Abstract
Background
Donation after circulatory death (DCD) is current clinical practice to increase the donor pool. Deleterious effects on renal graft function are described for hypothermic preservation. Therefore, current research focuses on investigating alternative preservation techniques, such as normothermic perfusion.
Methods
We compared continuous pressure-controlled normothermic ex vivo kidney perfusion (NEVKP) with static cold storage (SCS) in a porcine model of DCD autotransplantation. After 30 minutes of warm ischemia, right kidneys were removed from 30-kg Yorkshire pigs and preserved with 8-hour NEVKP or in 4°C histidine-tryptophan-ketoglutarate solution (SCS), followed by kidney autotransplantation.
Results
Throughout NEVKP, electrolytes and pH values were maintained. Intrarenal resistance decreased over the course of perfusion (0 hour, 1.6 ± 0.51 mm per minute vs 7 hours, 0.34 ± 0.05 mm Hg/mL per minute, P = 0.005). Perfusate lactate concentration also decreased (0 hour, 10.5 ± 0.8 vs 7 hours, 1.4 ± 0.3 mmol/L, P < 0.001). Cellular injury markers lactate dehydrogenase and aspartate aminotransferase were persistently low (lactate dehydrogenase < 100 U/L, below analyzer range; aspartate aminotransferase 0 hour, 15.6 ± 9.3 U/L vs 7 hours, 24.8 ± 14.6 U/L, P = 0.298). After autotransplantation, renal grafts preserved with NEVKP demonstrated lower serum creatinine on days 1 to 7 (P < 0.05) and lower peak values (NEVKP, 5.5 ± 1.7 mg/dL vs SCS, 11.1 ± 2.1 mg/dL, P = 0.002). The creatinine clearance on day 4 was increased in NEVKP-preserved kidneys (NEVKP, 39 ± 6.4 vs SCS, 18 ± 10.6 mL/min; P = 0.012). Serum neutrophil gelatinase-associated lipocalin at day 3 was lower in the NEVKP group (1267 ± 372 vs 2697 ± 1145 ng/mL, P = 0.029).
Conclusions
Continuous pressure-controlled NEVKP improves renal function in DCD kidney transplantation. Normothermic ex vivo kidney perfusion might help to decrease posttransplant delayed graft function rates and to increase the donor pool.
Kidney transplantation offers several advantages for patients suffering from end-stage renal disease when compared with dialysis.1–5 A major limitation is the worldwide shortage of organs available for transplantation.6–9 Therefore, various strategies have been explored to increase the pool of deceased donor kidney grafts.10–12 Besides standard criteria donor and living donor grafts,13–15 extended criteria donor (ECD) grafts and kidneys donated after circulatory death (DCD)16–18 are used for transplantation.
Donation after circulatory death is an important strategy to increase the donor pool and to shorten the recipient’s time on the waiting list10 and has gained more interest again since the 1990s.19 Since approval by the World Health Organization in 2011,20 it is current clinical practice in 10 of 27 countries of the European Union, North America, South America, Australia, and Japan.10,21,22 At present, controlled DCD accounts for about 40% of deceased donation in the UK, more than 50% in the Netherlands,23 and for 15% of all deceased kidneys transplanted in the US in 2013.7 Clinical trials and systematic reviews demonstrated comparable long-term graft and patient survival outcomes for DCD kidney transplantation when compared with donation after brain death (DBD). However, rates of primary nonfunction, delayed graft function (DGF), and early graft loss are increased in DCD transplantation.22,24–27 Various studies indicate that donor age, body mass index, and warm ischemia (WI) times longer than 40 minutes, especially in combination with increased age and prolonged cold ischemia times, are independent risk factors for primary nonfunction, DGF, and graft failure in DCD kidney transplantation.26,28,29 In particular, prolonged cold ischemia times have shown to negatively impact the long-term outcomes in DCD kidney transplantation.23,28,30
Modification of current kidney preservation techniques represents a novel approach to improve renal graft function after DCD kidney transplantation. The most widely used preservation techniques are static cold storage (SCS) and hypothermic machine perfusion (HMP). Some evidence supports better outcomes posttransplant with HMP compared with SCS.31–33 However, for controlled DCD kidney transplantation, 2 recently conducted multicenter randomized controlled trials demonstrated controversial results. Jochmans et al34 demonstrated a significant reduction for incidence and length of DGF in HMP preserved kidneys and higher creatinine clearance up to 1 month after transplantation versus SCS. One-year graft and patient survival were similar in both groups. In contrast, Watson et al35 found no significant differences between HMP and SCS preserved grafts for incidence of DGF, renal function, and graft and patient survivals. To further improve the outcome of DCD grafts, alternative preservation approaches, such as modification of HMP with oxygenation,36,37 subnormothermic storage,38,39 and normothermic techniques40,41 are being investigated.
We recently demonstrated the feasibility and safety of replacing hypothermic storage with a continuous pressure-controlled normothermic ex vivo kidney perfusion (NEVKP) technique in a model of heart-beating donation.42,43 The aim of the current study was to investigate the potential of continuous pressure-controlled NEVKP to improve renal graft function in DCD kidney transplantation.
MATERIALS AND METHODS
Study Design
We compared the impact of continuous pressure-controlled NEVKP versus SCS on kidney graft injury and function after autotransplantation in pigs. Kidney grafts were subjected to either 8 hours of normothermic ex vivo perfused graft preservation or storage in cold histidine-tryptophan-ketoglutarate (HTK) solution at 4°C (n = 5 pigs in each group). After renal autotransplantation, pigs were followed up for 10 days (Figure 1A). Perfusion parameters, graft injury, and renal function after transplantation were assessed. The study was approved by the Animal Care Committee of the Toronto General Research Institute, Ontario, Canada.
FIGURE 1.
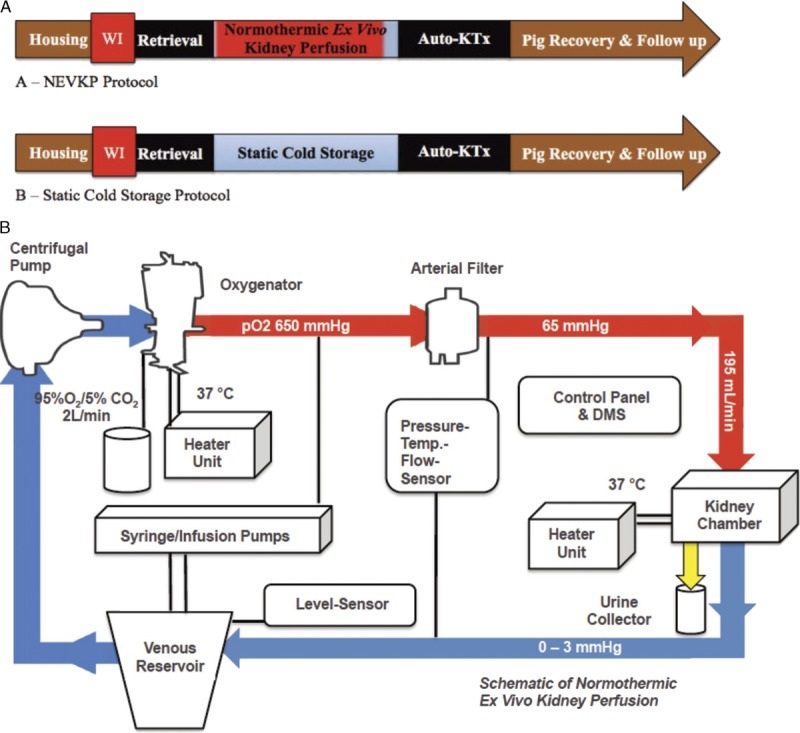
A, Study design. Animals were housed before planned procedures. Prior to kidney recovery, a WI time of 30 minutes was applied. Renal grafts were either preserved with NEVKP (A) or SCS (B). After preservation and graft transplantation, pigs were recovered and followed up for 10 days. B, Schematic of the NEVKP circuit. The circuit consists of neonatal cardiopulmonary bypass technology. The perfusion solution is collected in the venous reservoir. A centrifugal pump propels the solution into the oxygenator, where it is enriched with oxygen and warmed to 37°C. After passing the arterial filter, the perfusate is driven with a pressure of 65 mm Hg through the renal artery into the graft located in the customized double-walled kidney chamber. The venous outflow (0-3 mm Hg) leads the perfusate back into the venous reservoir. Syringe and infusion pumps secure the supply with additional compounds. The urine is collected throughout the perfusion. Control panel and DMS indicate and record perfusion parameters continuously. DMS, Data Management System.
Animals
Male Yorkshire pigs (30 kg) were used. Species adapted housing with water and food ad libitum was provided. All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care of Laboratory Animals” published by the National Institutes of Health.
Normothermic Ex Vivo Kidney Perfusion
Normothermic ex vivo kidney perfusion was conducted as described previously.42,43 Briefly, the perfusion equipment consisted of neonatal cardiopulmonary bypass technology and an S3 heart lung machine. A centrifugal pump with continuous flow mode was used to propel the solution through the circuit (Sorin Group Inc., Markham, Canada). A customized double-walled organ chamber served to provide sterility and a normothermic environment for the graft during preservation. Perfusion circuit parameters (temperature, arterial and venous pressure, and arterial flow) were recorded real-time using the Data Management System (Sorin Group Inc.) (Figure 1B). As previously described, the perfusion circuit was primed with Ringer lactate, Steen solution, washed erythrocytes, double reverse osmosis water, sodium bicarbonate (8.4%), calcium gluconate (10%, 100 mg/mL), and heparin to achieve a near physiologic perfusate composition (Tables 1 and 2).42,43
TABLE 1.
Ingredients in perfusate solution and amount or rate administered
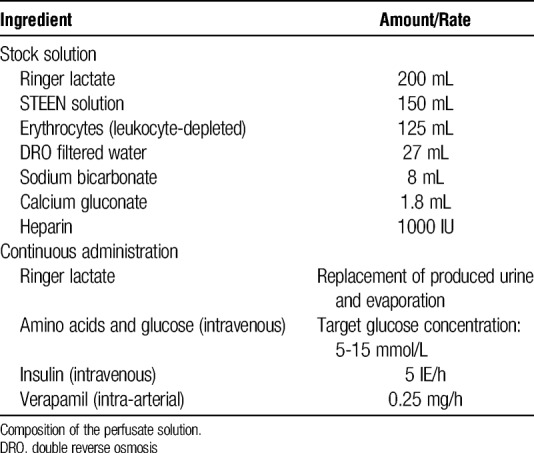
TABLE 2.
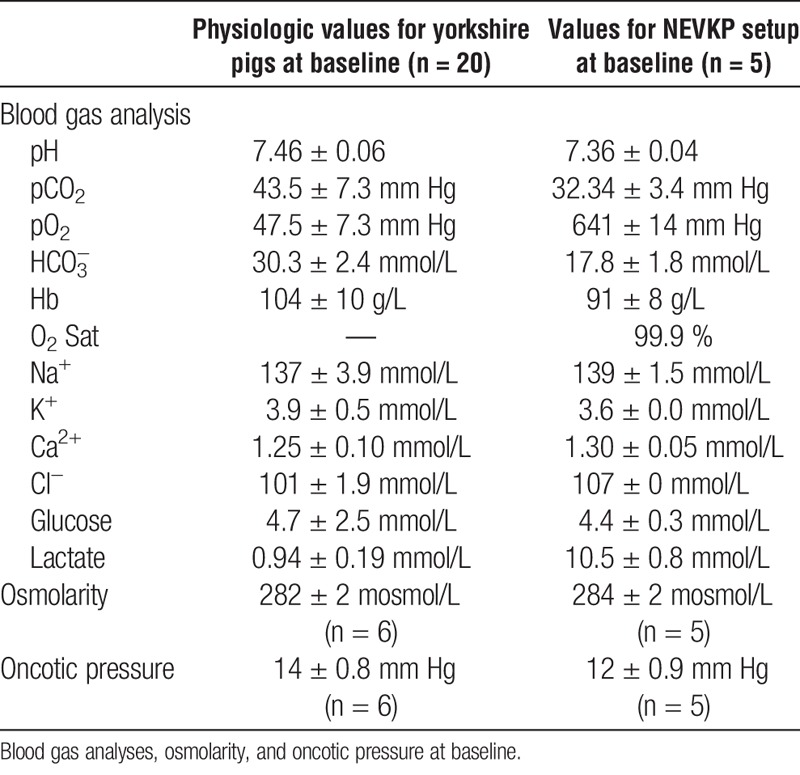
Blood gas analysis, osmolarity, and oncotic pressure measured at baseline in yorkshire pigs and at the start of normothermic Ex vivo Kidney perfusion
In the NEVKP preserved group, the hilum of the right kidney was clamped for 30 minutes in situ to induce WI. To simulate a DCD model most similar to the clinical scenario, the grafts vessels and ureter were cut shortly before the end of the WI period for vessel cannulation, but were kept warm intracorporeally. After cannulation of the renal artery (1.6 in., Sorin Group Inc.) and vein (1/4 × 1/8 in., Sorin Group Inc.) and fixation with 2-0 silk ties (Covidien, Mississauga, Canada), grafts were flushed for a short period (<5 minutes) with 4°C Ringer lactate (300-500 mL) containing 10 000 IU/L heparin (Sandoz Canada Inc., Toronto, Canada) at a pressure of 100 cm H2O. Immediately, grafts were connected to the primed normothermic perfusion (NP) circuit. As previously described, oxygen, verapamil (0.25 mg/h), amino acids and glucose (1 mL/h), and insulin (5 IU/h) were added continuously during perfusion (Table 1).42,43 Urine was replaced with Ringer lactate. Blood gas parameters, concentration of lactate, and the cell injury markers, aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) were measured in the perfusate hourly. Hourly collected perfusate samples were frozen at −80°C after centrifugation for further investigation. After NEVKP, renal grafts were flushed with 4°C cold HTK and stored on ice for anastomoses of the renal artery and vein.
Static Cold Storage
After induction of 30 minutes of WI, grafts were recovered and flushed with 300 to 500 mL of 4°C cold HTK containing 10 000 IU/L heparin with a pressure of 100 cm H2O. Up until the end of autotransplantation, renal grafts were statically stored in 4°C cold HTK solution for 8 hours in a sterile organ bag (CardioMed Supplies Inc., Lindsay, Canada).
Kidney Retrieval and Transplants
Porcine kidney retrieval and transplantation were conducted as previously described elsewhere by our group.44 Briefly, anesthesia was administered as intramuscular injection of ketamine (20 mg/kg; Bimeda-MTC Animal Health Inc., Cambridge, Canada), atropine (0.04 mg/kg; Rafter 8 Products, Calgary, Canada), and midazolam (0.3 mg/kg; Pharmaceutical Partners of Canada Inc., Richmond Hill, Canada). After intubation, isoflurane (2.5%; Pharmaceutical Partners of Canada Inc., Richmond Hill, Canada) was administered, a central venous catheter (9.5 French; Cook Medical Company, Bloomington, US) placed for administration of fluids and medication, and a midline incision was performed. Right kidneys were dissected and renal artery and vein were clamped with vascular clamps. After 30 minutes of WI, grafts were resected. Immediately, the renal artery and vein were cannulated, and kidneys were flushed with Ringer lactate and perfused at 37°C, or flushed with HTK and stored at 4°C as described above. After abdominal closure and recovery, pigs were reanesthetized for renal autotransplantation. After resection of the left kidney, grafts were taken from pump and flushed with 4°C HTK or taken from ice and renal anastomoses (vein end-to-side to cava, artery end-to-side to aorta, ureter side-to-side) sewed. Perioperative procedures, drug administration, and follow-up of the pigs were conducted as previously described.44
Whole Blood, Serum, and Urine Measurements
Blood gas analyses (RAPIDPoint 500 Systems; Siemens AG, Berlin, Germany) using perfusate samples were performed hourly during NEVKP and daily after kidney transplantation. Perfusate samples were analyzed for AST and LDH (Vitros DT60 II; Johnson & Johnson, Markham, Canada) during NEVKP and serum samples for measurement of creatinine and blood urea nitrogen (BUN)/urea (Piccolo Xpress, Union City, Canada) for follow-up after transplantation. Serum samples were also frozen down to −80°C until enzyme-linked immunosorbent assay analysis. Neutrophil gelatinase-associated lipocalin (NGAL), a specific renal injury marker, was measured using a porcine (NGAL) ELISA kit (Bioporto, Hellerup, Denmark). Twenty-four-hour urine collection was performed using a metabolic cage to investigate the creatinine clearance before transplantation (day 0), on postoperative day (POD) 4, and POD 10. Further serum and urine analyses were performed in the Core Laboratory on the Abbott Architect Chemistry Analyzer using the manufacturer’s reagents (Abbott Laboratories, Abbott Park, IL).
Histology
On the 10th POD, a wedge biopsy of renal tissue was performed under anesthesia. Tissue was placed in 10% neutral buffered formalin and transferred to 70% alcohol after 36 to 48 hours. After paraffin-embedding, sectioning, and staining, 3-μm periodic acid-Schiff–stained sections were used to score tubular injury, edema, fibrosis, and interstitial inflammation on a scale of 0 to 3 blinded to the experimental group as previously described.43 Tubular injury including brush border loss, tubular dilatation, epithelial vacuolation, thinning and sloughing, and luminal debris were scored in 10 high-power fields and averaged to assess overall tubular injury. Interstitial inflammation was scored in 10 low-power fields and averaged. Glomerular shrinkage was assessed. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed according to standard protocol. Because of the low rate of TUNEL-positive cells, the total number of positive cells were counted in 25 high-power fields and averaged.
Statistical Analysis
SPSS software version 23.0 (IBM, Armonk, NY) was used to perform statistical analysis. Fisher exact test was used for calculation of differences in mortality (2-sided). The Shapiro-Wilk test was used to test variables for normal distribution. Student t test was used to compare differences of parametric continuous variables, Mann-Whitney U test for ordinal and nonparametric data between the 2 groups. Significance was defined as P less than 0.05.
RESULTS
Animal Demographics and Surgical Procedure
Animal weights in the NEVKP group (30.2 ± 1.4 kg) were similar when compared with the SCS group (31.7 ± 0.9 kg) with t(7) = 1.907, P = 0.98. Total preservation times (NEVKP, 488.0 ± 8.5 minutes vs SCS, 492.4 ± 15.0 with t(8) = 0.570 and P = 0.585) and times for the anastomoses (NEVKP, 36.2 ± 3.5 min vs SCS, 32.2 ± 6.3 min with t(8) = −1.242 and P = 0.250) were not different in between both groups.
NEVKP Grafts Demonstrate Stable and Physiologic Perfusion Characteristics
Normothermic ex vivo kidney perfusion was initiated at an arterial pressure set to 75 mm Hg and a venous pressure between 0 and 3 mm Hg. After rewarming of the graft, the arterial pressure dropped to a physiological value of around 65 mm Hg without the need to regulate the speed of the centrifugal pump within about 1 hour. In case the pressure dropped below 65 mm Hg, the RPM of the centrifugal pump were increased to achieve a pressure of 65 mm Hg again (Figure 2A). Venous pressure was maintained at around 2 mm Hg by height regulation of the venous reservoir. Flow rates increased from 48 ± 13 mL/min at baseline to 194 ± 30 mL/min at hour 7 (t(4) = −11.005, P < 0.001) (Figure 2B). During perfusion, the intrarenal resistance (IRR) decreased from 1.60 ± 0.50 to 0.34 ± 0.05 mm Hg/mL per minute at hour 7 (t(4) = 5.662, P = 0.005) (Figure 2C). Urine production during NEVKP is demonstrated in Figure 2D. Arterial pressure, venous pressure, flow rates, and IRR were similar to physiologic values measured by our group in 30 healthy control pigs as previously described.43
FIGURE 2.
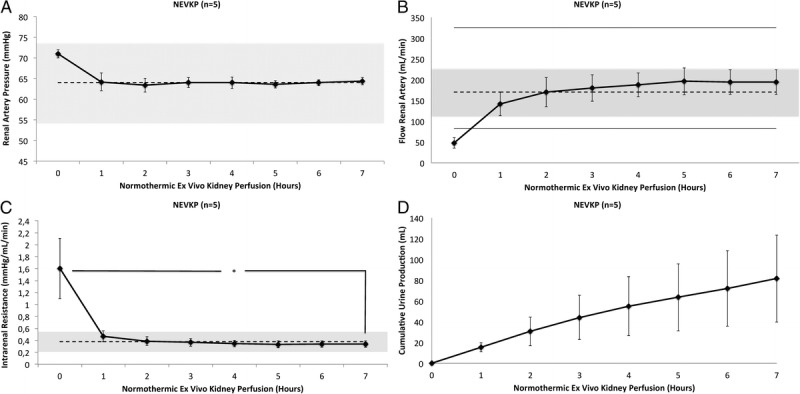
A, Renal artery blood pressure during normothermic ex vivo kidney perfusion. Values are presented as mean ± SD in mm Hg. Dashed line and grey area represent mean systemic blood pressure and SD measured invasively in situ in 30 anesthetized pigs by placing a catheter into the carotid artery. B, Renal artery flow during normothermic ex vivo kidney perfusion. Values presented as mean ± SD in mL/min Dashed line and grey area represent mean flow rate with SD measured in situ in 30 anesthetized pigs; upper and lower lines represent maximal and minimal renal artery flow rates in these pigs. The measurements were performed in control pigs following laparotomy and minimal dissection of the right renal artery with a flow probe. C, Intrarenal resistance during normothermic ex vivo kidney perfusion. Values presented as mean ± SD in mm Hg/mL per minute. Dashed line and grey area represent mean IRR with SD based on measurements performed in situ in 30 anesthetized pigs. The IRR decreased significantly over the course of perfusion (0 hour, 1.6 ± 0.51 mm Hg/mL per hour vs 7 hour, 0.34 ± 0.05 mm Hg/mL per hour, P = 0.005). D, Cumulative urine output during normothermic ex vivo kidney perfusion. Values presented as mean ± SD in mL.
NEVKP Grafts Maintain Physiologic Biochemical Parameters in the Perfusate
For the assessment of renal graft viability, hourly blood gas analyses were performed. Acid-base homeostasis was maintained without administration of bicarbonate after initiation of NEVKP and was physiologic when compared with baseline values measured in 20 healthy Yorkshire pigs (Figures 3A and B).
FIGURE 3.
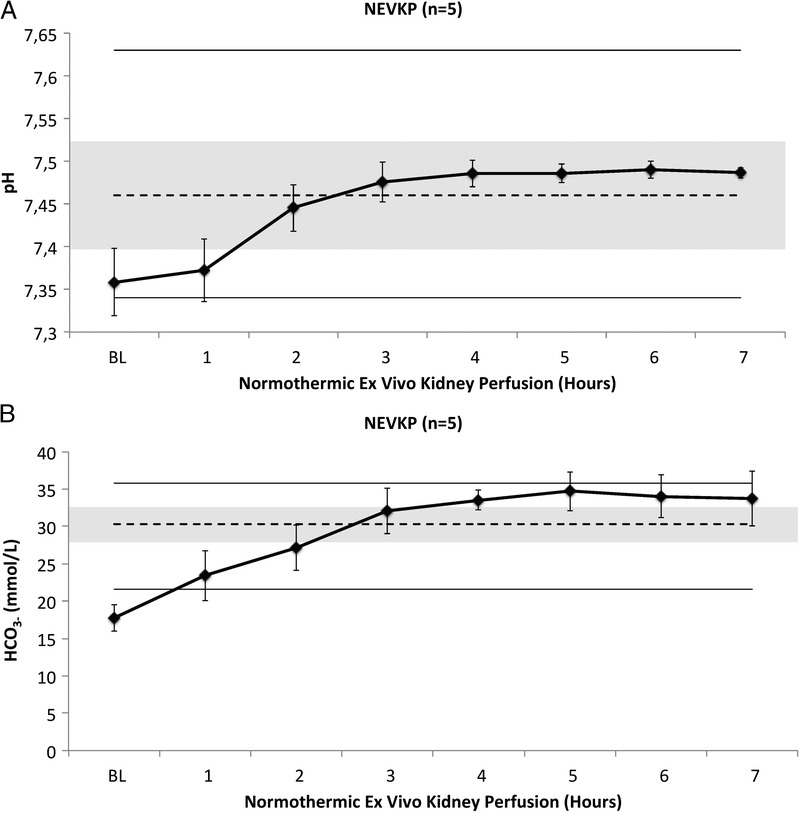
A, pH of the perfusate during normothermic ex vivo kidney perfusion. Values presented as mean ± SD. Dashed line and grey area represent mean serum pH with SD measured in situ in 20 anesthetized pigs; upper and lower lines represent maximal and minimal pH values in these pigs. B, HCO3− concentration in the perfusate during Normothermic Ex Vivo Kidney Perfusion. Values presented as mean ± SD in mmol/L. Dashed line and grey area represent mean serum HCO3− with SD measured in situ in 20 anesthetized pigs; upper and lower lines represent maximal and minimal HCO3− values in these pigs.
NEVKP Grafts Do Not Demonstrate Increased Injury During Perfusion
To assess renal graft injury during NEVKP, real-time marker LDH, and AST were measured hourly. Lactate dehydrogenase values were constantly below the level of detection of the analyzer (<100 U/L) and AST was stable throughout the perfusion (baseline: 19 ± 7 U/L vs hour 7: 25 ± 15 U/L, P = 0.272). Lactate decreased significantly over the 7 hours of perfusion (baseline: 10.5 ± 0.83 mmol/L vs hour 7: 1.39 ± 0.28 mmol/L, t(3) = 24.555, P < 0.0001) (Figure 4).
FIGURE 4.
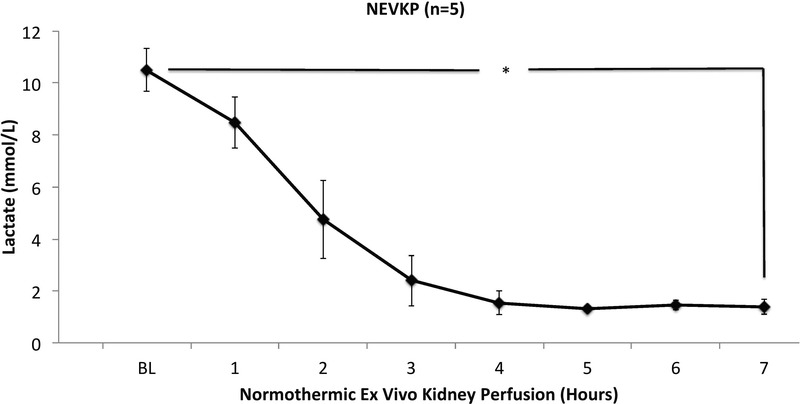
Lactate levels in renal perfusate during normothermic ex vivo kidney perfusion. Values presented as mean ± SD in mmol/L. Perfusate lactate concentration decreased over time (0 hour, 10.5 ± 0.8 mmol/L vs 7 hour, 1.4 ± 0.3 mmol/L, P < 0.001).
NEVKP Transplantation Results in Improved Renal Function and Reduced Injury
Normothermic ex vivo kidney perfusion preserved grafts demonstrated significantly improved kidney function after heterotopic autotransplantation when compared to SCS grafts. On POD 1 till 7, serum creatinine values were significantly lower in the NEVKP group when compared with SCS (P < 0.05, respectively). Also, peak values of 5.5 ± 1.8 mg/dL in the normothermic group and 11.1 ± 2.1 mg/dL in the hypothermic group were significantly different (t(8) = 4.611, P = 0.002) (Figure 5A). Serum BUN was significantly lower in the normothermic perfused kidneys on day 2 till 4 after transplant (P < 0.05, respectively) with a significantly lower peak value of 47 ± 6.2 versus 68 ± 24.2 mg/dL (P = 0.007) (Figure 5B). In addition, serum potassium levels were significantly lower on days 1 and 2 after transplant in the NEVKP versus SCS group (3.9 ± 0.1 vs 4.7 ± 0.4 mmol/L, P = 0.003, and 3.9 ± 0.7 vs 4.8 ± 0.4 mmol/L, P = 0.029) (Figure 5C). The 24-hour creatinine clearance of the normothermic perfused versus SCS kidneys was significantly higher on day 4 after transplant (39.0 ± 6.5 vs 18.2 ± 10.5 mL/min, P = 0.012) (Figure 5D). Serum NGAL, as a specific marker of graft injury, was significant lower at POD 3 in the NEVKP versus SCS group (1267 ± 372 vs 2697 ± 1145 ng/mL, P = 0.029) (Figure 6).
FIGURE 5.
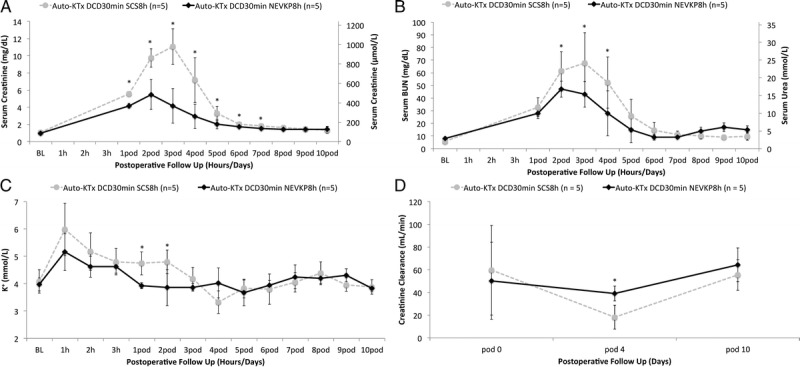
A, Serum creatinine of the transplanted animals during 10-day postoperative follow-up for autologous kidney transplantation after SCS and NEVKP. Values presented as mean ± SD in mg/dL and μmol/L. NEVKP demonstrated significantly lower serum creatinine on postoperative days 1 to 7 (P < 0.05) and lower peak values when compared to grafts preserved at 4°C (NEVKP 5.5 ± 1.7 mg/dL vs SCS 11.1 ± 2.1 mg/dL, P = 0.002). B, Serum BUN/urea during 10-day postoperative follow-up for autologous kidney transplantation after SCS and NEVKP. Values presented as mean ± SD in mg/dL and μmol/L. Serum BUN was significantly lower in the normothermic perfused kidneys on day 2 till 4 after transplant (P < 0.05, respectively) with a significantly lower peak value of 47 ± 6.2 mg/dL versus 68 ± 24.2 mg/dL (P = 0.007). C, Serum potassium during 10-day postoperative follow-up for autologous kidney transplantation following SCS and NEVKP. Values presented as mean ± SD in mmol/L. Serum potassium levels were significantly lower on day 1 and 2 after transplant in the NEVKP versus SCS group (3.9 ± 0.1 vs 4.7 ± 0.4 mmol/L, P = 0.003, and 3.9 ± 0.7 vs 4.8 ± 0.4 mmol/L, P = 0.029). D, 24-hour creatinine clearance during 10-day postoperative follow-up. Values presented as mean ± SD in mL/min. The creatinine clearance on postoperative day 4 was increased in NEVKP versus SCS preserved kidneys (NEVKP 39 ± 6.4 mL/min vs SCS 18 ± 10.6 mL/min, P = 0.012).
FIGURE 6.
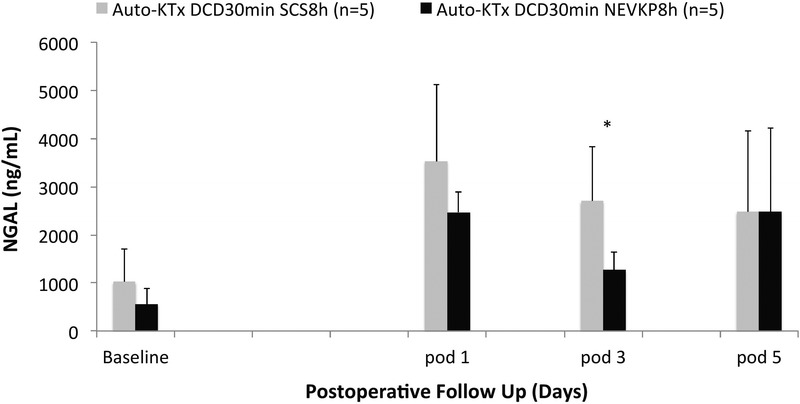
Serum NGAL during 5-day postoperative follow-up for autologous kidney transplantation after SCS and NEVKP. Values presented in mean ± SD in ng/mL. NGAL was significantly lower at postoperative day 3 in the NEVKP versus SCS group (1267 ± 372 ng/mL vs 2697 ± 1145 ng/mL, P = 0.029).
Renal histology was compared between the 2 groups on day 10 after transplantation. There were no significant differences in between NEVKP versus SCS preserved kidneys for tubular injury (0.5 [0.5-1.5] vs 0.5 [0.5-1.0], U = 9.5, P = 0.548), interstitial inflammation (1.0 [1.0-1.5] vs 0.5 [0.0-1.5], U = 4.5, P = 0.095), edema (0 [0-0] vs 0 [0-0], U = 12.5, P = 1.0), or fibrosis (0 [0-0] vs 0 [0-0], U = 12.5, P = 1.0) (Table 3; Figures 7A and B). In all cases, the glomeruli were mildly shrunken without statistical differences in between the 2 groups. TUNEL staining showed extremely low levels of apoptotic cells with no differences between NEVKP and SCS preserved kidneys (data not shown).
TABLE 3.
Histological findings 10 days after transplantation assessed by haematoxylin and eosin/PAS staining
FIGURE 7.
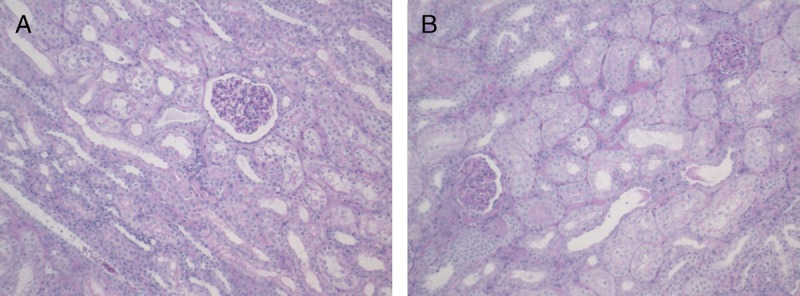
A, Wedge biopsy of a normothermic preserved kidney taken 10 days after transplantation (PAS). Corticomedullary junction showing minimal to mild tubular injury (100×). B, Wedge biopsy of a hypothermic preserved kidney taken ten days after transplantation (PAS staining). Corticomedullary junction showing minimal to mild tubular injury (100×). PAS, Periodic acid-Schiff.
NEVKP Did Not Compromise Animal Survival
Animal survival was similar in both groups (P = 1.000). In the NEVKP group, 1 pig had to be sacrificed due to a bowel obstruction with a consecutive bowel perforation on day 5 after transplant with a well-functioning renal graft.
DISCUSSION
Normothermic ex vivo organ perfusion is a novel technique to improve graft preservation before transplantation. Recently, we have demonstrated the feasibility and safety of continuous NEVKP over 8 hours in a model of heart-beating donor kidney transplantation.43 We now determined the superiority of NEVKP over SCS in a model of DCD kidney transplantation. Throughout normothermic preservation, physiologic perfusion parameters and biochemical values were maintained with low markers of injury, high clearance of lactate, and declining IRR. After renal autotransplantation, NEVKP preserved grafts demonstrated improved graft function with significantly lower serum creatinine values and significantly higher creatinine clearance when compared with SCS.
Donation after circulatory death is an effective strategy to reduce the worldwide severe organ shortage. The outcome after transplantation of organs donated after circulatory death has steadily improved throughout the last decades. However, persisting reservations result in discard rates as high as 51% for ECD/DCD renal grafts in North America.45 To improve DCD graft outcome and further expand the donor pool by accepting even more marginal grafts, emphasis is placed on modifying current preservation techniques and development of alternative organ storage strategies.
Static cold storage has been the criterion standard for decades in kidney transplantation. In clinical practice, 3 types of composition of solution for SCS are used: intracellular, intermediate, and extracellular. Which preservation solution is the most appropriate for DCD has not been conclusively determined.12 Several groups have investigated how to alter the composition of existing solutions to adapt them to the clinical needs.46 Since the 2000s, the expansion of the donor pool with acceptance of ECD and DCD kidney grafts has resulted in increased utilization of HMP in the United States.7 Some reports suggest beneficial effects for HMP versus SCS in kidney transplantation with respect to incidence of DGF.32 However, other studies have demonstrated equality for HMP versus SCS regarding rates of DGF and renal function after kidney transplantation, especially in DCD kidney transplantation.35,47–49 Also, systematic reviews demonstrated similar outcomes for HMP versus SCS in DCD kidney transplantation.50,51 Therefore, further research focuses on alterations in HMP using pulsatile perfusion,52 novel perfusate solutions,53,54 and oxygenation during preservation37 to further improve the grafts outcome. However, despite modification of cold storage solutions, several studies clearly demonstrate the detrimental impact of prolonged cold ischemia on DCD grafts which lead to increased rates of DGF.23,28,30
Normothermic preservation of renal grafts has yet to prove its superiority over hypothermic storage techniques in DCD kidney transplantation. The most recent recommendations for DCD kidney preservation and transplantation from North America21 and Europe22 do not discuss the potential to use normothermic preservation in clinical settings. First experiments investigating normothermic preservation techniques using heparinized whole blood were conducted in canine models already in the late 1970s and the early 1980s by Kootstra.55,56 Aiming for prolongation of preservation times, a combination of HMP (8°C) and normothermic ex vivo perfusion (38°C) was performed for up to 144 hours in heart-beating donation kidneys. After renal autotransplantation, grafts that underwent a phase of 3 or 4 hours of normothermic ex vivo perfusion during the preservation period demonstrated significantly lower serum creatinine values and improved animal survival when compared with continuously hypothermic perfused grafts. These beneficial effects of intermediate NP have also been demonstrated for renal grafts that were subjected to 30 minutes of WI.57 In the late 1990s and early 2000s, Brasile, Stubenitsky, and Kootstra58,59 investigated an alternative preservation technique, called exsanguinous metabolic support (EMS), based on subnormothermic acellular, low pressure, pulsatile ex vivo kidney perfusion. Canine kidneys were subjected to 30 minutes of WI and 24 hours of SCS. Grafts were then either reimplanted directly or perfused for 3 hours with EMS and then transplanted. The results demonstrated significantly lower 24-hour posttransplant serum creatinine and lower peak serum creatinine in the subnormothermic preserved kidneys.60 In another study from Brasile et al,38 canine kidneys were subjected to 30 minutes of WI followed by various duration of cold storage and sub-NP with EMS. Reduction of SCS and prolongation of sub-NP resulted in significantly improved renal graft function with decreased serum creatinine levels after graft autotransplantation. Further publications supported the beneficial findings of sub-NP on the EMS device.61,62 Unfortunately, subnormothermic temperature, low perfusion pressure, and nonphysiologic perfusate composition limits the options for graft assessment. Our technique of continuous NEVKP has several differences when compared to the subnormothermic studies. In contrast to the work of Brasile et al,58 our NEVKP model preserves grafts at close to physiologic temperature and pressure, resulting in physiologic renal flow rates and IRR. The physiologic metabolic activity of the preserved kidney allows graft assessment and application of additional repair strategies, such as gene transfer or stem cell therapy. Of note, since its development nearly 20 years ago, EMS has not been translated to clinical trials yet.63
Nicholson et al64,65 investigated NP for renal grafts using erythrocyte-based solutions in porcine models. They demonstrated beneficial results for the application of a short period of NP at the end of the preservation period when compared with hypothermic preservation. The postpreservation assessment was performed during reperfusion on a separate ex vivo perfusion system using whole-blood perfusion for 3 hours as a model for transplantation. In 1 study, this group investigated a short additional period of NP in a porcine autotransplantation model. Renal grafts were subjected to 30 minutes of WI and 22 hours of HMP, or 20 hours of HMP with an additional period of NP for 2 hours. No significant differences were found in graft survival or kidney function, but lower levels of lipid peroxidation were measured in the NP group 60 min after transplantation. This study demonstrated the feasibility of a short NP time of 2 hours in a porcine model of renal transplantation.40 Most recently, they translated their findings into a clinical study in which the first in man renal transplantation after a short ex vivo NP66 was performed. After SCS and 1 hour of NP at 34.6°C, ECD grafts demonstrated significantly lower rates of DGF when compared to a control group, where ECD grafts underwent SCS only.41 Despite the promising results demonstrated by Hosgood and Nicholson, in their studies, only additional short-term NPs of 1 or 2 hours have been investigated. Prolonged or continuous normothermic preservation with exclusion of hypothermic preservation has not yet been studied. In contrast, our technique of NEVKP preserves renal grafts at normothermic and more physiologic circumstances continuously for the entire preservation period. Other than Hosgood et al,40 our NEVKP technique demonstrates significantly superior outcome when compared with SCS for parameters of renal function post transplantation. It is possible that complete avoidance of cold ischemic injury has advantages when compared with short warm perfusion periods as an attempt to reverse cold ischemic damage before transplantation.
In this study, we demonstrate that continuous pressure-controlled NEVKP improves graft function in DCD kidney transplantation. Providing near physiologic conditions for DCD grafts throughout preservation with normothermic temperature, physiologic pressures, and flow rates as well as leukocyte-depleted perfusate composition—all based on measurements in healthy control animals—resulted in low cell injury markers (AST, LDH),60,67–69 and decrease of lactate and IRR70 during NEVKP. This resulted in significantly better outcomes after kidney transplantation with reduced serum creatinine, BUN, and NGAL, and increased creatinine clearance. The high metabolic graft activity afforded by NP during preservation provides a platform for graft assessment and repair. Prolonged, continuous NP might offer sufficient time for immunomodulation, gene transfer, microRNA administration, and other techniques to improve renal function after transplantation during preservation.
Our study has several limitations. No biopsies were taken during NEVKP due to technical reasons. Because the perfusate solution is heparinized and the graft is perfused extracorporeally using verapamil for vasodilation, sampling during preservation caused bleeding that could not be stopped by surgical manipulation. Excessive bleeding in turn might lead to loss of perfusate, which is prone to hemolysis if reintroduced into the circuit. Biopsies were only taken on day 10 after transplant, when potential differences may no longer be visible in this model. Furthermore, the total preservation time was kept short to have a control group with little cold ischemic injury. These short storage periods may be impossible to reach in several procurement areas, where prolonged preservation sometimes is needed. Although our study suggests superiority of continuous NP, clinically portable perfusion devices are not yet available for normothermic kidney preservation. However, because these devices are already available for ex vivo heart, liver, and lung perfusion, portable perfusion devices for kidneys might be developed in the near future. Static cold storage was chosen as control group, as it is still commonly used in renal graft preservation worldwide. Although some studies demonstrate superior outcome for HMP versus SCS, others did not suggest lower DGF rates for HMP, especially for DCD kidney transplantation.35,47–49 However, further experimental studies need to explore and directly compare outcomes between NEVKP and HMP, oxygenated HMP, slow graft rewarming, and subnormothermic machine perfusion. Only direct comparison of NEVKP versus HMP or sub-NP will determine if normothermia rather than machine perfusion is the crucial beneficial aspect in our setting.
In conclusion, we demonstrated that continuous, pressure-controlled normothermic ex vivo kidney perfusion improves renal graft function compared to SCS in a model of DCD kidney transplantation. Providing a physiologic environment during graft preservation results in significantly superior outcomes when compared with SCS. The metabolic activity during the perfusion period might allow assessment and repair during preservation. Continuous NEVKP might be capable of reducing DGF rates, resulting in improved long-term outcomes in DCD kidney transplantation and lower healthcare expenses. This in turn would extend the donor pool by allowing use of more marginal grafts and reduce the recipient’s waiting list.
ACKNOWLEDGMENTS
The authors thank the Sorin Group (Milano, Italy), XVIVO Perfusion Inc. (Goteborg, Sweden), Novartis, and Braun AG (Melsungen, Germany) for their support. The authors highly appreciate the support of the John David and Signy Eaton Foundation. This work is part of the Canadian National Transplant Research Program (CNTRP).
Footnotes
The authors declare no funding or conflicts of interest.
L.R. and M.S. share senior authorship.
J.M.K., J.E., D.B., I.M., A.G., D.G., L.R., and M.S. participated in research design. J.M.K., D.G., L.R., and M.S. participated in writing the article. J.M.K., J.E., Y.-M.C., D.C., N.G., I.L., and L.D. participated in performing the experiments. J.M.K., P.Y., and R.J. participated in data analysis.
Correspondence: Markus Selzner, MD, University of Toronto, General Surgery & Multi-Organ Transplant Program Toronto General Hospital, University Health Network 585 University Avenue, 11 PMB 178 Toronto, ON, Canada M5G 2N2. (markus.selzner@uhn.ca); Lisa A. Robinson, MD, FRCP(C), Division of Nephrology, The Hospital for Sick Children 555 University Avenue, Room 5265 Toronto, ON, Canada M5G 1X8. (lisa.robinson@sickkids.ca).
The authors demonstrate the feasibility and safety of a continuous pressure-controlled normothermic ex vivo kidney perfusion technique in a DCD model. It provides a physiologic environment during graft preservation and results in significantly superior outcomes when compared to static cold storage.
REFERENCES
- 1.Wolfe RA, Ashby VB, Milford EL. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant N Engl J Med 1999. 3411725–1730 [DOI] [PubMed] [Google Scholar]
- 2.Tonelli M, Wiebe N, Knoll G. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes Am J Transplant 2011. 112093–2109 [DOI] [PubMed] [Google Scholar]
- 3.Ingsathit A, Kamanamool N, Thakkinstian A. Survival advantage of kidney transplantation over dialysis in patients with hepatitis C: a systematic review and meta-analysis Transplantation 2013. 95943–948 [DOI] [PubMed] [Google Scholar]
- 4.Meier-Kriesche HU, Port FK, Ojo AO. Effect of waiting time on renal transplant outcome Kidney Int 2000. 581311–1317 [DOI] [PubMed] [Google Scholar]
- 5.Rana A, Gruessner A, Agopian VG. Survival benefit of solid-organ transplant in the United States JAMA Surg 2015. 150252–259 [DOI] [PubMed] [Google Scholar]
- 6.Global Observatory on Donation and Transplantation, WHO. Organ Donation and Transplantation Activities 2013 [Internet]. 2015 [cited 2015 Nov 18]. Available from: http://www.transplant-observatory.org/Documents/Data%20Reports/Basic%20slides%202013.pdf. [Google Scholar]
- 7.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2013 annual data report: kidney. Am J Transplant. 2015;(15 Suppl 2):1–34. [DOI] [PubMed] [Google Scholar]
- 8.Pippias M, Stel VS, Abad Diez JM. Renal replacement therapy in Europe: a summary of the 2012 ERA-EDTA Registry Annual Report Clin Kidney J 2015. 8248–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.America TTSOL, Caribbean T. Latin America Transplantation Report 2009. http://www.abto.org.br/abtov03/Upload/file/RBT/RBT_Latino_Americano/Latino_Americano_2009.pdf. 2009. [Google Scholar]
- 10.Maggiore U, Oberbauer R, Pascual J, et al. Strategies to increase the donor pool and access to kidney transplantation: an international perspective. Nephrol Dial Transplant. Oxford University Press. 2014:gfu212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schold JD, Segev DL. Increasing the pool of deceased donor organs for kidney transplantation Nat Rev Nephrol 2012. 8325–331 [DOI] [PubMed] [Google Scholar]
- 12.Bon D, Chatauret N, Giraud S. New strategies to optimize kidney recovery and preservation in transplantation Nat Rev Nephrol 2012. 8339–347 [DOI] [PubMed] [Google Scholar]
- 13.Hariharan S, Johnson CP, Bresnahan BA. Improved graft survival after renal transplantation in the United States, 1988 to 1996 N Engl J Med 2000. 342605–612 [DOI] [PubMed] [Google Scholar]
- 14.Reese PP, Boudville N, Garg AX. Living kidney donation: outcomes, ethics, and uncertainty Lancet 2015. 3852003–2013 [DOI] [PubMed] [Google Scholar]
- 15.Lam NN, Lentine KL, Levey AS. Long-term medical risks to the living kidney donor Nat Rev Nephrol 2015. 11411–419 [DOI] [PubMed] [Google Scholar]
- 16.Pascual J, Zamora J, Pirsch JD. A systematic review of kidney transplantation from expanded criteria donors Am J Kidney Dis 2008. 52553–586 [DOI] [PubMed] [Google Scholar]
- 17.Rao PS, Ojo A. The alphabet soup of kidney transplantation: SCD, DCD, ECD—fundamentals for the practicing nephrologist Clin J Am Soc Nephrol 2009. 41827–1831 [DOI] [PubMed] [Google Scholar]
- 18.Morrissey PE, Monaco AP. Donation after circulatory death: current practices, ongoing challenges, and potential improvements Transplantation 2014. 97258–264 [DOI] [PubMed] [Google Scholar]
- 19.Daemen JW, Kootstra G, Wijnen RM, et al. Nonheart-beating donors: the Maastricht experience. Clin Transpl. 1994:303–316. [PubMed] [Google Scholar]
- 20.WHO, Transplantation Society (TTS), Organizatión Nacional de Transplantes (ONT). Third WHO Global Consultation on Organ Donation and Transplantation: striving to achieve self-sufficiency, March 23–25, 2010, Madrid, Spain. Transplantation. 2011;91:S27–S28. [DOI] [PubMed] [Google Scholar]
- 21.Reich DJ, Mulligan DC, Abt PL, et al. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant. 2009:2004–2011. [DOI] [PubMed] [Google Scholar]
- 22.van Heurn LW, Talbot D, Nicholson ML, et al. Recommendations for donation after circulatory death kidney transplantation in Europe. Transpl Int. 2015. [DOI] [PubMed] [Google Scholar]
- 23.Summers DM, Watson CJ, Pettigrew GJ. Kidney donation after circulatory death (DCD): state of the art Kidney Int 2015. 88241–249 [DOI] [PubMed] [Google Scholar]
- 24.Weber M, Dindo D, Demartines N. Kidney transplantation from donors without a heartbeat N Engl J Med 2002. 347248–255 [DOI] [PubMed] [Google Scholar]
- 25.Cho YW, Terasaki PI, Cecka JM. Transplantation of kidneys from donors whose hearts have stopped beating N Engl J Med 1998. 338221–225 [DOI] [PubMed] [Google Scholar]
- 26.Summers DM, Johnson RJ, Allen J. Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study Lancet 2010. 3761303–1311 [DOI] [PubMed] [Google Scholar]
- 27.Hamed MO, Chen Y, Pasea L. Early graft loss after kidney transplantation: risk factors and consequences Am J Transplant 2015. 151632–1643 [DOI] [PubMed] [Google Scholar]
- 28.Locke JE, Segev DL, Warren DS. Outcomes of kidneys from donors after cardiac death: implications for allocation and preservation Am J Transplant 2007. 71797–1807 [DOI] [PubMed] [Google Scholar]
- 29.Ortiz J, Gregg A, Wen X. Impact of donor obesity and donation after cardiac death on outcomes after kidney transplantation Clin Transplant 2012. 26E284–E292 [DOI] [PubMed] [Google Scholar]
- 30.Summers DM, Johnson RJ, Hudson A. Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: a cohort study Lancet 2013. 381727–734 [DOI] [PubMed] [Google Scholar]
- 31.Moers C, Smits JM, Maathuis MH. Machine perfusion or cold storage in deceased-donor kidney transplantation N Engl J Med 2009. 3607–19 [DOI] [PubMed] [Google Scholar]
- 32.O’Callaghan JM, Morgan RD, Knight SR. Systematic review and meta-analysis of hypothermic machine perfusion versus static cold storage of kidney allografts on transplant, outcomes Br J Surg 2013. 100991–1001 [DOI] [PubMed] [Google Scholar]
- 33.Jochmans I, O’Callaghan JM, Pirenne J. Hypothermic machine perfusion of kidneys retrieved from standard and high-risk donors Transpl Int 2015. 28665–676 [DOI] [PubMed] [Google Scholar]
- 34.Jochmans I, Moers C, Smits JM. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial Ann Surg 2010. 252756–764 [DOI] [PubMed] [Google Scholar]
- 35.Watson CJ, Wells AC, Roberts RJ. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: a UK multicenter randomized controlled trial Am J Transplant 2010. 101991–1999 [DOI] [PubMed] [Google Scholar]
- 36.Thuillier R, Allain G, Celhay O. Benefits of active oxygenation during hypothermic machine perfusion of kidneys in a preclinical model of deceased after cardiac death donors J Surg Res 2013. 1841174–1181 [DOI] [PubMed] [Google Scholar]
- 37.Hoyer DP, Gallinat A, Swoboda S. Influence of oxygen concentration during hypothermic machine perfusion on porcine kidneys from donation after circulatory death Transplantation 2014. 98944–950 [DOI] [PubMed] [Google Scholar]
- 38.Brasile L, Stubenitsky BM, Booster MH. Hypothermia—a limiting factor in using warm ischemically damaged kidneys Am J Transplant 2001. 1316–320 [DOI] [PubMed] [Google Scholar]
- 39.Hoyer DP, Gallinat A, Swoboda S. Subnormothermic machine perfusion for preservation of porcine kidneys in a donation after circulatory death model Transpl Int 2014. 271097–1106 [DOI] [PubMed] [Google Scholar]
- 40.Hosgood SA, Barlow AD, Yates PJ. A pilot study assessing the feasibility of a short period of normothermic preservation in an experimental model of non heart beating donor kidneys J Surg Res 2011. 171283–290 [DOI] [PubMed] [Google Scholar]
- 41.Nicholson ML, Hosgood SA. Renal transplantation after ex vivo normothermic perfusion: the first clinical study Am J Transplant 2013. 131246–1252 [DOI] [PubMed] [Google Scholar]
- 42.Kaths JM, Spetzler VN, Goldaracena N, et al. Normothermic ex vivo kidney perfusion for the preservation of kidney grafts prior to transplantation. J Vis Exp. 2015:e52909–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaths JM, Echeverri J, Goldaracena N, et al. Eight hour continuous normothermic ex vivo kidney perfusion is a safe preservation technique for kidney transplantation: a new opportunity for the storage, assessment and repair of kidney grafts. Transplantation, in press. 2016;108. [DOI] [PubMed] [Google Scholar]
- 44.Kaths JM, Echeverri J, Goldaracena N, et al. Heterotopic renal autotransplantation in a porcine model: a step-by-step protocol. J Vis Exp. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh SK, Kim SJ. Epidemiology of kidney discard from expanded criteria donors undergoing donation after circulatory death. Clin J Am Soc Nephrol. 2016: CJN.07190715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hauet T, Eugene M. A new approach in organ preservation: potential role of new polymers Kidney Int 2008. 74998–1003 [DOI] [PubMed] [Google Scholar]
- 47.van der Vliet JA, Kievit JK, Héné RJ. Preservation of non-heart-beating donor kidneys: a clinical prospective randomised case-control study of machine perfusion versus cold storage. Transplant Proc. 2001;33:847. doi: 10.1016/s0041-1345(00)02343-5. [DOI] [PubMed] [Google Scholar]
- 48.Moustafellos P, Hadjianastassiou V, Roy D. The influence of pulsatile preservation in kidney transplantation from non–heart-beating donors Transplant Proc 2007. 391323–1325 [DOI] [PubMed] [Google Scholar]
- 49.Plata-Munoz JJ, Muthusamy A, Quiroga I. Impact of pulsatile perfusion on postoperative outcome of kidneys from controlled donors after cardiac death Transpl Int 2008. 21899–907 [DOI] [PubMed] [Google Scholar]
- 50.Wight J, Chilcott J, Holmes M. The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors Health Technol Assess 2003. 71–94 [DOI] [PubMed] [Google Scholar]
- 51.Wight JP, Chilcott JB, Holmes MW. Pulsatile machine perfusion vs. cold storage of kidneys for transplantation: a rapid and systematic review Clin Transplant 2003. 17293–307 [DOI] [PubMed] [Google Scholar]
- 52.Gill J, Dong J, Eng M. Pulsatile perfusion reduces the risk of delayed graft function in deceased donor kidney transplants, irrespective of donor type and cold ischemic time Transplantation 2014. 97668–674 [DOI] [PubMed] [Google Scholar]
- 53.Gallinat A, Lüer B, Swoboda S. Use of the new preservation solution Custodiol-N supplemented with dextran for hypothermic machine perfusion of the kidney Cryobiology 2013. 66131–135 [DOI] [PubMed] [Google Scholar]
- 54.Minor T, Paul A, Efferz P. Kidney transplantation after oxygenated machine perfusion preservation with Custodiol-N solution Transpl Int 2015. 281102–1108 [DOI] [PubMed] [Google Scholar]
- 55.van der Wijk J, Slooff MJ, Rijkmans BG. Successful 96- and 144-hour experimental kidney preservation: a combination of standard machine preservation and newly developed normothermic ex vivo perfusion Cryobiology 1980. 17473–477 [DOI] [PubMed] [Google Scholar]
- 56.Rijkmans BG, Buurman WA, Kootstra G. Six-day canine kidney preservation. Hypothermic perfusion combined with isolated blood perfusion Transplantation 1984. 37130–134 [DOI] [PubMed] [Google Scholar]
- 57.Maessen JG, van der Vusse GJ, Vork M. The beneficial effect of intermediate normothermic perfusion during cold storage of ischemically injured kidneys. A study of renal nucleotide homeostasis during hypothermia in the dog Transplantation 1989. 47409–414 [DOI] [PubMed] [Google Scholar]
- 58.Brasile L, Green E, Haisch C. Warm ex vivo perfusion prevents reperfusion injury in warm ischemically damaged kidneys Transplant Proc 1997. 293422–3423 [DOI] [PubMed] [Google Scholar]
- 59.Brasile L, Clarke J, Green E. The feasibility of organ preservation at warmer temperatures Transplant Proc 1996. 28349–351 [PubMed] [Google Scholar]
- 60.Stubenitsky BM, Booster MH, Brasile L. Exsanguinous metabolic support perfusion—a new strategy to improve graft function after kidney transplantation Transplantation 2000. 701254–1258 [DOI] [PubMed] [Google Scholar]
- 61.Brasile L, Stubenitsky BM, Booster MH. NOS: the underlying mechanism preserving vascular integrity and during ex vivo warm kidney perfusion Am J Transplant 2003. 3674–679 [DOI] [PubMed] [Google Scholar]
- 62.Brasile L, Stubenitsky BM, Haisch CE. Repair of damaged organs in vitro Am J Transplant 2005. 5300–306 [DOI] [PubMed] [Google Scholar]
- 63.Brasile L, Stubenitsky BM. Breonics Inc. [Internet]. 2016 [cited 2016 Jan 4]. Available from: http://www.breonics.com/ems.html#clinical. [Google Scholar]
- 64.Bagul A, Hosgood SA, Kaushik M. Experimental renal preservation by normothermic resuscitation perfusion with autologous blood Br J Surg 2008. 95111–118 [DOI] [PubMed] [Google Scholar]
- 65.Hosgood SA, Bagul A, Kaushik M. Application of nitric oxide and carbon monoxide in a model of renal preservation Br J Surg 2008. 951060–1067 [DOI] [PubMed] [Google Scholar]
- 66.Hosgood SA, Nicholson ML. First in man renal transplantation after ex vivo normothermic perfusion Transplantation 2011. 92735–738 [DOI] [PubMed] [Google Scholar]
- 67.Jochmans I, Lerut E, van Pelt J. Circulating AST, H-FABP, and NGAL are early and accurate biomarkers of graft injury and dysfunction in a preclinical model of kidney transplantation Ann Surg 2011. 254784–791 [DOI] [PubMed] [Google Scholar]
- 68.Danpure CJ. Lactate dehydrogenase and cell injury Cell Biochem Funct 1984. 2144–148 [DOI] [PubMed] [Google Scholar]
- 69.Mahboub P, Ottens P, Seelen M. Gradual rewarming with gradual increase in pressure during machine perfusion after cold static preservation reduces kidney ischemia reperfusion injury. PLoS One. 2015;10:e0143859. doi: 10.1371/journal.pone.0143859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jochmans I, Moers C, Smits JM. The prognostic value of renal resistance during hypothermic machine perfusion of deceased donor kidneys Am J Transplant 2011. 112214–2220 [DOI] [PubMed] [Google Scholar]


