survival in favor of the lower concentrations. Notably, 1- and 5-year graft survival were significantly better in the group with lower concentrations (at 1 year, 84% vs 72% [P = 0.019] and at 5 years 79% vs 66% [P = 0.015]).
DISCUSSION
Many studies have sought to identify possible relations between measurable biomolecules and organ injury, posttransplant graft function or survival. It has been shown that kidneys release a plethora of biomolecules over time during machine perfusion. The release of these molecules appears to reflect the pathophysiologic processes that occur during organ donation.
Interest in extracellular histones has grown rapidly during the last decade. Studies have demonstrated a crucial role of extracellular histones multiple pathophysiologic processes like sepsis, inflammation, vascular dysfunction, and thrombosis.15,16,18,22,26–28 It has been shown that extracellular histones are in fact cytotoxic molecules that can kill various cell types, including endothelial cells, and contribute to organ failure.15,18,20,22,28–30 Hence, histones once released into the circulation may initiate an amplifying cascade of cell death and histone release culminating in microcirculatory damage and consequently organ failure. Extracellular histones have not yet been studied in the pathophysiologic process of kidney injury and organ viability in a clinical setting of organ donation and transplantation. In this study, we addressed the relevance of measuring extracellular histones in the machine perfusate of hypothermic machine perfused DCD kidneys before transplantation.
The incidence of PNF in this group of DCD kidneys is high. Donor kidneys in this group were procured in the period that the waitlists exceeded 5 years for kidney transplantation and we were able to extend the donor pool threefold by transplanting marginal donor kidneys. These kidneys were further characterized by a very long CIT due to allocation issues and were mainly transplanted in fragile recipients in whom a prolonged waiting time for transplantation was considered not desirable. Despite the relatively good graft survival of functioning grafts and although transplantation of marginal kidneys with a high incidence of PNF may provide a survival benefit to recipients who alternatively would wait for a standard criteria donor kidneys, we considered this percentage as too high and adapted our acceptance criteria for marginal donor kidneys.31
Essentially, every nucleated cell contains core histones, which include the histones H2A, H2B, H3, and H4 in an equimolar ratio (Figure 1). Considering the equimolar presence of the cytotoxic histones H2B, H3, and H4, measurement of histone H3 is considered a proxy-measurement of H2B and H4 as well. Recently, we were able to show a correlation between disease severity and mortality in sepsis and extracellular histone H3 levels in patients in the intensive care unit.26 Therefore, we decided to measure the levels of histone H3 in our collection of machine perfusates.
To verify that differences in perfusion protocols and time of storage did not influence extracellular histone H3 measurements, we compared mean histone H3 concentrations for IGF, DGF, and PNF between the different perfusion protocols used here, and analyzed a possible influence of storage time. We neither observed differences between perfusion protocols nor did we observe an influence of storage time. This notion is supported by several studies that show that long-term storage only results in minor decreases of nucleosome concentrations and repeated freeze-thaw cycles do not affect measured concentrations of nucleosomal DNA fragments.32–34 We also verified the latter observation by measuring histones repeatedly in our set-up (mean average coefficient of variation, 7.24 ± 0.08% for 3 independent experiments; data not shown).
The average extracellular histones concentration is strikingly higher in kidneys which showed posttransplant dysfunction. The apparent prognostic benefit that correlates with lower concentrations of extracellular histones in perfusate was especially seen in graft survival analysis, where we found a 12% higher one-year survival for the lower concentration half (Figure 4). The survival of functioning grafts appears to be equal. Moreover, in contrast to three recent studies,12,35,36 which did not find a significant association of any molecule measured in machine perfusate to 1-year graft failure, extracellular histones in this study were associated with an increased risk of 1 year graft failure. Also, we found that extracellular histones are an independent predictor of DGF when adjusted for relevant confounding factors. Subsequent subgroups analyses yielded similar results for controlled DCD kidney transplantation. Analyses on uncontrolled DCD only showed a significant association to graft function in the multivariable prediction model for DGF and PNF, likely due to a small group size of uncontrolled DCD and the lower amount of IGF (n = 12) in this group. We have shown that extracellular histone concentration is associated with graft function and graft survival, however its value to predict the outcome after transplantation is very limited and equivalent to the predictive value of most other machine perfusate biomolecules.12–14,35,36 Therefore, the currently available data on perfusate histones concentration does not aid in the decision to either accept or refuse a kidney for transplantation.
Here, we have shown for the first time that extracellular histones are present in the perfusate of hypothermically machine perfused DCD kidneys. We observe that extracellular histones are likely to represent an independent risk factor for DGF and 1-year graft failure and that there is an association to PNF. DCD machine perfused kidneys with low histone concentrations have a significantly better survival after transplantation. Future studies are necessary to assess if graft survival can be improved if the extracellular histones concentration is reduced either by better organ preservation or by therapies that may filter or neutralise these potentially cytotoxic agents.
ACKNOWLEDGMENTS
The authors thank all recipient centers for providing follow-up data.
Footnotes
This work was supported by the Cardiovascular Research Institute Maastricht (to G.A.F.N.) and the Hemker foundation (to G.A.F.N. and C.P.R.)
T.C.v.S. and D.M.H.B. contributed equally.
T.C.v.S. participated in the design of the study, sample and data collection and analysis and writing of the manuscript. D.M.H.B. participated in the design of the study, sample and data collection, and analysis and writing of the article. E.R.P.H. participated in the design of the study, sample and data collection, and analysis and writing of the article. B.W. participated in data analysis and writing of the article. M.H.L.C. participated in data collection and writing of the article. C.P.R. participated in design of the study and writing of the article. L.W.E.v.H. participated in the design of the study, sample and data collection and writing of the article. G.A.F.N. Participated in the design of the study, sample and data collection and writing of the article.
Correspondence: Tim C. van Smaalen, MD, MSc, Department of Surgery, Maastricht University Medical Centre, PO Box 5800, 6202 AZ Maastricht, The Netherlands. (vansmaalen@gmail.com).
The authors investigate the association between the histone concentration from machine perfusates and graft function in deceased donor kidney transplantation. The cytotoxic extracellular histones may contribute to graft dysfunction and poor survival.
REFERENCES
- 1.Jochmans I, O’Callaghan JM, Pirenne J. Hypothermic machine perfusion of kidneys retrieved from standard and high-risk donors Transpl Int 2015. 28665–676 [DOI] [PubMed] [Google Scholar]
- 2.O’Callaghan JM, Morgan RD, Knight SR. Systematic review and meta-analysis of hypothermic machine perfusion versus static cold storage of kidney allografts on transplant outcomes Br J Surg 2013. 100991–1001 [DOI] [PubMed] [Google Scholar]
- 3.De Deken J, Kocabayoglu P, Moers C. Hypothermic machine perfusion in kidney transplantation Curr Opin Organ Transplant 2016. 21294–300 [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Fu Z, Zhong Z. Hypothermic machine perfusion decreases renal cell apoptosis during ischemia/reperfusion injury via the ezrin/AKT pathway Artif Organs 2016. 40129–135 [DOI] [PubMed] [Google Scholar]
- 5.Yuan X, Theruvath AJ, Ge X. Machine perfusion or cold storage in organ transplantation: indication, mechanisms, and future perspectives Transpl Int 2010. 23561–570 [DOI] [PubMed] [Google Scholar]
- 6.Paredes-Zapata D, Ruiz-Arranz A, Rodriguez-Villar C. Does the pulsatile preservation machine have any impact in the discard rate of kidneys from older donors after brain death? Transplant Proc 2015. 472324–2327 [DOI] [PubMed] [Google Scholar]
- 7.Moers C, Pirenne J, Paul A. Machine perfusion or cold storage in deceased-donor kidney transplantation N Engl J Med 2012. 366770–771 [DOI] [PubMed] [Google Scholar]
- 8.Moers C, Smits JM, Maathuis MH. Machine perfusion or cold storage in deceased-donor kidney transplantation N Engl J Med 2009. 3607–19 [DOI] [PubMed] [Google Scholar]
- 9.Jochmans I, Moers C, Smits JM. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial Ann Surg 2010. 252756–764 [DOI] [PubMed] [Google Scholar]
- 10.Treckmann J, Moers C, Smits JM. Machine perfusion versus cold storage for preservation of kidneys from expanded criteria donors after brain death Transpl Int 2011. 24548–554 [DOI] [PubMed] [Google Scholar]
- 11.Dare AJ, Pettigrew GJ, Saeb-Parsy K. Preoperative assessment of the deceased-donor kidney: from macroscopic appearance to molecular biomarkers Transplantation 2014. 97797–807 [DOI] [PubMed] [Google Scholar]
- 12.Parikh CR, Hall IE, Bhangoo RS. Associations of perfusate biomarkers and pump parameters with delayed graft function and deceased donor kidney allograft function Am J Transplant 2016. 161526–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Smaalen TC, Hoogland ER, van Heurn LW. Machine perfusion viability testing Curr Opin Organ Transplant 2013. 18168–173 [DOI] [PubMed] [Google Scholar]
- 14.Bhangoo RS, Hall IE, Reese PP. Deceased-donor kidney perfusate and urine biomarkers for kidney allograft outcomes: a systematic review Nephrol Dial Transplant 2012. 273305–3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen R, Kang R, Fan XG. Release and activity of histone in diseases. Cell Death Dis. 2014;5:e1370. doi: 10.1038/cddis.2014.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mas VR, Le TH, Maluf DG. Epigenetics in kidney transplantation: current evidence, predictions, and future research directions Transplantation 2016. 10023–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, Zhang X, Monestier M. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury J Immunol 2011. 1872626–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J, Zhang X, Pelayo R. Extracellular histones are major mediators of death in sepsis Nat Med 2009. 151318–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen Z, Liu Y, Li F. Circulating histones exacerbate inflammation in mice with acute liver failure J Cell Biochem 2013. 1142384–2391 [DOI] [PubMed] [Google Scholar]
- 20.Allam R, Scherbaum CR, Darisipudi MN. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4 J Am Soc Nephrol 2012. 231375–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allam R, Darisipudi MN, Tschopp J. Histones trigger sterile inflammation by activating the NLRP3 inflammasome Eur J Immunol 2013. 433336–3342 [DOI] [PubMed] [Google Scholar]
- 22.Mena HA, Carestia A, Scotti L. Extracellular histones reduce survival and angiogenic responses of late outgrowth progenitor and mature endothelial cells J Thromb Haemost 2016. 14397–410 [DOI] [PubMed] [Google Scholar]
- 23.Mulay SR, Kumar SV, Lech M. How kidney cell death induces renal necroinflammation Semin Nephrol 2016. 36162–173 [DOI] [PubMed] [Google Scholar]
- 24.Kootstra G, Daemen JH, Oomen AP. Categories of non-heart-beating donors Transplant Proc 1995. 272893–2894 [PubMed] [Google Scholar]
- 25.Wind J, Hoogland ER, van Heurn LW. Preservation techniques for donors after cardiac death kidneys Curr Opin Organ Transplant 2011. 16157–161 [DOI] [PubMed] [Google Scholar]
- 26.Wildhagen KC, Wiewel MA, Schultz MJ. Extracellular histone H3 levels are inversely correlated with antithrombin levels and platelet counts and are associated with mortality in sepsis patients Thromb Res 2015. 136542–547 [DOI] [PubMed] [Google Scholar]
- 27.Kessenbrock K, Krumbholz M, Schonermarck U. Netting neutrophils in autoimmune small-vessel vasculitis Nat Med 2009. 15623–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allam R, Kumar SV, Darisipudi MN. Extracellular histones in tissue injury and inflammation J Mol Med (Berl 2014. 92465–472 [DOI] [PubMed] [Google Scholar]
- 29.Chaput C, Zychlinsky A. Sepsis: the dark side of histones Nat Med 2009. 151245–1246 [DOI] [PubMed] [Google Scholar]
- 30.Wildhagen KC, García de Frutos P, Reutelingsperger CP. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis Blood 2014. 1231098–1101 [DOI] [PubMed] [Google Scholar]
- 31.Snoeijs MG, Schaubel DE, Hene R. Kidneys from donors after cardiac death provide survival benefit J Am Soc Nephrol 2010. 211015–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holdenrieder S, Von Pawel J, Nagel D. Long-term stability of circulating nucleosomes in serum Anticancer Res 2010. 301613–1615 [PubMed] [Google Scholar]
- 33.Holdenrieder S, Mueller S, Stieber P. Stability of nucleosomal DNA fragments in serum Clin Chem 2005. 511026–1029 [DOI] [PubMed] [Google Scholar]
- 34.Holdenrieder S, Stieber P, Bodenmuller H. Nucleosomes in serum as a marker for cell death Clin Chem Lab Med 2001. 39596–605 [DOI] [PubMed] [Google Scholar]
- 35.Moers C, Varnav OC, van Heurn E. The value of machine perfusion perfusate biomarkers for predicting kidney transplant outcome Transplantation 2010. 90966–973 [DOI] [PubMed] [Google Scholar]
- 36.Hoogland ER, de Vries EE, Christiaans MH. The value of machine perfusion biomarker concentration in DCD kidney transplantations Transplantation 2013. 95603–610 [DOI] [PubMed] [Google Scholar]


