Abstract
Background
De novo use of mammalian target of rapamycin inhibitors after kidney transplantation is associated with a concentration-dependent incidence of wound healing adverse events (WHAE). The objective of this analysis was to compare the incidence of WHAE in patients receiving everolimus (EVR) or mycophenolate sodium (MPS).
Methods
This was a predefined subanalysis of a single-center prospective randomized study in which 288 kidney transplant recipients receiving tacrolimus and prednisone were randomized for 3 different regimens: rabbit antithymocyte globulin (r-ATG)/EVR (N = 85); basiliximab (BAS)/EVR (N = 102); BAS/MPS (N = 101). Clinical WHAE were prospectively collected using a prespecified case report form in all study visits. Abdominal ultrasound was performed at 30 days posttransplant to capture subclinical abnormalities. Surgeons were blinded to randomized treatment and no specific surgical procedures were implemented.
Results
A higher proportion of patients in BAS/EVR showed at least 1 clinical WHAE (22.3% vs 35.3% vs 22.0%, P = 0.03) and total clinical and subclinical WHAE (35% vs 42% vs 26%, P = 0.014) compared with BAS/MPS, respectively. A higher proportion of patients in r-ATG/EVR showed subclinical WHAE (13% vs 7% vs 4%, P = 0.025) compared with BAS/MPS, respectively. Patients receiving EVR showed a higher risk of developing clinical or subclinical WHAE (r-ATG/EVR vs BAS/MPS hazard ratio 1.30; BAS/EVR vs BAS/MPS hazard ratio 1.73, P = 0.028).
Conclusions
In this cohort of de novo kidney transplant recipients receiving tacrolimus and prednisone, the use of EVR was associated with higher incidence of combined clinical and subclinical WHAE compared with MPS.
Wound healing adverse events (WHAE) are considered the most common type of posttransplant surgical complication and a major cause of morbidity.1 This adverse event is associated with delayed hospital discharge, rehospitalization, and increased costs.1,2 Immunosuppressive regimens, containing mammalian target of rapamycin inhibitors (mTORi), everolimus (EVR), and sirolimus (SRL), have demonstrated efficacy for the prevention of acute rejection but have been associated with increased incidence of WHAE compared with mycophenolate.2
These drugs target signal transduction pathways involved in cell-cycle progression, inhibiting even fibroblast proliferation.1,3 These effects may be further exacerbated by its ability to inhibit angiogenesis.2 All these data in addition to previous clinical trials experiences presupposed the association between mTORi use and WHAE.3 However, in the last years, the dosing regimens for mTORi have evolved, with growing avoidance of loading doses and adoption of concentration-controlled dosing, challenging the veracity of the relationship previously established.2,3
To evaluate the real impact of mTORi on the main aspects of wound healing, we prospectively compared patients who received 2 different de novo EVR-based immunosuppression regimens to patients receiving a standard regimen based on mycophenolate sodium (MPS).
MATERIALS AND METHODS
Study Design
This was a prospective, randomized, single-center trial comparing the efficacy and safety of 2 new immunosuppressive treatments with the current standard of care therapy. Detailed methodology has been published previously.4 The study was approved by the local institutional review board, and it was conducted according to good clinical practices and the Declaration of Helsinki guidelines. All subjects signed an approved written informed consent before enrollment, and they were monitored for 12 months after transplantation. The core study was registered at ClinicalTrials.gov number NCT01354301.
Study Endpoints
The primary endpoint of this analysis was the incidence of patients with at least 1 clinical WHAE. Secondary endpoints included the incidence of patients who required surgical intervention due to WHAE, subclinical WHAE detected by abdominal ultrasound, total incidence of clinical and subclinical WHAE, and risk factors associated with the development of WHAE.
Population
The study included 288 recipients of living or deceased donor kidney transplants performed between July 11, 2011, and May 7, 2013. Eligible patients underwent randomization after the transplant surgery stratified according to donor type (living or deceased donor).
Surgical Technique
All transplants were performed by 1 of 13 trained surgeons. The number of surgical procedures per surgeon ranged from 1 to 57, with no differences according to treatment allocation. All surgeons used similar technique, including muscle-sparing modified Gibson incision and extraperitoneal approach to place the kidney in the iliac fossa. The lymphatic vessels were ligated with cotton 3-0 sutures and end-to-side vascular anastomoses preferably to common iliac vessels with polypropylene 6-0 sutures. The type of ureteral anastomosis and the use of drains were left at the discretion of the surgeon, including the use of double-J ureteral stent. Nonabsorbable continuous suture was used in all cases for reapproximation of the posterior and anterior rectus abdominis fascial layers. The subcutaneous tissue was approximated using continuous absorbable suture, and the skin was closed with nonabsorbable stitches. All patients received prophylaxis against wound infections consisted of intravenous cefazolin 2 g during the surgery followed by 4 doses of 1 g every 8 hours. Surgeons were not aware of treatment allocation, and therefore only routine standard surgical procedures were used in all patients.
Immunosuppression
In the first group (rabbit antithymocyte globulin [r-ATG/EVR], n = 85), patients received a single intravenous 3 mg/kg dose of r-ATG followed by oral doses of tacrolimus (TAC) adjusted to maintain whole blood trough concentration below 5 ng/mL, oral doses of EVR to maintain whole blood trough concentrations between 4 and 8 ng/mL and prednisone. In the second group (basiliximab [BAS]/EVR, n = 102), patients received 2 intravenous 20 mg doses of BAS at days 0 and 4, TAC doses adjusted to maintain whole blood trough concentration around 6 ng/mL for the first 3 months then reduced below 5 ng/mL, EVR doses adjusted to maintain whole blood trough concentrations between 4 and 8 ng/mL and prednisone. In the third group (BAS/MPS, n = 101), patients received 2 intravenous 20 mg dose of BAS at days 0 and 4, TAC doses adjusted to maintain whole blood trough concentration between 6 and 8 ng/mL, MPS (1440 mg/d), and prednisone. Changes in the initial randomized immunosuppressive therapy were permitted either due to lack of efficacy or adverse events. All drugs were started within 24 hours of graft revascularization.
Corticosteroids
All patients received a single 1-g intravenous dose of methylprednisolone (MP) before graft revascularization. Patients in r-ATG/EVR group also received 300 mg of hydrocortisone before r-ATG infusion. Within 24 hours, all patients received 0.5 mg/kg oral dose (maximum of 30 mg) of prednisone that was reduced to 20 mg/d by day 7, 15 mg/d by day 14, 10 mg/d by day 21, and 5 mg/d by day 30, which was maintained up to 12 months.
Wound Healing Adverse Events
The wound incision was inspected daily by a study designated transplant physician during hospital stay and during the study visits at outpatient clinic. All WHAE, symptomatic or not, were individually and prospectively identified and classified according to common terminology criteria for adverse events with the assistance of a pharmacist using a special template form developed for this purpose. In this form, onset date, best terminology according to common terminology criteria for adverse events, grade of severity, treatment, need for hospitalization, and end date were collected. Imaging tests were performed to confirm clinical diagnosis when necessary. A scheduled abdominal ultrasound was performed in all patients 30 days after the transplant surgery to detect subclinical WHAE. To compare the incidence of WHAE among the 3 groups, all events were grouped as wound healing dehiscence, wound healing infection, incisional hernia, lymphorrhea, fluid collections, perigraft hematoma, and urine leak. All fluid collections were diagnosed by either ultrasound examination or computed tomography (CT) and were categorized according to the composition as hematomas or fluid collections. Wound dehiscence was defined as any spontaneous separation in the skin and/or fascia. Urine leaks were defined by the presence of a high concentration of creatinine relative to plasma or in drainage fluid. Each complication was reviewed with respect to the presence or absence of surgical site infection, confirmed by cultures and treatment with specific antibiotics.
Clinical Events During the First Month
All episodes of acute rejection were confirmed by histological analysis and categorized according the Banff 2009 classification.5 Mild and moderate acute rejection episodes (Banff IA or IB) were treated with 0.5 to 1 g methylprednisolone for 1 to 5 days while severe episodes (Banff IIA, IIB, III) were treated with 1 mg/kg doses of r-ATG for 7 to 14 days. All causes of graft losses and deaths were recorded. The value of creatinine at day 30 after transplantation was used to calculate the estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease formula.6
Statistical Analysis
Descriptive analyses were summarized as mean and standard deviation or proportions. Categorical variables were compared using χ2 test. Continuous variables were compared using analysis of variance and Kruskal-Wallis test according to data distribution. The cumulative incidences of first clinical WHAE and first clinical and subclinical WHAE were calculated using the Kaplan-Meier method, and the differences between the groups were identified using the Log Rank test. Hazard ratios and 95% confidence intervals were calculated comparing each of the EVR groups with the MPS group. For the purposes of this analysis, all corticosteroids doses were normalized to equivalent doses of prednisone.7 Univariable and multivariable logistic regressions analyses were performed to identify risk factors associated with WHAE. Variables included in the model were: (1) recipient: sex, age, race, abdominal circumference, body mass index (BMI), cause of chronic renal disease, duration of dialysis, and dialysis modality; (2) donor: age and type (living or deceased); (3) transplant: cold ischemia time, type of ureteral anastomosis and number of surgeries per surgeon; (4) immunosuppression: type of induction agent (r-ATG vs BAS), adjunctive therapy (EVR vs MPS), median whole blood concentrations of EVR and average prednisone dose during month 1; (5) transplant outcomes: incidence and duration of DGF, treated acute rejection within the first month, and renal function eGFR at 30 days. Variables associated with clinical and subclinical WHAE on univariable analyses (P values < 0.10) and other clinically relevant covariates associated with wound healing in previous studies were included in the multivariable logistic regression modeling. All statistical analyses were performed using the SPSS 18 standard software (SPSS Inc., Chicago, IL), and differences between the groups were considered significant at the a P value less than 0.05.
RESULTS
Patient Population
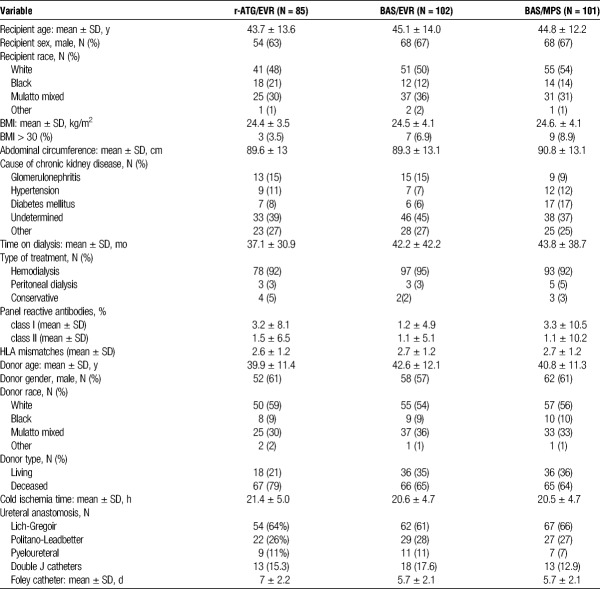
The demographic characteristics of the study population are presented in Table 1. Overall, the study population was constituted by relatively young, white, and nonobese patients with mean time on dialysis over 3 years. Prevalence of diabetes mellitus was low, and 69% of the patients received grafts from deceased donors.
TABLE 1.
Demographic characteristics of the study population
In relation to surgical aspects, the most frequent ureteral anastomosis technique used was Lich-Gregoir (64%) with low overall use of double “J” catheters (15%). Altogether, 80 (94%), 94 (92%), and 90 (89%) patients completed 12 months of follow-up in ATG/EVR, BAS/EVR, and BAS/MPS, respectively.
Wound Healing Adverse Events
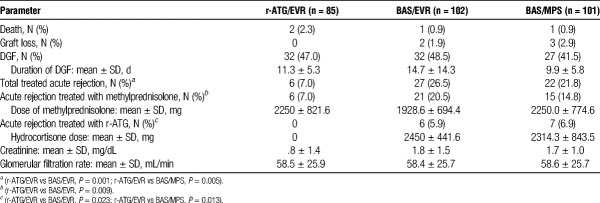
Overall, 26.7% of the patients presented at least 1 clinical WHAE. Higher incidence was observed in the BAS/EVR group compared with BAS/MPS group (35.3 vs 22.0%, P = 0.033, Table 2), but the cumulative relative risk did not reach statistical significance (Figure 1A). Furthermore, there was a higher number of patients with more than 1 clinical WHAE (4 vs 18 vs 11) and higher number of clinical WHAE (28 vs 57 vs 38) in BAS/EVR group. On the other hand, the proportion of patients requiring 1 (11.8% vs 10.8% vs 10.9%, P = 0.974) or more surgical reinterventions was similar among the groups. Of 123 clinical WHAE, 81 did not require surgical intervention and were represented primarily by fluid collections (42.9%) and wound infections (21.0%). Of 42 clinical WHAE that required surgical reintervention, the most frequent was urinary leak, with an overall incidence of 6.3% (Table 2).
TABLE 2.
Wound healing adverse events
FIGURE 1.
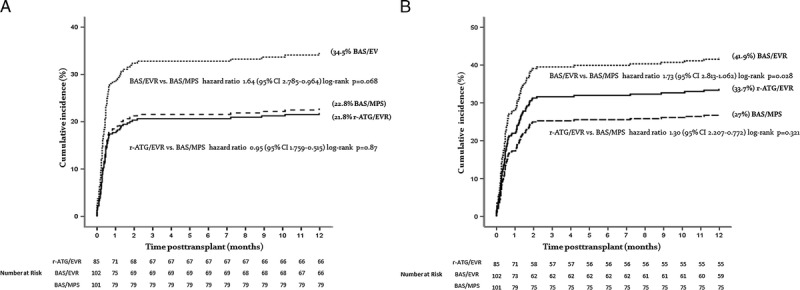
A, Cumulative incidence of clinical wound healing adverse events during the first year after kidney transplantation. B, Cumulative incidence of clinical and subclinical wound healing adverse events during the first year after kidney transplantation.
Abdominal ultrasound was performed in 99.6% of patients who completed 30 days of transplant. A higher proportion of patients with subclinical WHAE was observed in the r-ATG/EVR compared with BAS/MPS group (13 vs 4%, P = 0.025), respectively. The most frequent findings were fluid collections (Table 2). The proportion of patients with clinical or subclinical WHAE was higher in the BAS/EVR compared with BAS/MPS group (42 vs 26%, P = 0.014), respectively, with a cumulative risk of 1.73 (Figure 1B). Finally, a total of 145 clinical and subclinical WHAE were recorded, with a higher number observed in the BAS/EVR group. Even pooling together data from r-ATG/EVR and BAS/EVR groups, a significant difference in WHAE compared with BAS/MPS (P = 0.163) was not detected. Summary of the clinical characteristics of all WHAE, including type, severity, treatment, need for hospital readmission and surgical intervention are shown in Table S1 (SDC, http://links.lww.com/TP/B325). Independent risk factors for the development of clinical and subclinical WHAE were BMI (odds ratio [OR], 1.03; P < 0.01), abdominal circumference (OR, 1.03; P < 0.01), use of EVR (OR, 1.84; P = 0.03), and higher average EVR whole blood trough concentration (OR, 1.84; P = 0.03). Using multivariable logistic regression analysis, no association between independent clinical variables with the incidence of clinical and subclinical WHAE was observed, perhaps due to the small sample size.
Immunosuppression
Tacrolimus doses (5.6 ± 2.9 mg/d vs 7.7 ± 4.9 mg/d vs 8.0 ± 4.5 mg/d) and concentrations (5.1 ± 1.7 ng/mL vs 7.4 ± 2.6 ng/mL vs 8.1 ± 3.1 ng/mL) at 30 days were lower in the r-ATG/EVR compared with BAS/MPS group, respectively. There were no differences in EVR doses (3.7 ± 0.8 mg/d vs 3.7 ± 1.5 mg/d) and concentrations (5.2 ± 2.1 ng/mL vs 4.5 ± 1.5 ng/mL) at 30 days in r-ATG/EVR and BAS/EVR groups, respectively. Doses of MPS were reduced during the first month but no significant changes were observed in MPA plasma concentration. There were no differences in mean prednisone doses at days 7, 14, 21, and 30. In addition, no significant difference was observed comparing average of normalized prednisone dose during the first month (68.9 ± 26.4 mg/d vs 78.8 ± 36.5 mg/d vs 76.1 ± 36.9 mg/d; P = 0.056). Data regarding doses and concentrations of the immunosuppressive drugs are shown in Table S2 (SDC, http://links.lww.com/TP/B325).
Clinical Outcomes During the First 30 Days
There were 4 deaths and 5 graft losses during the first month after transplantation. Among them 3 patients had developed WHAE, 1 in each group (Table 3). The overall incidence of delayed graft function was 46% with no significant differences among the groups. Lower incidence of acute rejection was observed in r-ATG/EVR group. No significant difference in mean methylprednisolone doses per patient was observed among the 3 groups. Finally, we did not observe any difference in mean eGFR 30 days after transplantation.
TABLE 3.
Clinical outcomes during the first 30 days after transplantation
DISCUSSION
This is the first prospective controlled study to evaluate wound healing complications comparing 2 EVR containing regimens with mycophenolate in de novo kidney transplant recipients receiving TAC. The incidence of surgical complications was 26.7%, within the range of previous reported incidences of 15% to 32%.2 Including clinical and subclinical WHAE, the observed incidence of 34.3% is lower than that observed in another study with similar design, ranging from 40% to 41.9%.8 The incidence of major ureteric complications (6.2%) and overall reinterventions (11.1%) were within previously reported ranges.9,10
A direct comparison indicates that patients receiving EVR showed higher number of WHAE compared to those receiving MPS. Although patients receiving r-ATG/EVR showed similar incidence of clinical WHAE compared to the standard of care therapy, higher incidence was observed in patients receiving BAS/EVR. Interestingly, the proportion of patients with more than 1 WHAE was lower among patients receiving r-ATG/EVR. Importantly, the proportion of patients with WHAE leading to surgical reinterventions was essentially the same among the 3 groups. On the other hand, subclinical WHAE were observed more frequently in both EVR treatment groups. Overall, among clinical and subclinical WHAE, the differences between EVR and MPS groups were largely due to fluid collections, which accounted for a small fraction of surgical reinterventions.
The higher incidence of clinical WHAE in the BAS/EVR group compared with r-ATG/EVR group is intriguing because no difference in mean EVR concentrations during the first month was observed between these 2 groups. It was speculated if the higher incidence of acute rejection episodes treated with methylprednisolone could account for this observation. Nevertheless, multivariable analysis did not identify treatment of rejection or cumulative dose of steroids during the first 30 days as independent covariates associated with WHAE.
Several risk factors have been associated with the incidence and severity of surgical complications.11,12 Among demographic risk factors associated with wound healing reported in the scientific literature,2,3 BMI and abdominal circumference were associated with clinical and subclinical WHAE on univariable analysis in our study. White recipient race,11 BMI,1,13 and diabetes14,15 have also been associated with WHAE in previous studies. Nevertheless, none of these demographic characteristics was associated with WHAE after multivariable analysis, perhaps due to the low prevalence of patients with diabetes and high BMI in our study cohort.
The use of r-ATG has been associated with an increase incidence of WHAE.16,17 The effect of thymoglobulin reduces peripheral T cells, which significantly influence impair breaking strength of wounds and reduce collagen disposition.11 In our study, we were unable to associate the use of r-ATG with increased incidence of WHAE, perhaps because a single 3 mg/kg dose was used. Mycophenolate is potent inhibitor of fibroblast proliferation and has been associated with higher incidence of WHAE compared with azathioprine.2,14 Several studies indicate that use of corticosteroids is another factor that affects wound healing processes.2,18,19 Finally, among clinical outcomes, delayed graft function20,21 and acute rejection22 have been associated with increased incidence of WHAE.
The de novo use of mTORi has been associated with increased incidence of WHAE.2 Yet, this observation occurred primarily in early trials in patients receiving high loading and maintenance doses of sirolimus.10 Since then, the de novo use of mTORi evolved considerably over time, with most centers adopting a concentration-controlled synergistic dosing strategy with low exposure of both calcineurin and mTORi.1,23 A pooled analysis of data from 3 prospective, multicenter trials with similar design including 1996 de novo kidney transplant recipients receiving cyclosporine showed that the incidence of WHAE by day 90 was higher in patients receiving EVR 3.0 mg/d but not in those receiving EVR 1.5 mg/d, compared with patients receiving mycophenolate (21.8 vs 16.6% vs 14.3%) Importantly, the risk of any wound healing complication was associated with increasing EVR concentrations.15 In our study, the use of EVR or its concentration during the first month was not independently associated with higher incidence of clinical and subclinical events WHAE after multivariable analysis.
Finally, early conversion strategies have been used to avoid WHAE associated with de novo use of mTORi. Nevertheless, in a small prospective study comparing immediate (n = 65) or delayed (from week 5, n = 74) use of EVR in de novo kidney transplant recipients receiving cyclosporine, the incidence of any WHAE by week 4 was higher among patients receiving mycophenolate (24.6% vs 33.8%). From week 4 til month 3, the incidence was higher in the immediate versus delayed group (15.4% and 8.1%), reaching similar cumulative incidence by month 3 (40.0 vs 41.9%), respectively.23 In another prospective open-label, multicenter, randomized trial, early conversion from a cyclosporine-based to a sirolimus-based regimen 10 to 24 days after renal transplantation showed no significant differences in the incidence of lymphocele (23.9% vs 27.5%), technical surgical complications (9.9% vs 10.1%), or wound healing disorders (11.3% vs 10.1%), respectively.24 Three other prospective randomized trials investigating the efficacy and safety of early conversion from CNI to mTORi did not analyze in detail the incidence of WHAE.25–27 Although the incidence of wound healing events would be intuitively lower if mTORi were started after 30 days, the data provided in these studies do not allow us to provide robust scientific evidence.
Limitations
There are limitations in the interpretation and extrapolation of the results of this study. It is a single-center study despite the fact that 13 surgeons were involved. The study has no sufficient power to detect differences in WHAE among the 3 groups, as sample size was not calculated for this primary objective as higher incidence of combined clinical and subclinical WHAE were observed in both EVR groups compared with mycophenolate after univariable but not after multivariable analysis. The low prevalence of patients with diabetes and higher BMI also preclude direct extrapolation to populations with diverse demographic characteristics.
CONCLUSIONS
In summary, in this cohort of de novo kidney transplant recipients receiving TAC and prednisone, the use of EVR was associated with higher incidence of combined clinical and subclinical WHAE compared with MPS.
Footnotes
Clinical Trial Notation: Efficacy and Safety of Induction Strategies Combined With Low Tacrolimus Exposure in Kidney Transplant Recipients Receiving Everolimus or Sodium Mycophenolate. Number NCT01354301.
The following authors of this manuscript have conflicts of interest to disclose: H.T.-S. has received speaker’s fees travel or accommodation expenses for development of educational presentations scientific advice from Novartis, Pfizer Roche. Jose Medina-Pestana has received speaker’s fees travel or accommodation expenses for development of educational presentations scientific advice from Bristol- Myers Squibb, Novartis, Pfizer Roche. Claudia Felipe has received speaker’s fees for development of educational presentations travel or accommodation expenses from Novartis Pfizer. Marina Cristelli has received speaker’s fees for development of educational presentations travel or accommodation expenses from Novartis Pfizer. The institution, Hospital do Rim, has received Research Grants for clinical studies from Novartis, Pfizer, Astellas, Bristol-Myers Squibb, Roche LifeCycle Pharma.
P.U., C.F., H.T.-S., J.M.-P. participated in research design. P.U., C.F., A.F., M.C., L.V., J.M., G.B., P.H., W.A., H.T.-S., J.M.-P. participated in the writing of the article. P.U. C.F., A.F., M.C., L.V., J.M., G.B., P.H., H.T.-S., J.M.-P. participated in the performance of the research. P.U., C.F., H.T.-S., J.M.-P. participated in data analysis.
Correspondence: Helio Tedesco-Silva Jr, MD, Nephrology Division, Hospital do Rim, Universidade Federal de São Paulo, Rua Borges Lagoa, 960-11° Andar, Zip code: 04038-002, São Paulo, Brazil. (heliotedesco@medfarm.com.br).
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
In a single-center prospective randomized study, 288 de novo kidney transplant recipients receiving everolimus, tacrolimus and prednisone show higher incidence of combined clinical and subclinical wound healing adverse events compared to mycophenolate sodium. Supplemental digital content is available in the text.
REFERENCES
- 1.Mehrabi A, Fonouni H, Wente M. Wound complications following kidney and liver transplantation Clin Transplant 2006. 2097–110 [DOI] [PubMed] [Google Scholar]
- 2.Nashan B, Citterio F. Wound healing complications and the use of mammalian target of rapamycin inhibitors in kidney transplantation: a critical review of the literature Transplantation 2012. 94547–561 [DOI] [PubMed] [Google Scholar]
- 3.Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors Transplant Rev (Orlando 2014. 28126–133 [DOI] [PubMed] [Google Scholar]
- 4.Tedesco-Silva H, Felipe C, Ferreira A. Reduced incidence of cytomegalovirus infection in kidney transplant recipients receiving everolimus and reduced tacrolimus doses Am J Transplant 2015. 152655–2664 [DOI] [PubMed] [Google Scholar]
- 5.Sis B, Mengel M, Haas M. Banff ’09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups Am J Transplant 2010. 10464–471 [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Bosch JP, Lewis JB. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group Ann Intern Med 1999. 130461–470 [DOI] [PubMed] [Google Scholar]
- 7.Asare K. Diagnosis and treatment of adrenal insufficiency in the critically ill patient Pharmacotherapy 2007. 271512–1528 [DOI] [PubMed] [Google Scholar]
- 8.Albano L, Berthoux F, Moal MC. Incidence of delayed graft function and wound healing complications after deceased-donor kidney transplantation is not affected by de novo everolimus Transplantation 2009. 8869–76 [DOI] [PubMed] [Google Scholar]
- 9.Dalgic A, Boyvat F, Karakayali H. Urologic complications in 1523 renal transplantations: the Baskent University experience Transplant Proc 2006. 38543–547 [DOI] [PubMed] [Google Scholar]
- 10.Valente JF, Hricik D, Weigel K. Comparison of sirolimus vs. mycophenolate mofetil on surgical complications and wound healing in adult kidney transplantation Am J Transplant 2003. 31128–1134 [DOI] [PubMed] [Google Scholar]
- 11.Knight RJ, Villa M, Laskey R. Risk factors for impaired wound healing in sirolimus-treated renal transplant recipients Clin Transplant 2007. 21460–465 [DOI] [PubMed] [Google Scholar]
- 12.Seow YY, Alkari B, Dyer P. Cold ischemia time, surgeon, time of day, and surgical complications Transplantation 2004. 771386–1389 [DOI] [PubMed] [Google Scholar]
- 13.Dean PG, Lund WJ, Larson TS. Wound-healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus Transplantation 2004. 771555–1561 [DOI] [PubMed] [Google Scholar]
- 14.Humar A, Ramcharan T, Denny R. Are wound complications after a kidney transplant more common with modern immunosuppression? Transplantation 2001. 721920–1923 [DOI] [PubMed] [Google Scholar]
- 15.Cooper M, Wiseman AC, Zibari G. Wound events in kidney transplant patients receiving de novo everolimus: a pooled analysis of three randomized controlled trials Clin Transplant 2013. 27E625–E635 [DOI] [PubMed] [Google Scholar]
- 16.Benavides C, Mahmoud KH, Knight R. Rabbit antithymocyte globulin: a postoperative risk factor for sirolimus-treated renal transplant patients? Transplant Proc 2005. 37822–826 [DOI] [PubMed] [Google Scholar]
- 17.Pourmand GR, Dehghani S, Saraji A. Relationship between post-kidney transplantation antithymocyte globulin therapy and wound healing complications Int J Organ Transplant Med 2012. 379–84 [PMC free article] [PubMed] [Google Scholar]
- 18.Anstead GM. Steroids, retinoids, and wound healing Adv Wound Care 1998. 11277–285 [PubMed] [Google Scholar]
- 19.Rogers CC, Hanaway M, Alloway RR. Corticosteroid avoidance ameliorates lymphocele formation and wound healing complications associated with sirolimus therapy Transplant Proc 2005. 37795–797 [DOI] [PubMed] [Google Scholar]
- 20.Tiong HY, Flechner SM, Zhou L. A systematic approach to minimizing wound problems for de novo sirolimus-treated kidney transplant recipients Transplantation 2009. 87296–302 [DOI] [PubMed] [Google Scholar]
- 21.Flechner SM, Zhou L, Derweesh I. The impact of sirolimus, mycophenolate mofetil, cyclosporine, azathioprine, and steroids on wound healing in 513 kidney-transplant recipients Transplantation 2003. 761729–1734 [DOI] [PubMed] [Google Scholar]
- 22.Goel M, Flechner SM, Zhou L. The influence of various maintenance immunosuppressive drugs on lymphocele formation and treatment after kidney transplantation J Urol 2004. 1711788–1792 [DOI] [PubMed] [Google Scholar]
- 23.Dantal J, Berthoux F, Moal MC. Efficacy and safety of de novo or early everolimus with low cyclosporine in deceased-donor kidney transplant recipients at specified risk of delayed graft function: 12-month results of a randomized, multicenter trial Transpl Int 2010. 231084–1093 [DOI] [PubMed] [Google Scholar]
- 24.Guba M, Pratschke J, Hugo C. Early conversion to a sirolimus-based, calcineurin-inhibitor-free immunosuppression in the SMART trial: observational results at 24 and 36 months after transplantation Transpl Int 2012. 25416–423 [DOI] [PubMed] [Google Scholar]
- 25.Weir MR, Mulgaonkar S, Chan L. Mycophenolate mofetil-based immunosuppression with sirolimus in renal transplantation: a randomized, controlled Spare-the-Nephron trial Kidney Int 2011. 79897–907 [DOI] [PubMed] [Google Scholar]
- 26.Budde K, Becker T, Arns W. Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: an open-label, randomised, controlled trial Lancet 2011. 377837–847 [DOI] [PubMed] [Google Scholar]
- 27.Silva HT, Felipe CR, Garcia VD. Planned randomized conversion from tacrolimus to sirolimus-based immunosuppressive regimen in de novo kidney transplant recipients Am J Transplant 2013. 133155–3163 [DOI] [PubMed] [Google Scholar]