Abstract
Background
A number of corticosteroid minimization and avoidance protocols for post–solid organ transplant have been developed. The study objective was to examine the effect of corticosteroid withdrawal/avoidance on growth and safety parameters in pediatric solid organ transplant recipients.
Methods
A systematic review using Medline and Embase was performed. All randomized controlled trials (RCT) and observational studies comparing corticosteroid withdrawal/avoidance to controls receiving corticosteroids in pediatric transplant recipients which reported growth as change in height or final height were included. Two reviewers independently abstracted study data and assessed quality.
Results
The search yielded 930 records, 14 separate studies involving 1146 patients. Renal RCTs (n = 5) showed that corticosteroid withdrawal/avoidance was associated with a significant increase in growth (mean difference in height standard deviation score [SDS], 0.18; 95% confidence interval [95% CI], 0.07-0.29; P = 0.001) compared with those remaining on steroids. In liver RCTs (n = 2), mean difference in height SDS was −0.20 (95% CI, −1.08 to 0.68; P = 0.66). Results for renal observational studies (n = 5) was 0.34 (95% CI, 0.03-0.65; P = 0.03). The most pronounced effect was seen in prepubertal children with SDS of 0.28 (95% CI, 0.14-0.41; P < 0.0001). In pubertal participants this was not observed (SDS, 0.06; 95% CI, −0.04 to 0.15; P = 0.24). Corticosteroid withdrawal/avoidance was not associated with acute rejection (odds ratio [OR], 0.87; P = 0.63), graft failure (OR, 0.45; P = 0.08), or death (OR, 0.34; P = 0.16) in renal trials.
Conclusions
Corticosteroid withdrawal/avoidance in pediatric renal transplantation is associated with a significant improvement in height. Prepubertal patients appeared to have the greatest benefit. Importantly, the improvement in growth was not accompanied by increased rejection or worsening patient/allograft survival in the short term.
Solid organ transplantation is the only therapy for children with end-stage organ failure. As immunosuppressive therapies have evolved, patient and transplant allograft survival have also improved substantially in the pediatric population.1,2 However, much of the evidence and guidelines for posttransplant care stems from adult studies which are not always appropriate for the pediatric population as children often have very different needs and comorbidities compared with adults.3,4 For example, the goal of optimizing growth posttransplant is unique to young children and may represent an often overlooked patient-centered outcome. Children with end-stage renal disease who have moderate to severe growth failure have an increased risk of morbidity and mortality5 as well as reduced self-esteem.6
Multiple factors affect recovery of growth after transplantation including the type of solid organ transplanted and age.7 Kidney recipients between 1 and 6 years and liver transplant recipients less than 2 experience significant catch-up growth compared with other ages.8,9 Transplant function is important especially in renal transplantation with those displaying reduced function and those requiring a new organ having reduced growth velocity after transplantation. Height deficit at the time of transplantation also impacts potential growth recovery7 and pubertal status, with prepubertal children having greater gains in height.10 Lastly, but perhaps most importantly because it is modifiable is the use of corticosteroids. Corticosteroids form the backbone of most immunosuppressive regimens however they have many potential adverse effects.11 Children receiving excess glucocorticoids often have a reduced growth rate which appears to be mediated by suppression of growth hormone as well as a direct effect on the growth plate.12 Corticosteroid withdrawal and complete avoidance have been used posttransplantation in an effort to reduce potential steroid toxicity. The effect of these alternative regimens on growth posttransplantation has not been determined.
The objective of this systematic review was to identify and analyze the data on corticosteroid withdrawal and avoidance after pediatric solid organ transplantation specifically focusing on the outcome of growth. Secondary objectives were the safety of corticosteroid withdrawal/avoidance by examining the outcomes of acute rejection, graft function, graft and patient survival. We hypothesized that corticosteroid withdrawal/avoidance would be associated with greater growth without compromising graft function or survival compared with those who remain on steroid therapy.
METHODS
Search Strategy
We conducted systematic electronic searches using Ovid Medline (1946 to 2014 May 6) and Embase (1947 to 2014 May 6). Bibliographies from recent reviews and published studies were searched as well as abstracts from scientific meetings. There was no restriction based on language.
Study Selection
An initial screen of identified titles and abstracts was performed by 1 investigator (A.T.) with clearly irrelevant records removed at this stage. A second screen to identify potentially relevant studies was independently performed by 2 reviewers (A.M. and A.T.). If no abstract was available, the full text was obtained. Full-text versions of potentially eligible studies were obtained and independently screened by 2 reviewers (N.F. and A.T.) to determine their eligibility based on the selection criteria. Any disagreements during the screening process were resolved through discussion among the authors in accordance with the selection criteria. We included studies that met the following criteria: (1) randomized controlled trial (RCT), prospective nonrandomized interventional study, prospective or retrospective cohort or case control study involving solid organ transplant recipients; (2) participants were all 21 years or younger or study included adults but presented pediatric and adult data separately; (3) compared early (within 7 days posttransplant), intermediate (7 days to 1 year), or late (after 1 year post transplant) steroid withdrawal or complete steroid avoidance as defined by Grenda et al,13 to participants who were still on steroids (either daily or alternate day); (4) the study reported growth as either change in height (using a standard deviation score or absolute change) or final study height in both groups.
We excluded (1) any study that used historical controls because the immunosuppressive medications may be significantly different between periods, (2) cross-sectional studies and any other study that lacked a control arm, (3) animal studies, (4) abstracts without a full-text publication, and (5) studies before the year 1990 due to changes in immunosuppressive protocols.
Data Abstraction
Data were abstracted independently by 2 investigators (A.T. and N.F.) using a standardized data abstraction form. Any disagreement was resolved by a third investigator (G.K.). Our primary outcome was growth as assessed by either change in height standard deviation score (SDS) or change in cm/inches also known as growth velocity if height SDS was not provided. Height SDS can be used to compare a child’s height to a normal population standard typical for age, race, and sex. In children with chronic diseases, a negative height SDS can measure how much a child deviates from the average population. If the change was not calculated or provided, then final height reported (SDS) was used and compared between corticosteroid withdrawal/avoidance and the control group. Secondary outcomes included acute rejection, transplant failure, and death. For renal transplant recipients, estimated glomerular filtration rate (eGFR) or creatinine clearance was abstracted. We also collected information on the following characteristics: primary author, publication year, country, solid organ type, and language. For data related to the study participants, we abstracted: age, sex, race, pubertal status, bone age, deceased versus living organ, history of dialysis, baseline body mass index and whether or not participants received growth hormone therapy. Immunosuppressive medications used during the induction and maintenance phases were abstracted. When data was only available in figures, the GNU image manipulation program (GIMP 2.8; http://www.gimp.org/) was used to extract data. If individual studies had more than 1 published report we extracted all relevant data and combined it as 1 study but referenced all published reports.
Risk of Bias Assessment
Study quality was independently assessed by 2 reviewers (A.T. and N.F.). Randomized controlled trials were assessed using the Cochrane Collaboration Risk of Bias assessment tool14 which evaluates randomization, allocation concealment, blinding, and follow-up. The overall assessment was based on what was determined from the individual section. If 1 or more of the domains was rated as high risk of bias, the study was classified as being at high risk of bias. If all domains were classified as low risk, then the trial was considered low risk and if there was a mix of unclear and low risk it was classified as unclear. Observational studies were assessed using the Ottawa Newcastle scale15 which assesses selection, comparability and outcome of a study and uses a star point system. The maximum number of stars that can be assigned is 9. We categorized the studies based on the following cutoffs which have been previously used in a meta-analysis by Wallis et al16; more than 7 low risk of bias, scores of 5 to 7 indicated moderate risk of bias, and scores less than 5 indicated high risk of bias.
Statistical Analysis
Data were analyzed using Review Manager, version 5.2 (RevMan; The Cochrane Collaboration, Oxford, UK). Continuous data were expressed as mean difference with 95% confidence intervals (95% CIs) and dichotomous data were presented as an odds ratio with 95% CIs. For RCTs, mean differences were pooled using a random effects model.17 Heterogeneity was evaluated using the I2 index.18 Sensitivity analyses were performed to identify sources of heterogeneity based on the following subgroups (if data was available): organ type (eg, kidney transplant, liver transplant), prepubertal participants, late corticosteroid withdrawal separate from early withdrawal/avoidance, length of follow up, pubertal status, and methodological quality (low risk vs high risk of bias). Analyses for each organ type were kept separate. This study was conducted and prepared using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (www.prisma-statement.org). In the case where SEM was provided, we calculated the SD based on the following equation: SD = SE * √N. For most of the observational studies, the baseline and final height were provided however not the mean change. The mean change was therefore obtained by subtracting the final mean height SDS from the baseline. The SD of the change was obtained for each group by calculating a correlation coefficient as per the Cochrane handbook (http://handbook.cochrane.org).
RESULTS
Literature Search
The initial search identified 930 unique records (Figure 1) and 48 citations were initially eligible for full-text review. Upon review of the full publications, 33 were excluded. Thus, 15 full-text publications reporting on 14 separate studies (n = 1146 pediatric patients) met eligibility and were included.
FIGURE 1.
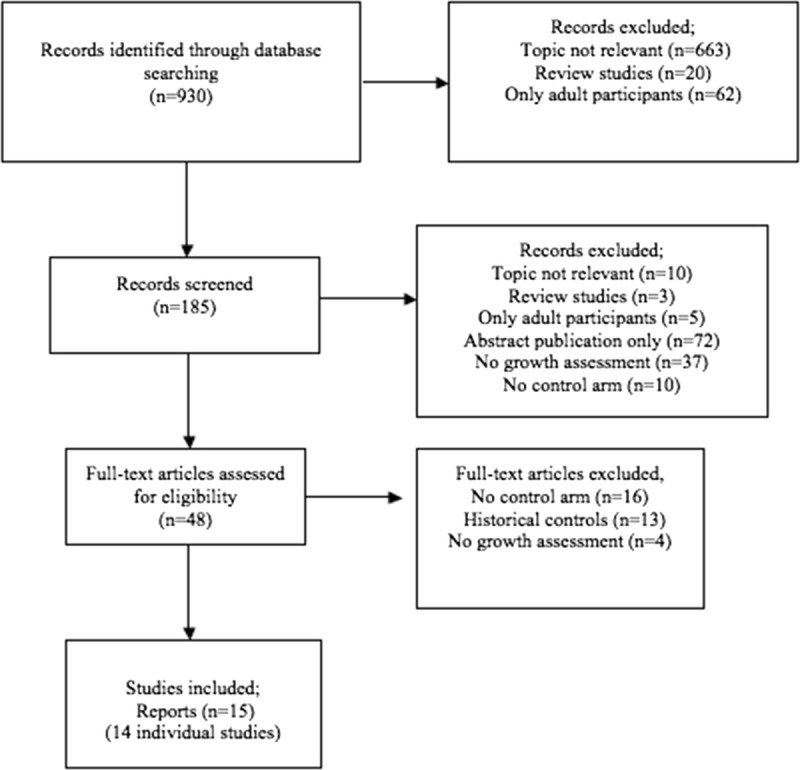
Flow diagram demonstrating electronic search results.
Description of Included Studies
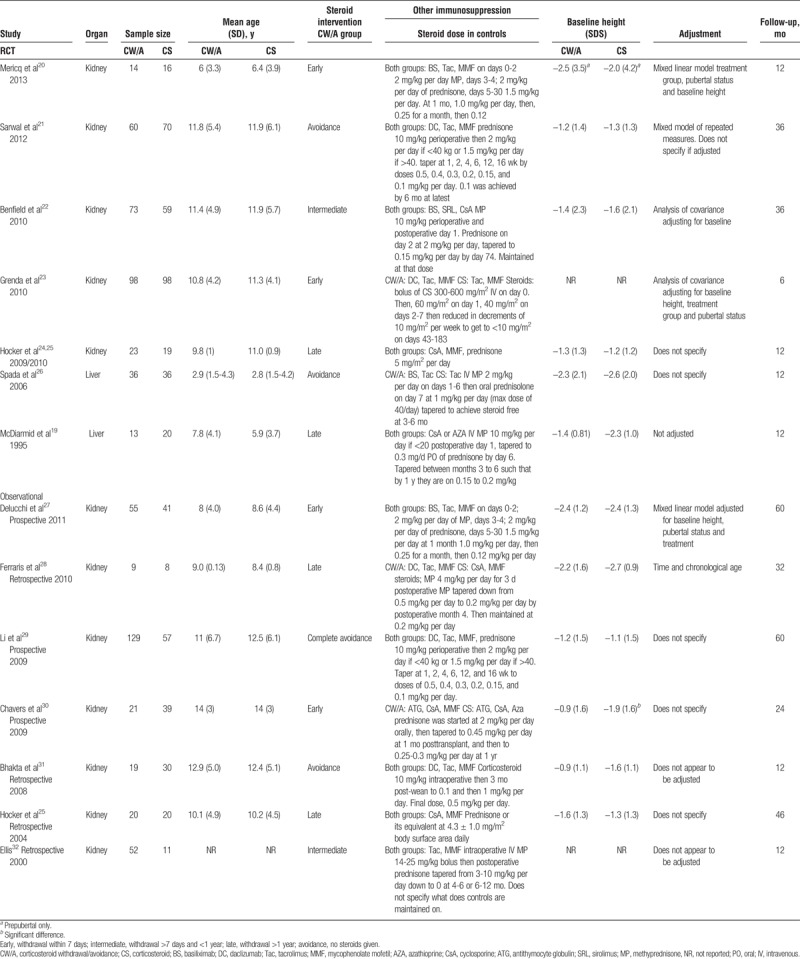
There were 7 RCTs (n = 635 patients) and 7 observational studies (n = 511 patients) included in the review. Five studies originated from the United States, 5 from Europe, 3 from South America, and 1 from both the US and Mexico. Twelve studies assessed growth in kidney transplant recipients and 2 in liver transplantation patients. There were no studies assessing growth in heart, lung, pancreas, or small bowel transplant recipients. All reports included pediatric patients only with the exception of a liver trial that was primarily adult based but included a subset of children (n = 33).19 Growth was evaluated as the primary outcome for 5 RCTs and as a secondary outcome in 1 study. Three studies excluded patients receiving growth hormone, whereas the rest of the studies made no mention of growth hormone. The timing of follow up growth assessment ranged from 6 months posttransplant to 5 years (Table 1).
TABLE 1.
Characteristics of included studies
Risk of Bias Assessment
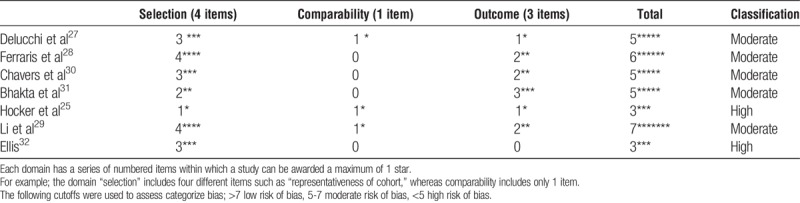
All RCTs had some degree of bias (Table 2) mainly due to a lack of blinding of participants and personnel (n = 4 trials), unclear blinding of the outcome assessment (n = 5 trials) or selective outcome reporting (n = 4 trials). Because all trials were classified overall as high risk of bias, sensitivity analyses could not be performed. All observational studies had bias, most of which was moderate. This was mainly in the comparability of the 2 groups. The baseline height was not always comparable between the groups, and the studies did not always adjust for these differences. In some studies, the steroid protocol was chosen by the physician, likely introducing selection bias (Table 3).
TABLE 2.
Risk of bias and methodological quality assessment of included RCTs
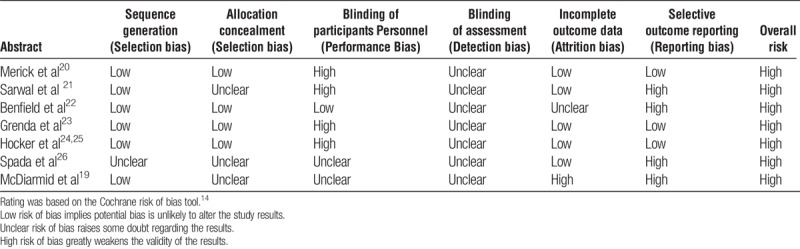
TABLE 3.
Risk of bias in observational studies
Growth in Corticosteroid Withdrawal/Avoidance Trials
When we compared growth among the 5 renal RCTs, the mean difference was 0.18 (95% CI, 0.07-0.29; P = 0.001; I2 = 37%, χ2 = 6.35, P = 0.1). When we compared growth among the 2 liver trials, the mean difference was −0.20 (95% CI, −1.08 to 0.68; P = 0.66; I2 = 76%; χ2 = 4.12, P = 0.04; Figure 2). When analyzed by corticosteroid regimen, renal RCTs using an early withdrawal/avoidance strategy20,21,23 (n = 3) had a pooled mean difference in height SDS of 0.16 (95% CI, −0.04 to 0.36; P = 0.13; I2 = 50%; χ2 = 4.03; P = 0.13) compared with those remaining on steroids and among the renal studies with intermediate/late protocols22,24 (n = 2), the mean difference in height SDS was 0.22 (95% CI, 0.09-0.36; P = 0.0008; I2 = 0; χ2 = 0.40; P = 0.52). Test for subgroup difference was not significant (χ2 = 0.15; P = 0.70; I2 = 0%). Length of follow-up differed among the 5 renal trials; 2 renal trials had follow up for 12 months and had a pooled mean difference in height SDS of 0.37 (95% CI, 0.12-0.63; P = 0.004; I2 = 7%; χ2 = 1.07; P = 0.3). Two renal trials had 36 months of follow-up with a pooled mean difference in height SDS of 0.15 (95% CI, −0.01 to 0.31; P = 0.07; I2 = 22%; χ2 = 1.29; P = 0.26). Test for subgroup difference was not significant (χ2 = 2.31; P = 0.13; I2 = 56.8%).
FIGURE 2.
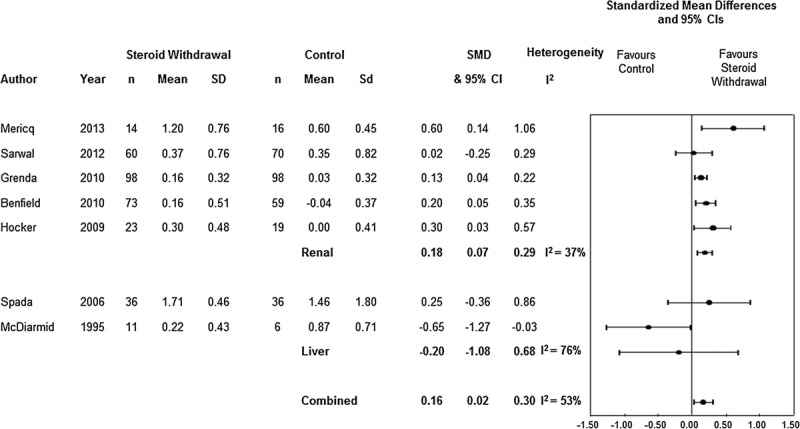
Standardized height results for pooled renal and liver randomized controlled trials.
In 1 observational study Li et al,29 the growth data were presented by age categories without group sample sizes and thus the data could not be pooled. Elli32 provided the mean change in height SDS without a measure of variance which precluded pooling. When the 5 remaining observational studies were pooled (n = 262 patients), corticosteroid withdrawal/avoidance was associated with a significant improvement in the end of study height SDS at 1 year 0.72 (95% CI, 0.37-1.07; P < 0.0001; I2 = 16%; χ2 = 4.73; P = 0.32; Figure 3A). Similarly, when change in height SDS was analyzed, corticosteroid withdrawal/avoidance was associated with a significant improvement 0.34 (95% CI, 0.03-0.65; P = 0.03; I2 = 75%; χ2 = 16.15; P = 0.003; Figure 3B).
FIGURE 3.
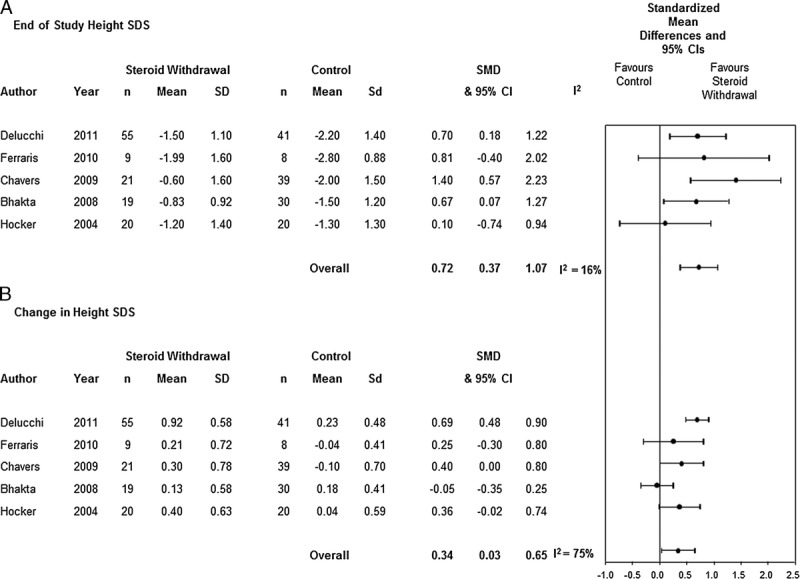
Standardized height results for pooled renal observational studies.
Growth During Puberty
Eight studies (n = 5 RCTs20,21,23,24,26; n = 3 observational25,27,28]; n = 339 patients) reported growth data in prepubertal children, defined either by Tanner stage or aged younger than 5 years. Five studies reported change in height SDS over 12 months (n = 4) or 6 months (n = 1), whereas 3 studies reported the 12-month final height SDS. Pooled data for the 4 renal RCTs demonstrated a mean difference in the change in height (SDS, 0.33; 95% CI, 0.13-0.53; P = 0.001; I2 = 21%; χ2 = 3.81; P = 0.28) for prepubertal patients in the corticosteroid withdrawal/avoidance group. Only 2 of these RCTs21,22 provided data for pubertal participants. Pooled data from these studies demonstrated that the change in height SDS was not significantly different between corticosteroid withdrawal/avoidance compared with controls (SDS, 0.05; 95% CI, −0.04 to 0.15; P = 0.24; I2 = 0; χ2 = 0.17; P = 0.68). Test for subgroup difference demonstrated was significantly different (χ2 = 5.94; P = 0.01; I2 = 83.2%). Pooled data for the 2 observational studies25,28 that provided 12-month final height showed that corticosteroid withdrawal/avoidance was associated with a significant improvement in height (SDS, 1.07; 95% CI, 0.11-2.04; P = 0.03; I2 = 0; χ2 = 0.01; P = 0.92). Final height for pubertal patients was only provided in the study by Hocker et al precluding any meta-analysis.
Li et al29 demonstrated that for both younger age categories (<6 years, 6 to 12 years) the growth velocity was significantly better in the steroid free groups. This finding was not consistent in those >12 years. Hocker et al27 reported height velocity in prepubertal patients was 9.1 ± 0.9 versus 5.7 ± 0.8 cm/year, respectively, in those who had stopped taking steroids compared with controls, whereas the height velocity in pubertal patients was 4.4 ± 0.9 versus 3.7 ± 0.9 cm/year for those who remained on steroids. In the observational study by Ferraris et al,28 height velocity in prepubertal children over 12 months after late steroid withdrawal was 5.85 (±3.06) cm compared with 3.23 (±1.17) cm in the corticosteroid maintenance group but the significance between groups was not reported. The study included only prepubertal patients and thus growth in pubertal patients was not evaluated.
Acute Rejection
All reported episodes of acute rejection were confirmed by biopsy with the exception of 1 episode in the RCT by Hocker et al in the steroid withdrawal group. Five renal RCTs reported acute rejection and 1 liver RCT.20–24,26 There were 30 independent episodes of acute rejection out of 305 participants in the corticosteroid withdrawal/avoidance group compared to 41 of 301 (1 patient had 2 rejection episodes) in the control group. Pooling of the renal data showed no significant effect of corticosteroid regimen on rejection (odds ratio [OR], 0.87; 95% CI, 0.50-1.52; P = 0.63; I2 = 0%; χ2 3.97; P = 0.41). When we repeated the analyses excluding the study by Hocker et al, the results remained unchanged (OR, 0.87; 95% CI, 0.5-1.54). In the 3 observational studies that adequately reported acute rejection,25,28,31 pooling of the data showed no significant effect of corticosteroid regimen on rejection (OR, 0.91; 95% CI, 0.27-3.07; P = 0.88; I2 = 57%; χ2 = 4.66; P = 0.1).
Renal Function
Four renal RCTs provided eGFR using Schwartz formula.21–24 There was no significant difference between the corticosteroid withdrawal/avoidance group and the control group (mean difference, −0.16 mL/min per 1.73 m2; 95% CI, −6.09 to 5.77; P = 0.96; I2 = 85%; χ2 = 20.49; P = 0.0001). Five observational studies reported an eGFR using Schwartz formula at the final visit or after 12 months of follow-up.25,27,28,30,31 There was no significant difference between the corticosteroid withdrawal/avoidance group and the control group (mean difference, −0.43 mL/min per 1.73 m2; 95% CI, −12.52 to 11.66; P = 0.94; I2 = 72%; χ2 = 14.29; P = 0.006).
Patient and Allograft Survival
Six RCTs reported allograft survival.20–24,26 Thirteen transplants failed of 304 participants in the corticosteroid withdrawal/avoidance group compared with 19 of 298 in the control group. This difference was not statistically significant among the 5 renal RCTs (OR, 0.45; 95% CI, 0.18-1.10; P = 0.08; I2 = 36%; χ2 = 3.10; P = 0.21). Four observational studies reported allograft failure.28,30–32 There were 5 of 117 allograft failures in the corticosteroid withdrawal/avoidance group and 13 of 85 in the control arm. The difference was significant with the corticosteroid withdrawal/avoidance group associated with less graft loss (OR, 0.29; 95% CI, 0.09-0.94; P = 0.04; I2 = 76%; χ2 = 4.09; P = 0.04). Six RCTs reported patient survival.20–24,26 Overall, there were 6 deaths among 304 participants in the corticosteroid withdrawal/avoidance group and 8 among 298 in the control group. The difference was not statistically significant among the 5 renal RCTs (OR, 0.34; 95% CI, 0.07-1.54; P = 0.16; I2 = 64%; χ2 = 2.80; P = 0.09). Six observational studies reported patient survival.27–32 There were 6 deaths among 285 corticosteroid withdrawal/avoidance participants and 5 of 186 among the control arm. The difference was not statistically significant (OR, 0.81; 95% CI, 0.22-2.93; P = 0.75; I2 = 39%; χ2 = 4.94; P = 0.18).
DISCUSSION
This systematic review and meta-analysis included data from 7 randomized trials and 7 observational studies involving 1146 pediatric transplant recipients. We observed a significant improvement in growth posttransplant when corticosteroid withdrawal or avoidance protocols were used among renal studies. This finding was consistent in both the renal randomized and observational studies. The improvement in growth was most pronounced in the studies whose participants were younger. Importantly, this improvement in growth did not occur at the expense of more rejection, allograft loss, or patient death.
In addition to being statistically significant, the findings from our analysis are clinically important to both patients and clinicians. Overall, we found a mean change in height (SDS, 0.18; 95% CI, 0.07-0.29) for those renal transplant recipients on a corticosteroid withdrawal/avoidance regimen. For an average female renal pediatric transplant recipient with a height SDS of −1.5, receiving a transplant with a corticosteroid withdrawal/avoidance protocol, this would result in a gain in height that was 1.0 cm more over a year than the gain in height if they were on a steroid-containing regimen. This gain in height could be as low as 0.3 cm but as high as 2.0 cm. Similarly, for an average male renal pediatric transplant recipient with a height SDS of −1.5, receiving a transplant with a corticosteroid withdrawal/avoidance protocol would result in a gain in height that was 1.3 cm more over a year than the gain in height if they were on a steroid-containing regimen. This gain in height could be as low as 0.2 cm but as high as 2.5 cm. Gains in height such as these are slightly lower compared to values obtained in growth hormone trials for short stature33; however, the baseline height of those receiving growth hormone therapy was much lower than in the steroid withdrawal trials. The lack of a growth benefit with steroid withdrawal/avoidance from the 2 pooled liver trials may be explained by the small sample size included as well as the shorter follow-up time. The study by McDiarmid et al19 also noted that the control sample was maintained on a very low of prednisone and thus may be similar to the withdrawal group.
With respect to safety, there was no difference in eGFR, acute rejection, graft or patient survival between the corticosteroid withdrawal/avoidance group and the control arm. Most RCTs required that a biopsy be performed if rejection was suspected, whereas others also mandated routine protocol biopsies. Only 1 study labeled a rejection episode without biopsy evidence.24 It is important to note that selection criteria varied among the trials we evaluated with 2 studies23,24 including higher risk patients, whereas 1 study21 excluded those with PRA levels less than 20%. This may explain the nonsignificant difference in acute rejection rates.
To date, no systematic review has pooled data on growth measurements in pediatric transplant recipients using corticosteroid withdrawal/avoidance immunosuppression. The Cochrane group34 conducted a systematic review on corticosteroid withdrawal/avoidance in adult and pediatric kidney transplant recipients. The review included only 1 pediatric RCT published as an abstract in 200535 and the remaining studies included were observational. The primary focus of the Cochrane review was safety including rates of acute rejection and graft failure. The growth parameters were only reported in a descriptive fashion. As there was only 1 pediatric RCT, there was no pooling of data. A meta-analysis published by Wu et al33 in 2013, assessed the efficiency and safety of growth hormone therapy post-renal transplant. It included 5 pediatric RCTs and found that those receiving growth hormone therapy demonstrated significantly higher growth velocity rates than those not receiving growth hormone (mean difference SDS, 0.52; 95% CI, 0.37-0.68). They did not assess whether pubertal status impacted potential growth and none of the RCTs included used corticosteroid withdrawal/avoidance protocols posttransplant.
Recently, Franke et al36 published the results of a large prospective study which followed growth (median follow up of 3.4 years) among 389 pediatric renal transplant recipients. Mean height during the observation period was −1.74 SDS and was significantly lower compared with healthy children (P < 0.001). The authors found that prolonged steroid exposure significantly impaired catch-up growth, that is, mean annual steroid dosage (in mg/kg) was negatively associated with linear growth (ß, −1.89; 95% CI, −2.68 to −1.10; P < 0.01). A recent retrospective single center study by Klare et al,10 followed up 74 kidney transplant patients (there was no comparison group) who had been weaned off steroids within 6 months after transplant. Mean follow-up was 8.5 years. They found complete catch up growth occurred in all prepubertal patients (standardized height velocity was 2.9 SDS for the first year compared with healthy children) but was incomplete in 20% of those who were pubertal. Significant predictors of catch up growth in this study were age and height deficit at time of transplant. The study was able to conclude that prepubertal patients weaned off steroids were able to have sustained catch-up growth and reached adult height that was within normal limits. Similarly, in our meta-analysis, the studies which assessed growth separately by pubertal status demonstrated a greater growth benefit from steroid withdrawal in the prepubertal patients.
There have been other observational studies that have assessed long-term growth after steroid withdrawal. Many of these studies were retrospective in nature and lacked a comparator group and were therefore not included in this systematic review. Most of the studies included in our meta-analysis reported a negative baseline height SDS suggesting a growth restricted state; however, most were greater than −2.0 suggesting the baseline height was not below the 2nd percentile. This is in keeping with recent data from the North American Pediatric Renal Transplant Cooperative Study that have shown the height SDS at time of transplant have improved from −2.5 in 1987 to between −1 and −1.5 in 2009.37
The main strength of our review was our ability to meta-analyze data and make conclusions with regard to an important clinical question. The main limitation of our study was the moderate heterogeneity found with respect to the growth data. This is likely related to the variability in the population studied, interventions used, outcomes assessed as well as the methodological quality of the included studies. For example, most of the studies included in our review made no mention of whether participants received growth hormone therapy. The length of follow-up was variable with 1 study reporting height at 6 months postwithdrawal and another reporting it at 3 years. However, almost all studies reported the change after 1 year of intervention, and these data were consistent and included. As with all systematic reviews, we were limited by the quality of the individual studies. In this particular review, most of the studies were not blinded. At the participant level, this could have led to differential adherence given the known side effects of corticosteroids. This, however, would have biased our findings toward the null. Finally, follow-up of individual RCTs was relatively short, and none provided achieved adult height, which is the most clinically relevant outcome.7
In conclusion, corticosteroid withdrawal or avoidance in pediatric renal transplantation is associated with a clinically significant improvement in height. The findings were consistent across renal study designs. As with other studies examining pediatric growth, prepubertal patients had the greatest benefit from corticosteroid withdrawal/avoidance, whereas pubertal patients do not appear to have the same benefit. Importantly, the improvement in growth was not accompanied by increased rejection or worsening patient/allograft survival in the short term. Although these findings are currently relevant to patients and clinicians, future studies need to focus on longer-term outcomes, such as achieved adult height and late-onset acute rejection.
Footnotes
A.T. is supported by the Kidney Research Scientist Core Education and National Training Program.
G.A.K. reports grants from Astellas Canada, Novartis Canada and Pfizer Canada outside the submitted work. The other authors declare no conflicts of interest.
A.T. was responsible for the conception and design of the study, analysis and interpretation of data, and drafted and finalized the article. G.K. was responsible for conception and design, analysis and interpreting the data, and drafting and approval of the final article. A.M. was involved with acquisition, analysis and interpretation of data, and drafting and final approval of the article. N.F. responsible for data abstraction and interpretation, drafting and final approval of the article. D.F. was involved in the conception and design of the work, analysis and interpretation of data, and drafting and final approval of the article. All authors approved the final article as submitted and agree to be accountable for all aspects of the work.
Correspondence: Greg Knoll, MD, The Ottawa Hospital, Riverside Campus, 1967 Riverside Drive, Ottawa, Ontario, Canada K1H 7W9. (gknoll@ottawahospital.on.ca).
Corticosteroid withdrawal/avoidance in pediatric renal transplantation, especially in prepubertal patients is associated with a significant improvement in height without any adverse events, including increased rejection or worsening patient/allograft survival in the short term.
REFERENCES
- 1.Horslen S, Barr ML, Christensen LL. Pediatric transplantation in the United States, 1996-2005 Am J Transplant 2007. 71339–1358 [DOI] [PubMed] [Google Scholar]
- 2.LaRosa C, Baluarte HJ, Meyers KE. Outcomes in pediatric solid-organ transplantation Pediatr Transplant 2011. 15128–141 [DOI] [PubMed] [Google Scholar]
- 3.Brooks RJ, Higgins GY, Webster AC. Systematic review of randomized controlled trial quality in pediatric kidney transplantation Pediatr Nephrol 2010. 252383–2392 [DOI] [PubMed] [Google Scholar]
- 4.Wilson JT. An update on the therapeutic orphan Pediatrics 1999. 104585–590 [PubMed] [Google Scholar]
- 5.Furth SL, Hwang W, Yang C. Growth failure, risk of hospitalization and death for children with end-stage renal disease Pediatr Nephrol 2002. 17450–455 [DOI] [PubMed] [Google Scholar]
- 6.Qvist E, Jalanko H, Holmberg C. Psychosocial adaptation after solid organ transplantation in children Pediatr Clin North Am 2003. 501505–1519 [DOI] [PubMed] [Google Scholar]
- 7.Laster ML, Fine RN. Growth following solid organ transplantation in childhood Pediatr Transplant 2014. 18134–141 [DOI] [PubMed] [Google Scholar]
- 8. North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) Annual report 2010. [Google Scholar]
- 9.Al-Sinani S, Dhawan A. Corticosteroids usage in pediatric liver transplantation: to be or not to be! Pediatr Transplant 2009. 13160–170 [DOI] [PubMed] [Google Scholar]
- 10.Klare B, Montoya CR, Fischer DC. Normal adult height after steroid-withdrawal within 6 months of pediatric kidney transplantation: a 20 years single center experience Transpl Int 2012. 25276–282 [DOI] [PubMed] [Google Scholar]
- 11.Schacke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids Pharmacol Ther 2002. 9623–43 [DOI] [PubMed] [Google Scholar]
- 12.Mazziotti G, Giustina A. Glucocorticoids and the regulation of growth hormone secretion Nat Rev Endocrinol 2013. 9265–276 [DOI] [PubMed] [Google Scholar]
- 13.Grenda R. Steroid withdrawal in renal transplantation Pediatr Nephrol 2013. 282107–2112 [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Altman DG, Gotzsche PC. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells GA SB, O’Connell D, Peterson J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, ON: Ottawa Hospital Research Institute; 2011. [Google Scholar]
- 16.Wallis CJ, Mahar AL, Choo R. Second malignancies after radiotherapy for prostate cancer: systematic review and meta-analysis. BMJ. 2016;352:i851. doi: 10.1136/bmj.i851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borenstein MHL, Higgings JPT, Rothstein HR. Introduction to Meta-Analysis. John Wiley & Sons; 2009. [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ. Measuring inconsistency in meta-analyses BMJ 2003. 327557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDiarmid SV, Farmer DA, Goldstein LI. A randomized prospective trial of steroid withdrawal after liver transplantation Transplantation 1995. 601443–1450 [DOI] [PubMed] [Google Scholar]
- 20.Mericq V, Salas P, Pinto V. Steroid withdrawal in pediatric kidney transplant allows better growth, lipids and body composition: a randomized controlled trial Horm Res Paediatr 2013. 7988–96 [DOI] [PubMed] [Google Scholar]
- 21.Sarwal MM, Ettenger RB, Dharnidharka V. Complete steroid avoidance is effective and safe in children with renal transplants: a multicenter randomized trial with three-year follow-up Am J Transplant 2012. 122719–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benfield MR, Bartosh S, Ikle D. A randomized double-blind, placebo controlled trial of steroid withdrawal after pediatric renal transplantation Am J Transplant 2010. 1081–88 [DOI] [PubMed] [Google Scholar]
- 23.Grenda R, Watson A, Trompeter R. A randomized trial to assess the impact of early steroid withdrawal on growth in pediatric renal transplantation: the TWIST study Am J Transplant 2010. 10828–836 [DOI] [PubMed] [Google Scholar]
- 24.Hocker B, Weber LT, Feneberg R. Prospective, randomized trial on late steroid withdrawal in pediatric renal transplant recipients under cyclosporine microemulsion and mycophenolate mofetil Transplantation 2009. 87934–941 [DOI] [PubMed] [Google Scholar]
- 25.Hocker B, John U, Plank C. Successful withdrawal of steroids in pediatric renal transplant recipients receiving cyclosporine A and mycophenolate mofetil treatment: results after four years Transplantation 2004. 78228–234 [DOI] [PubMed] [Google Scholar]
- 26.Spada M, Petz W, Bertani A. Randomized trial of basiliximab induction versus steroid therapy in pediatric liver allograft recipients under tacrolimus immunosuppression Am J Transplant 2006. 61913–1921 [DOI] [PubMed] [Google Scholar]
- 27.Delucchi A, Valenzuela M, Lillo AM. Early steroid withdrawal in pediatric renal transplant: five years of follow-up Pediatr Nephrol 2011. 262235–2244 [DOI] [PubMed] [Google Scholar]
- 28.Ferraris JR, Pasqualini T, Alonso G. A study on strategies for improving growth and body composition after renal transplantation Pediatr Nephrol 2010. 25753–762 [DOI] [PubMed] [Google Scholar]
- 29.Li L, Chang A, Naesens M. Steroid-free immunosuppression since 1999: 129 pediatric renal transplants with sustained graft and patient benefits Am J Transplant 2009. 91362–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chavers BM, Chang YC, Gillingham KJ. Pediatric kidney transplantation using a novel protocol of rapid (6-day) discontinuation of prednisone: 2-year results Transplantation 2009. 88237–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhakta N, Marik J, Malekzadeh M. Can pediatric steroid-free renal transplantation improve growth and metabolic complications? Pediatr Transplant 2008. 12854–861 [DOI] [PubMed] [Google Scholar]
- 32.Ellis D. Growth and renal function after steroid-free tacrolimus-based immunosuppression in children with renal transplants Pediatr Nephrol 2000. 14689–694 [DOI] [PubMed] [Google Scholar]
- 33.Wu Y, Cheng W, Yang XD. Growth hormone improves growth in pediatric renal transplant recipients—a systemic review and meta-analysis of randomized controlled trials Pediatr Nephrol 2013. 28129–133 [DOI] [PubMed] [Google Scholar]
- 34.Pascual J, Zamora J, Galeano C, et al. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev. 2009:CD005632. [DOI] [PubMed] [Google Scholar]
- 35.Benfield MR, Warshaw BL, Bartosh SM, et al. A Randomized controlled double-blind trial of steroid withdrawal in pediatric renal transplantation: a study of the Cooperative Clinical trials in pediatric transplantation (CPT). Paper presented at: American Journal of Transplantation. 2005. [DOI] [PubMed] [Google Scholar]
- 36.Franke D, Thomas L, Steffens R. Patterns of growth after kidney transplantation among children with ESRD Clin J Am Soc Nephrol 2015. 10127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) Annual Report. NAPRTCS 2010 Annual Transplant Report. https://web.emmes.com/study/ped/annlrept/2010_Report.pdf. Published 2010. Accessed May 9th, 2016. [Google Scholar]


