Abstract
Background
Single antigen beads (SAB) are used for monitoring HLA antibodies in pretransplant and posttransplant patients despite the discrepancy between virtual and actual crossmatch results and transplant outcomes. This discrepancy can be attributed to the presence of conformational variants of HLA-I on SAB, assessment of which would increase the concordance between SAB and flow cytometry crossmatch (FCXM) results, thus enabling improved organ accessibility for the waiting list patients and a better prediction of antibody-mediated rejection.
Methods
The conformational variants were examined on HLA-I beads, iBeads, acid-/alkali-treated beads, and T cells using HLA-I monoclonal antibodies (W6/32, TFL-006, and heavy chain (HC)-10).
Results
The affinity of the monoclonal antibodies against HLA-I beads confirmed the presence and heterogeneous density of peptide-associated β2-microglobulin–associated HLA HC (pepA-β2aHC), peptide-free-β2aHC (pepF-β2aHC), and β2-free HC (β2fHC) on every single antigen-coated bead. In contrast, iBeads harbor a high density of pepA-β2aHC, low density of pepF-β2aHC, and are lacking β2fHC. The FCXM analyses confirmed the prevalence of pepA-β2aHC, but not pepF-β2aHC or β2fHC on resting T cells.
Conclusions
The strength of a donor-specific antibody should be assessed with a bead-specific mean fluorescence intensity cutoff based on TFL-006 reactivity against HLA-I beads, and HC-10 against iBeads, where the β2fHC or pepF-β2aHC normalized donor-specific antibody level would reveal the true anti–pepA-β2aHC reactivity associated with positive FCXM.
HLA-I and HLA-II antibodies are monitored with a multitude of techniques, including Luminex multiplex HLA-coated single antigen beads (SAB), complement-dependent cytotoxicity (CDC), and flow cytometry (FC) crossmatching (XM) for preexisting and de novo donor-specific antibodies (DSA) in transplant patients.1,2 Although increased sensitivity and accuracy of SAB is beneficial for clinical monitoring of HLA antibodies and for performing virtual XMs to improve organ allocation on highly sensitized patients,3–5 CDCXM are more reliable and have higher specificity in predicting acute antibody-mediated rejection (AMR).6 The increased sensitivity of SAB has denied transplants to a growing number of sensitized patients on waiting lists, due to the inability to distinguish between clinically relevant and irrelevant HLA antibodies.7
HLA-I SAB are coated with both β2-microglobulin (β2m)-associated HLA heavy chain (β2aHC) and β2m-free HLA heavy chain (β2fHC).8,9 Anti-β2fHC HLA-I antibodies were identified in nonalloimmunized men and in cord blood.8,10 The prevalence of anti-β2fHC antibodies in HLA-I–sensitized patients awaiting a donor kidney was 39% (which accounted for 6% of the HLA-I antibodies detected), these antibodies did not correlate with a positive FCXM11 because the β2aHC variant is the most prevalent HLA-I expressed on normal cells, tissues, and donor organs. Anti-β2fHC DSA are found in 20% of renal allograft recipients, but only anti-β2aHC DSAs were predictive of graft failure.12 iBeads, devoid of β2fHC, detected mostly anti-β2aHC.13 Using iBeads in conjunction with HLA-I and acid-treated beads, the prevalence of anti-β2fHC DSA was found to be 12%, which least affected renal allograft survival.9 In another cohort, 11% of recipients had anti-β2fHC DSA. None of the patients with only anti-β2fHC DSA displayed positive T-cell FCXMs, nor did they develop acute AMR in the first year or suffer deleterious effects during prolonged graft survival.14
Antibodies to both β2aHC and β2fHC HLA-I variants are indiscriminately considered unacceptable for organ allocation, despite their differential immunologic risk. Unless the conformational variants for each one of the HLA-I antigens represented in the SAB’s panel are characterized and their relative density assessed, neither the DSA “strength” (determined by mean fluorescence intensity [MFI]) nor the MFI cutoff (used to correlate DSA “strength” to AMR) may distinguish the pathogenic DSAs that recognize β2aHC expressed by the allograft in vivo.
The distribution and density of HLA-I conformational variants which may appear on HLA-I beads, iBeads and acid-treated beads may be revealed by measuring the relative binding of well-characterized anti–HLA-I monoclonal antibodies (mAbs) to these variants. The results of this investigation will determine the reactivity of SAB against anti-β2aHC or β2fHC DSAs and will validate whether SAB other than conventional HLA-I beads are needed for distinguishing pathogenic from nonpathogenic HLA-I DSA.
MATERIALS AND METHODS
Monoclonal Antibodies
W6/32 (IgG2a) reacted with a wide range of cells (except for Daudi Burkitt lymphoma, lacking β2aHCs). It also immunoprecipitated both a 43kDA HLA heavy chain (HC) and a 12-kDa β2m.15 W6/32 formed immune complexes with β2aHC but not with β2fHC16 and bound to both peptide-associated (pepA) β2aHC and peptide-free (pepF) β2aHC.17,18 We obtained W6/32 from One Lambda (Canoga Park, CA).
HC-10, (IgG2a) developed by immunization with the β2fHC of HLA-B7 and HLA-B40,19 immunoprecipitated β2fHC from the cell lysates. The epitope of HC-10 is identified between amino acid positions 57 and 62 of the HLA α1 HC; arginine at position 62 (R62) is crucial for HC-10 binding.20 HC-10 repeatedly immunoprecipitated a small fraction of β2aHC from cell lysate.21 HC-10 recognized cell surface β2aHC devoid of a peptide (pepF-β2aHC), whereas the presence of peptide reduced the HC-10 reactivity.22 HC-10 was purchased from Nordic MUbio (Susteren, Netherlands, Cat MUB2037P).
TFL-006 (IgG2a) was developed by immunizing a properly folded HLA-E β2fHC.23–25 TFL-006 bound to β2fHC of both HLA-E and HLA-Ia and was inhibited by peptides from the amino acid sequences shared by all HLA-I antigen-coated beads and masked by β2m. TFL-006 was IgG-purified from ascites fluids.
SAB Luminex-Based Immunoassay
The HLA-I reactivity of the aforementioned mAbs was screened using LabScreen SAB multiplex Luminex Flow cytometry.2 The SAB used are: (i) HLA-I beads (Cat LS1A04, Lot 8 and Lot 9, One Lambda); (ii) iBeads (One Lambda)23; (iii) acid-treated beads, generated by incubating the HLA-I beads in 0.1 M glycine buffer (pH = 2.8) with 1% bovine serum albumin for 30 minutes, then washed thrice with LabScreen Wash Buffer9,11,14; (iv) alkali-treated beads, generated by incubating the HLA-I beads in 50 mM Tris-HCl (pH = 8.0) with 6 M guanidium chloride for 30 minutes, then washed thrice as previously described.26
All SAB kits covered the same panel of HLA-I molecules (31 HLA-A, 50 HLA-B, and 16 HLA-Cw antigens). The mAbs were tested on the same day and the same tray for all 4 SAB sets. Although all mAbs were titrated (10, 5, 2.5 μg/mL) to assess the optimal concentration saturating the SAB, only the data at 10 μg/mL is presented.
The Luminex immunoassay involved incubation of 20 μL of mAbs with 2 μL of SAB for 30 minutes at room temperature. The SAB were then washed thrice with LabScreen Wash Buffer. The mAbs binding were monitored using phycoerythrin-conjugated Goat Anti-Mouse IgG2a (γ2a chain specific; Concentration: 0.5 mg/mL; Cat 1080-09; SouthernBiotech, Birmingham, AL), diluted 1 to 100 in wash buffer. The SAB were incubated with 50 μL of the second antibody for 30 minutes at room temperature. The SAB were washed thrice as previously described, then resuspended in 1× phosphate buffered saline before acquisition. For each sample, at least 100 beads were counted. The IgG reactivity against each HLA-I antigens were recorded as Trimmed MFI. The MFI values were normalized against the negative control bead (bead 1) and sample (1× PBS). The MFI cutoff used for a positive reaction was 1000.
T-Cell FCXM Assay
On the day of the FCXM assay, 40 mL of whole blood from a healthy male (HLA-I typing: A*11:01/A*24:02, B*15:10/B*44:05, Cw*02:02/Cw*07:04, using LABType SSO Typing Test Kit (One Lambda, following manufacturer’s instructions) was collected after obtaining consent and institutional review committee approval. T cells were isolated using Dynabeads (Invitrogen by Life Technologies, Carlsbad, CA, Cat 11344D), following manufacturer’s negative selection protocol.
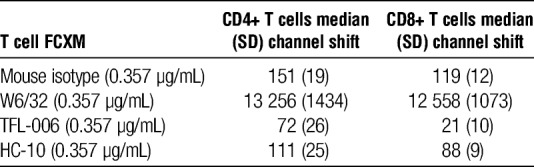
The purified T cells were aliquoted (2 × 105 cells) to a U-bottom 96-well tissue culture plate and washed thrice with serum-free AIM-V Medium (Life Technologies-Gibco, Cat 12055-083) with 1% 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH = 7.4). Before staining, the Fc receptors were blocked using Human TruStain FcX (BioLegend, San Diego, CA, Cat 422302). Seventy microliters of primary mAb (W6/32 or TFL-006 or HC-10; concentration, 0.357 μg/mL), were added to the T cells and incubated for 30 minutes (4°C, on a shaker). Then, the cells were washed thrice and stained with 50 μL of fluorescein isothiocyanate-conjugated goat antimouse IgG (Jackson ImmunoResearch Laboratories, Inc. West Grove, PA, Cat. 115-095-003; Concentration, 1.5 mg/mL) diluted 1 to 800. The plates were incubated for 30 minutes (4°C, on a shaker), and after washing, the cells were stained with 50 μL of serum-free AIM-V medium with 1% HEPES, containing 2.5 μL PE-conjugated antihuman CD4 (BioLegend, Cat 300539) and 2.5 μL peridinin-chlorophyll protein-conjugated antihuman CD8 (BioLegend, Cat 344708). After incubating and washing as previously described, the cells were resuspended in 90 μL of 1× PBS, then acquired with the BD Accuri C6 flow cytometer (BD Biosciences, San Jose, CA). The cells were analyzed using BD Accuri C6 software, version 1.0.264.21. The median channel shift demarcates the immune-affinity of the mAbs for HLA-I antigens.
Statistical Analysis
Statistical analyses were performed using STATA 13. All data were tested for normality using the Shapiro-Wilk, and Shapiro-Francia tests. Analysis for significance was performed using the Mann-Whitney U and Kruskal-Wallis tests. Two-tailed P values less than 0.05 were considered significant.
RESULTS
HLA-I Reactivity of the mAbs Against Different SAB
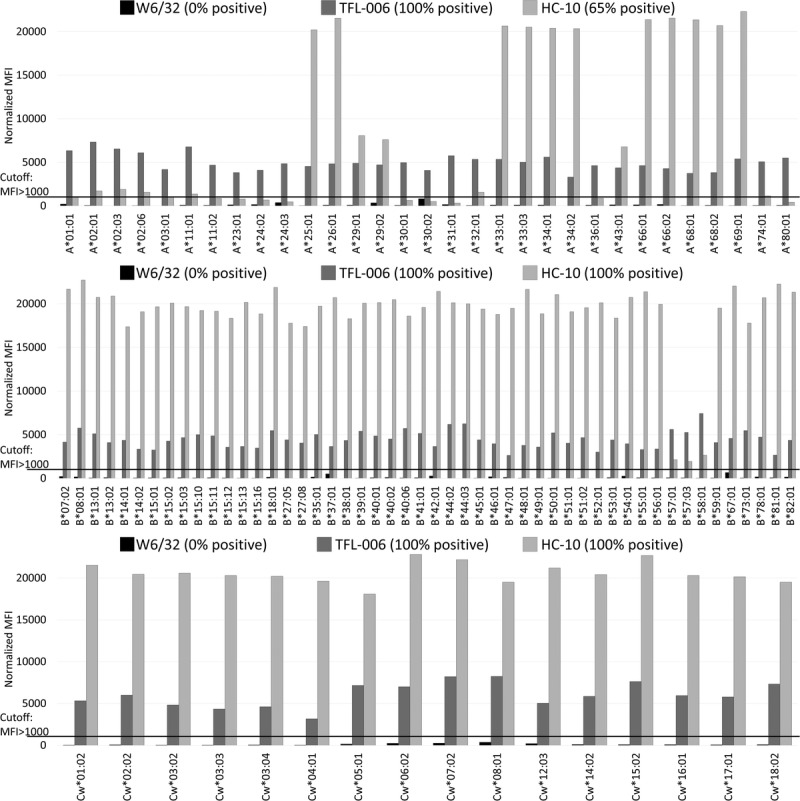
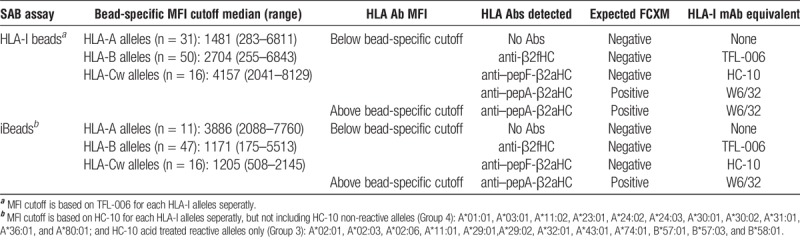
On HLA-I beads (Figure 1), W6/32 binds to 100% of HLA-A, HLA-B, and HLA-Cw antigens with MFI ranges: 15 357-20 956, 11 923-21 644, 6609-21 785, respectively, confirming the heterogeneous density of β2aHC of the different HLA-I antigens. TFL-006 binds to 68% of HLA-A, 88% of HLA-B, and 100% of HLA-Cw antigens with MFI ranges: 283-6811, 255-6843, 2041-8129, respectively, indicating the heterogeneous density of β2fHC on HLA-I beads. Some HLA-A and -B antigen-coated beads are devoid of β2fHC. HC-10 binds to 39% of HLA-A, 94% of HLA-B, and 100% of HLA-Cw antigens with MFI ranges: 0-18 962, 114-18 868, 10 002-19 918, respectively, showing the heterogeneous density of β2fHC on HLA-I beads, whereas higher reactivity of HC-10 compared with TFL-006 suggests the presence pepF-β2aHC.
FIGURE 1.

HLA-I beads. Anti–HLA-I mAbs (W6/32, TFL-006, HC-10) reactivity against all HLA-A (n = 31), HLA-B (n = 50) and HLA-Cw (n = 16) antigens in HLA-I beads. The MFI cutoff used for a positive reaction is 1000. Note that W6/32 binds to 100% of HLA-A, HLA-B, and HLA-Cw antigens and HC-10 binds to 39% of HLA-A, 94% of HLA-B and 100% of HLA-Cw antigens with high MFI. In contrast, TFL-006, though binds to 100% of HLA-A, HLA-B and HLA-Cw antigens, the MFI for the antigens remained lower than that of W6/32 and HC-10.
On iBeads (Figure 2), W6/32 binds to 100% of HLA-A, HLA-B, and HLA-Cw antigens with MFI ranges: 8811-19 006, 8314-20 958, 3544-16 377, respectively, confirming the heterogeneous density of β2aHC of the different HLA-I antigens in iBeads. In contrast, TFL-006 poorly recognized HLA-A (10%; MFI range, 66-1400), and failed to recognize HLA-B and HLA-Cw (0%; MFI range, 3-251, 8-118, respectively) antigens, confirming the absence of β2fHC on all HLA-B, HLA-Cw and most HLA-A-coated beads. A low density of β2fHC exists on the following HLA-A beads: A*11:01, A*24:02, and A*24:03 (MFI = 1400, 1005, and 1035, respectively). HC-10 recognized 35% of HLA-A, 56% of HLA-B and 63% of HLA-Cw antigens with MFI ranges: 6-7760, 18-5513, 508-2145, respectively, confirming the heterogeneous density of pepF-β2aHC on iBeads, as HC-10 identifies a variant of β2aHC that was recognized by W6/32 but not by TFL-006. Thus, HC-10’s epitope occurs on both β2fHC and pepF-β2aHC.
FIGURE 2.

iBeads. Anti–HLA-I mAbs (W6/32, TFL-006, HC-10) reactivity against all HLA-A (n = 31), HLA-B (n = 50) and HLA-Cw (n = 16) antigens in iBeads. The MFI cutoff used for a positive reaction is 1000. W6/32 binds to 100% of HLA-A, HLA-B and HLA-Cw antigens with high MFI. TFL-006 poorly recognizes of HLA-A antigens (10%), while failing to recognize HLA-B and -Cw antigens (0%). HC-10 binds to 35% of HLA-A, 56% of HLA-B and 63% of HLA-Cw antigens.
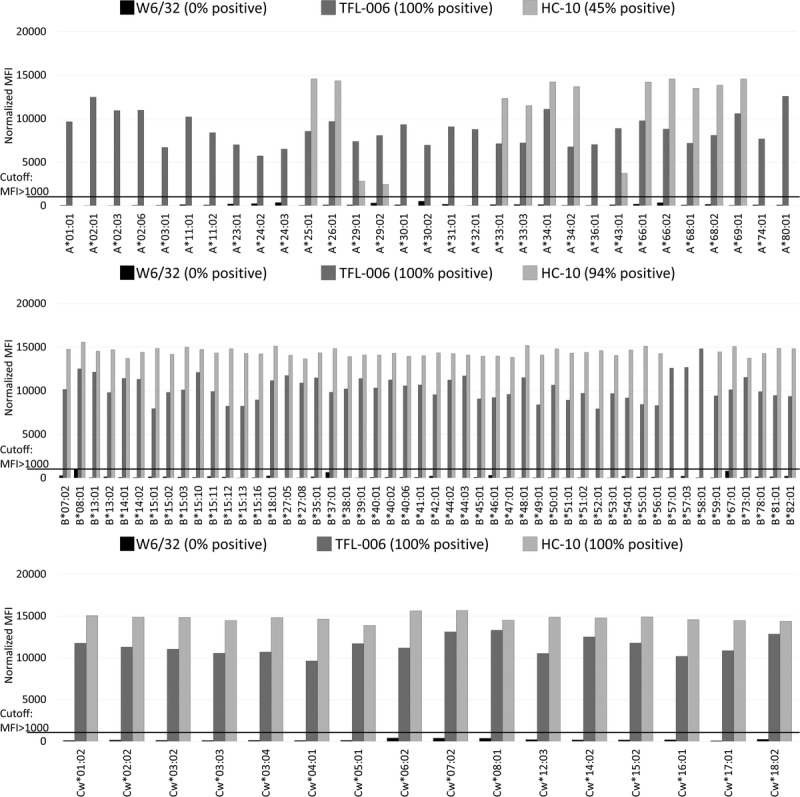
On acid-treated and alkali-treated beads (Figure 3 and 4), W6/32 failed to recognize HLA-A, HLA-B, and HLA-Cw antigens (0%, MFI < 1000), confirming its lack of affinity for β2fHC. In contrast, TFL-006 exhibited 100% reactivity to the HLA-A, HLA-B, and HLA-Cw antigens in both acid-treated (100%; MFI range, 3304-7335, 2633-7430, 3164-8255, respectively) and alkali-treated (100%; MFI range: 5731-12 569, 7930-14 817, 9630-13 299, respectively) beads, confirming TFL-006–specific affinity for β2fHC. Thus, both W6/32 and TFL-006 recognized all HLA-I antigens, but different conformational variants. In contrast to TFL-006, HC-10 binds to 65% of HLA-A (MFI range, 306-22 314) on acid-treated beads and 45% (MFI range, 0-14 572) on alkali-treated beads. HC-10 bound to 100% of HLA-B and -Cw antigens on acid-treated beads with MFI ranges: 1940-22 710, 18 075-22 828, respectively, and to 94% of HLA-B and 100% of HLA-Cw antigens on alkali-treated beads with MFI ranges: 0-15 560, 13 873-15 660, respectively. HLA-I antigens with low MFI (<3000) in acid-treated beads became negative in alkali-treated beads.
FIGURE 3.
Acid-treated beads. Anti–HLA-I mAbs (W6/32, TFL-006, HC-10) reactivity against all HLA-A (n = 31), HLA-B (n = 50) and HLA-Cw (n = 16) antigens in acid-treated beads. The MFI cutoff used for a positive reaction is 1000. W6/32 failed to recognize HLA-A, HLA-B and HLA-Cw in acid-treated beads (0%). TFL-006 shows 100% reactivity to the HLA-A, HLA-B and HLA-Cw antigens. HC-10 binds to 65% of HLA-A and 100% of HLA-B and HLA-Cw antigens.
FIGURE 4.
Alkali-treated beads. Anti–HLA-I mAbs (W6/32, TFL-006, HC-10) reactivity against all HLA-A (n = 31), HLA-B (n = 50) and HLA-Cw (n = 16) antigens in alkali-treated beads. The MFI cutoff used for a positive reaction is 1000. The binding pattern of W6/32 and TFL-006 on alkali-treated beads are similar to the binding on acid treated beads. HC-10 binds to 45% of HLA-A on alkali treated beads, 94% of HLA-B and 100% of HLA-Cw on alkali-treated beads.
Figure 5 shows that the reactivity of the mAbs across the HLA-I antigens represented in the SAB’s panels of 2 lots is not consistent. MFI differs between HLA-I beads lot 8 and lot 9 in the 3 different loci (HLA-A, -HLA-B, and HLA-Cw). The observations point out that lot-to-lot variability exists in the amount of β2aHC and β2fHC on HLA-I beads.
FIGURE 5.
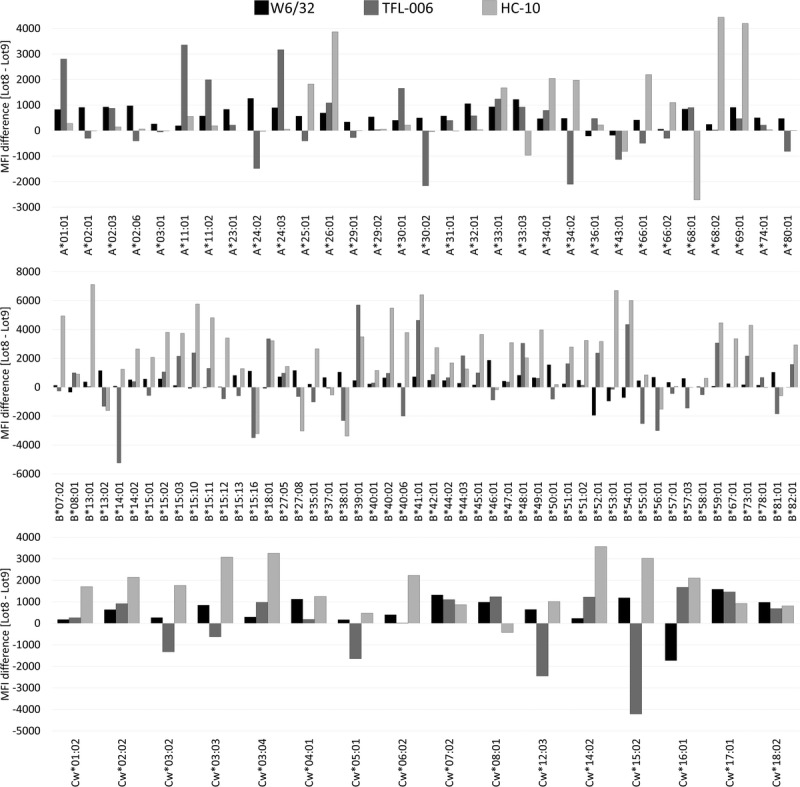
HLA-I beads lot-to-lot variability expressed as the difference between anti–HLA-I mAbs (W6/32, TFL-006, HC-10) reactivity against HLA-I beads lot 8 and lot 9 (lot 8 MFI – lot 9 MFI). Positive values illustrate higher reactivity with lot 8, while negative values illustrate higher reactivity with lot 9.
Specific Characteristics of HC-10 Reactivity Against HLA-I Antigens
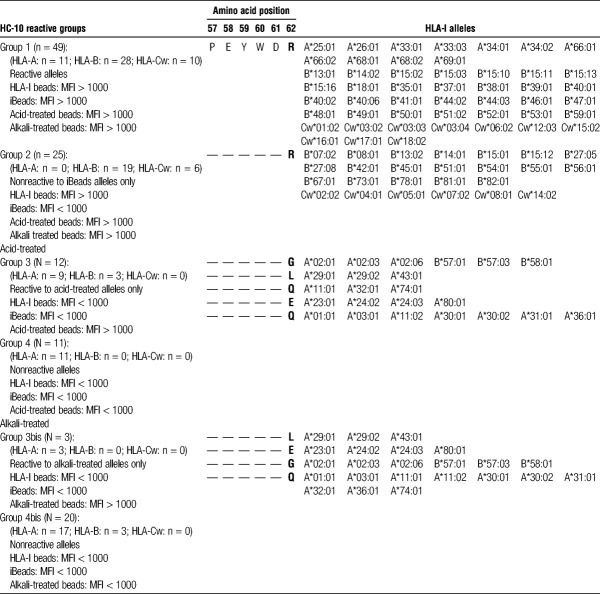
HC-10 reactivity against HLA-I are characterized into 4 different groups (Table 1):
TABLE 1.
Classification of HLA-I antigens based on HC-10 reactivity against 4 different SAB, (HLA-I beads, iBeads, acid-treated, and alkali-treated beads) coated with 31 antigens of HLA-A, 50 antigens of HLA-B, and 16 antigens of HLA-Cw)
Group 1. HC-10-reactive antigens on the different SAB: A shared characteristic of the HC-10-reactive antigens is the presence of the amino acid sequence motif 57PEYWDR62 in the α1 HC.
Group 2. HC-10-non-reactive antigens on iBeads only: HC-10-reactive antigens are found on HLA-I beads and acid-treated beads but not to the same antigens on iBeads. This group also bears the sequence 57PEYWDR62.
Group 3. HC-10-reactive antigens on acid-treated beads only: These antigens become reactive to HC-10 upon acid treatment. Interestingly, R62 is either replaced by glycine (G), leucine (L), or asparagine (Q) in the peptide sequence (57PEYWDR62). Upon alkali treatment, HC-10 failed to recognize the antigens with G62 and Q62 (group 3bis).
Group 4. HC-10 nonreactive antigens on all SAB: Several antigens are not recognized by HC-10 on all SAB. R62 is replaced by aspartic acid (E) or Q. Upon alkali-treatment, HC-10 fails to recognize the antigens with E62 and Q62, as well as antigens with G62 (group 4bis).
HC-10 recognizes, on iBeads, the antigens in group 1 but not in group 2. Because the antigens, in both groups, are recognized by W6/32 but not by TFL-006 on iBeads, HC-10 recognize one of the W6/32-positive variant, namely, pepA-β2aHC or pepF-β2aHC. Because HC-10 binds to the antigens in groups 1 and 2 on acid-treated beads, pepF-β2aHC may be the unique variant recognized by HC-10 on iBeads. Because groups 3 and 4 antigens are not recognized by HC-10 on HLA-I beads and iBeads, they are not included for further characterization of the SAB.
Density of β2fHC Coated on HLA-I Beads and iBeads
HLA-I molecules coated on SAB includes: β2aHC, represented by the sum of pepA-β2aHC and pepF-β2aHC, recognized by W6/32; and β2fHC recognized by TFL-006. Since there is no overlap between the HLA-I variants recognized by these 2 mAbs, the percentage of β2fHC for a specific HLA-I antigen on HLA-I beads and iBeads (Figure 6) is calculated as follows:
FIGURE 6.
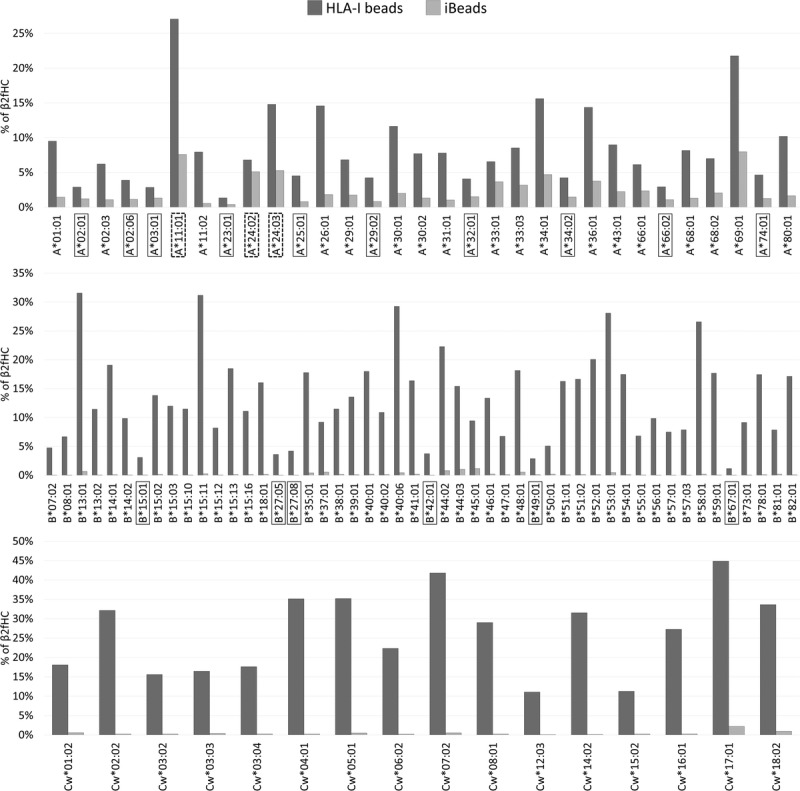
Density (%) of β2fHC coated on HLA-I beads and iBead, as assessed by the following formula: %β2fHC = (TFL-006 MFI)/(TFL-006 MFI + W6/32 MFI). Boxed antigens represent beads coated with non-detectable β2fHC with MFI < 1000 on HLA-I beads, whereas dotted-boxed antigens represent beads coated with detectable β2fHC with MFI > 1000 on iBeads.
Most HLA-I beads harbor detectable amounts (MFI > 1000) of β2fHC on their surface. However, no detectable amounts (MFI < 1000) of β2fHC are observed on the beads coated with A*02:01, A*02:06, A*03:01, A*23:01, A*25:01, A*29:02, A*32:01, A*34:02, A*66:02, A*74:01, B15:01, B*27:05, B*27:08, B*42:01, B*49:01, or B*67:01 (boxed antigens in Figure 6). HLA-Cw antigens coated on HLA-I beads always had a MFI greater than 1000, representing greater than 10% of β2fHC coated on the beads’ surface. In contrast, iBeads mostly harbor β2aHC, with nondetectable (MFI < 1000) β2fHC. Only the following HLA-A beads, A*11:01, A*24:02 and A*24:03 (dotted boxed antigens in Figure 6), harbor detectable amounts of β2fHC (1000 < MFI < 1500), with a single exception, A*69:01, that has nondetectable β2fHC (MFI < 1000) with density of 8%.
Table 2 shows that, in HLA-I beads, the median percentages of β2fHC in HLA-A, HLA-B, and HLA-Cw loci is 7% ± 5.7%, 11.7% ± 7.5%, and 28.2% ± 10.6%, respectively, while being lower in iBeads (HLA-A = 1.5% ± 1.9%, HLA-B = 0.1% ± 0.3%, HLA-Cw = 0.2% ± 0.5%) and higher in acid-treated beads (HLA-A = 98.3% ± 3.2%, HLA-B = 98.6% ± 2.7%, HLA-Cw = 98.8% ± 1.2%). In HLA-I beads, the density of β2fHC present on the HLA-A beads is significantly lower compared with HLA-B beads (P < 0.005) and HLA-Cw beads (P < 0.0001).
TABLE 2.
Density (%) of β2fHC coated on HLA-A, HLA-B and HLA-Cw antigen-coated beads in HLA-I beads, iBeads and acid-treated beads; and density (%) of pepF-β2aHC on coated on HLA-A, HLA-B, and HLA-Cw antigen-coated beads in iBeads
Relative Density of pepF-β2aHC on iBeads
W6/32 binds to both pepA-β2aHC and pepF-β2aHC and HC-10 binds to pepF-β2aHC and β2fHC, suggesting that there is an overlap between the HLA-I conformation recognized by both mAbs. Therefore, the percentage of pepF-β2aHC coated on iBeads is derived only for the HLA-I antigens expressing the antigenic determinant R62 recognized by HC-10 (Table 1, groups 1 and 2), because these antigens on iBeads are free of β2fHC and are coated with HLA-I variants restricted to pepA-β2aHC and pepF-β2aHC. The percentage of pepF-β2aHC for a specific antigen on iBeads (Figure 7) was calculated as follows:
FIGURE 7.
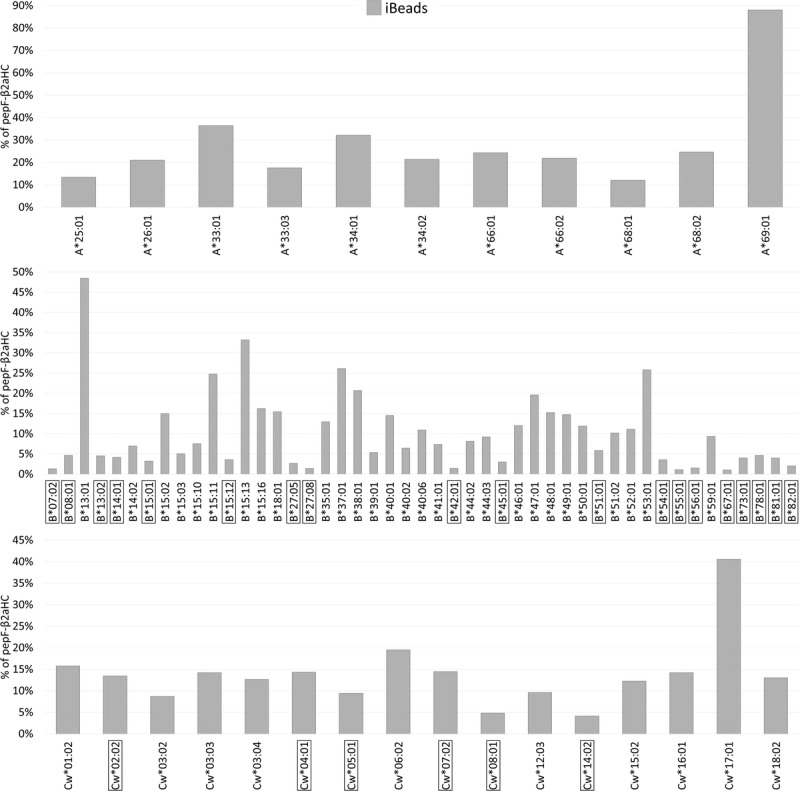
Density (%) of pepF-β2aHC coated on iBeads as assessed by the following formula: %pepF-β2aHC = (HC-10 MFI)/(W6/32 MFI). Boxed antigens represent beads coated with nondetectable pepF-β2aHC with MFI < 1000 on iBeads.
Most beads found in the iBeads’ panel have detectable (MFI > 1000) pepF-β2aHC. Only 19 HLA-B and 6 HLA-Cw have nondetectable (MFI < 1000) pepF-β2aHC (boxed-antigens in Figure 7). All HC-10-reactive HLA-A antigens had MFI greater than 1000, demonstrating greater than 10% of β2fHC coated on the beads. Table 2 shows that the median percentage of pepF-β2aHC on HLA-A, HLA-B, and HLA-Cw loci is 21.9% ± 21%, 7.4% ± 9.5%, and 13.2% ± 8.1% respectively. HLA-A antigens have significantly higher density of pepF-β2aHC compared with HLA-B (P < 0.001) and HLA-Cw (P < 0.005).
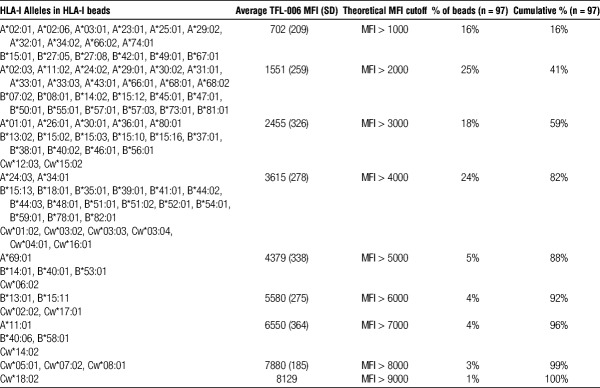
Ranking of HLA-I Antigen-Coated Beads Based on β2fHC Density in HLA-I Beads
Table 3 shows the ranking of all HLA-I antigens (n = 97) in the HLA-I beads’ panel, based on TFL-006 reactivity and categorized in 1000 MFI increment (from 1000 to 9000). This stratification points out the heterogeneous density of β2fHC coated on HLA-I beads and shows how the use of a standard cutoff across all beads may not distinguish anti-β2aHC from anti-β2fHC. Indeed, if 1000 is used, then only 16% of the beads will truly detect anti-β2aHC, the remaining beads may detect both anti-β2aHC and -β2fHC. If 5000 is used, then 88% of the beads will truly detect anti-β2aHC, whereas 72% of the beads may be false negative for anti-β2aHC and 12% may detect both anti-β2aHC and anti-β2fHC. This ranking suggests that each individual bead should have a bead-specific MFI cutoff to critically evaluate and distinguish anti-β2aHC from anti-β2fHC when using HLA-I beads.
TABLE 3.
Ranking of HLA-I antigens in the HLA-I beads’ panel based on their TFL-006 reactivity: bead-specific MFI cutoff of HLA-I beads’ panel
Ranking of HLA-I Antigen-Coated Beads Based on pepF-β2aHC Density in iBeads
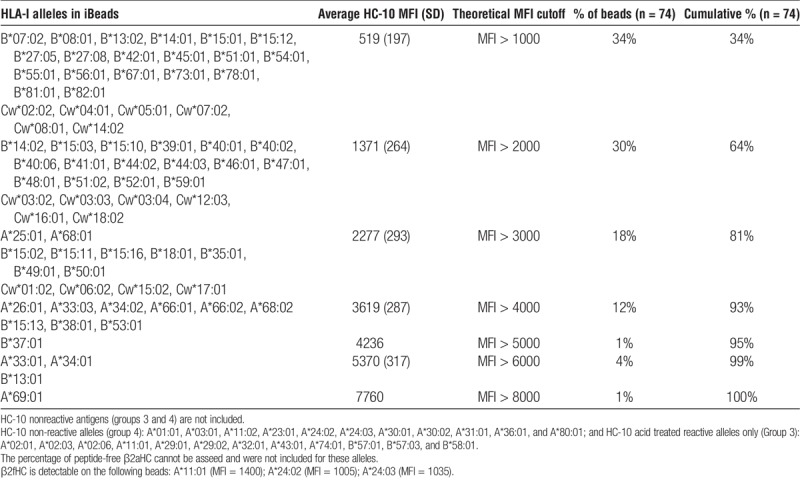
Table 4 shows the ranking of all HC-10–reactive antigens (n = 74) in the iBeads’ panel, based on HC-10 reactivity and categorized in 1000 MFI increments (from 1000 to 8000). The heterogeneity of pepF-β2aHC density on iBeads is obvious, as the MFI of HC-10 against antigens on iBeads ranged from 175 to 7760. Such a categorization is useful for assessing meaningful cutoffs to evaluate the HLA antibodies against pepA-β2aHC or pepF-β2aHC.
TABLE 4.
Ranking of HLA-I antigens in the iBeads’ panel based on their HC-10 reactivity: bead-specific MFI cutoff of ibeads’ panel
T-cell FCXM Using the Anti–HLA-I mAbs
Although W6/32 and TFL-006 can bind to all of the donor’s HLA-I antigens and HC-10 only to HLA-B and HLA-Cw ones, Table 5 reveals that W6/32 but not TFL-006 and HC-10 showed reactivity to HLA-I expressed on the surface of resting T cell, confirming the prevalence of pepA-β2aHC on the on the surface of resting T cells (CD4+ or CD8+). Explicitly resting T cells express neither β2fHC nor pepF-β2aHC.
TABLE 5.
T cell FCXM using anti–HLA-I mAbs W6/32, TFL-006, and HC-10
DISCUSSION
This investigation reveals the distribution and relative density of the conformational variants of the HLA-I on SAB by comparing the reactivities of mAbs, W6/32, TFL-006, and HC-10 on HLA-I beads, iBeads, acid, and alkali-treated beads. Figure 8 elucidates the specificity of the mAbs used, the relative density of the different conformational variants of HLA-I present on the surface of SAB and the variant that is associated with positive FCXM. The assessment of the intrinsic characteristics of the beads is critical for the reliability of virtual XMs in predicting CDCXM and FCXM outcomes and to increase accessibility of donor organs for highly sensitized patients on the waiting list.
FIGURE 8.
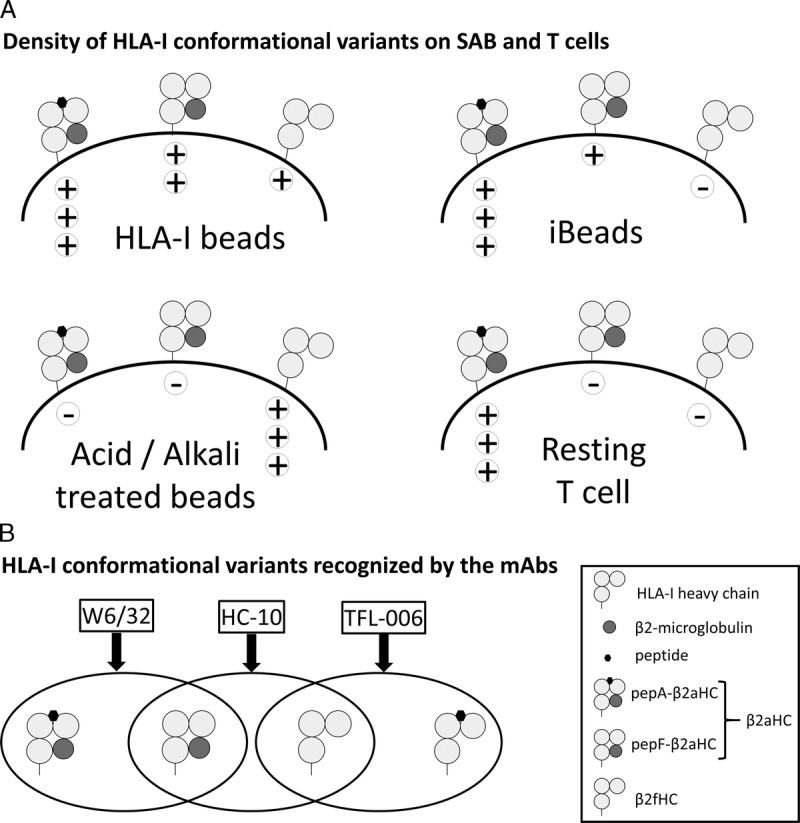
A, Density of HLA-I conformational variants on SAB and T-cells. HLA-I beads has highest density of pepA-β2aHC followed by pepF-β2aHC and then low density of β2fHC. iBeads has highest density of pepA-β2aHC and low density pepF-β2aHC and minimal density of β2fHC. Acid/Alkali-treated beads only bear β2fHC. T-cells only bear pepA-β2aHC. B, HLA-I conformational variants recognized by the mAbs. W6/32 recognize both pepA-β2aHC and pepF-β2aHC. HC-10 recognize pepF-β2aHC and pepF-β2fHC. TFL-006 recognize pepF-β2fHC and possibly pepA-β2fHC.
The discrepancy between virtual XMs and actual XMs is influenced by the conventionally used SAB (HLA-I beads) for monitoring HLA-I antibodies. HLA-I antibodies in an allograft recipient can detect different conformations of HLA-I,9,11,27 namely, β2aHC and β2fHC. Anti-β2aHC IgGs are associated with AMR and/or graft loss,28,29 whereas anti-β2fHC IgGs may not have a deleterious effect on graft function.9,12,14 These observations influence the assumption that SAB only detect and identify clinically relevant HLA-I antibodies,30 without examining the HLA variants (antigen integrity, conformation, and orientation) on the beads.31
Anti-β2fHC IgGs were documented in nonalloimmunized men, cord blood, and in CDCXM-negative transplant candidates without prior immunizations.8,10,32 Similarly, HLA-I–sensitized kidney recipients had anti-β2fHC IgGs without positive FCXM.11,14 Therefore, HLA-I beads can detect antibodies that may not be clinically relevant, such as anti-β2fHC not associated with a positive FCXM.27,30,33,34
Strategies to minimize the “false SAB reactions” (as implicated by Gombos et al32) are critical in maximizing the specificity of SAB that correlate with positive FCXM results. One approach is to define an MFI cutoff that is associated with transplant outcome, which is challenging to reach.7,30 The 2 aspects of MFI cutoffs are: a cutoff for a positive reaction (or sensitivity) and a cutoff for clinical relevance (or specificity); both contributing to the divergence and nonagreement of a specific “standard” MFI cutoff. Indeed, the MFI cutoffs used can range from 30035 to 6000,36 while 1000 is most common,30,32,37–39 particularly when the sera are used neat or minimally diluted, or in the presence of IgM and other interfering serum factors. Both the sensitivity and the specificity of SAB for detecting serum antibodies is related to the antigenic density of the HLA-I proteins displayed on their surface.40 Our results emphasize that the cutoff should be based on the density of β2fHC, as indicated by the MFI of the mAb TFL-006. Because the MFIs obtained with TFL-006 vary among different antigen-coated beads, a specific cutoff should be attributed for each antigen in the panel of HLA-I beads. Therefore, when an anti–HLA-I DSA is identified, the raw MFI obtained for a DSA should be normalized not only against the negative control bead and a negative control sample, but also against a positive control for DSA reacting to β2fHC, monitored with TFL-006. Only the β2fHC-normalized MFI would represent IgG directed against β2aHC. This approach using a bead-specific MFI cutoff is supported by the findings in literature, where the levels of anti-β2fHC were documented to vary from low (MFI < 1000) to high (MFI > 9000) levels.14,32 In the case of low MFI DSA, when TFL-006 MFI is higher than the DSA’s MFI, then there is a possibility of underestimating the DSA, if it recognizes an epitope present in both β2aHC and β2fHC, as described earlier.8,11,14 This limitation is applicable only to HLA-I beads, because this issue is eliminated if iBeads are used because they express predominantly β2aHC.
The other strategy to reduce the amount of “false SAB reactions” is to revive the production of iBeads (by the vendor of HLA-I beads), which eliminates “false positive reactions” caused by the presence of β2fHC on SAB.13 iBeads are not routinely used in clinical settings; however, promising data suggest that iBeads mainly detect anti-β2aHC9,41 associated with positive FCXM.11,14,42 Otten et al9 has described low levels of “denatured” HLA on iBeads, using mAb HC-10. However, our study shows that iBeads bear an even lower density of β2fHC, as assessed by β2fHC-specific mAb TFL-006. Assessing the reactivities of TFL-006 and HC-10 to iBeads shows evidence that HC-10 reacts to the pepF-β2aHC on iBeads. Visentin et al11,14,42 have also noted that DSA against “denatured” HLA can be iBeads-positive, and such DSAs were not associated with AMR, graft loss, and/or positive FCXM. In this context, our results clarify the DSA detected by Visentin et al may be directed against pepF-β2aHC, equivalent to HC-10–positive DSA on iBeads that are FCXM negative. Therefore, the HC-10 reactivity against specific antigens on iBeads will provide the cutoff for monitoring a DSA reacting to the pepF-β2aHC. Therefore, this study recommends the use of iBeads, when made available, in clinical monitoring of anti–HLA-I DSA, because the DSA detected with iBeads is more reliable than those detected with conventional HLA-I beads. Owing to the work of Visentin et al,11,14,42 DSA identified with iBeads have a propensity for allograft rejection and positive FCXM, and this DSA can be considered as pathogenic DSA.
Another aspect, emerging from the results, is the impact of a peptide, loaded in the peptide-binding groove of HLA-I HC, on the ability of HC-10 to bind to HLA-I antigens. It is known that R62 is the antigenic determinant of HC-1020 which is located in the second helix of the α1 domain.43 More precisely, R62 establishes interactions inside the A pocket anchoring the peptide N-terminus (P1), and its orientation could be strongly influenced by the P1 residues of a peptide.44 Although HC-10 recognizes both β2fHC and pepF-β2aHC, these variants are not expressed on resting T cells. Indeed, the deleterious effect of DSA is confined to the recognition of HLA-I HC loaded with peptides by anti–pepA-β2aHC,45 which is supported by the flexibility of the peptide-binding groove in the presence or absence of a peptide26,45,46 and their implication for immune recognition.46
In conclusion, while using HLA-I beads for monitoring DSA, it is important to be aware that the DSA detected by the SAB may or may not correlate with positive FCXM and rejection. Two strategies can reduce the discrepancy between SAB and FCXM results while increasing the concordance of virtual and actual XMs. Table 6 illustrates the strategies to follow while seeking “pathogenic” DSA using HLA-I beads or iBeads: a bead-specific MFI cutoff based on either TFL-006 reactivity with HLA-I beads or that based on HC-10 reactivity with iBeads. Essentially, using a cutoff based on TFL-006 and/or HC-10 MFI enables the normalization of DSA “strength” against β2aHC by removing “false SAB reactions” triggered by the possible presence of HLA antibodies against β2fHC and/or pepF-β2aHC, which are not associated with positive FCXM.
TABLE 6.
The use of a bead-specific MFI cutoff to increase the concordance between SAB and FCXM results
ACKNOWLEDGMENTS
The authors wish to express their sincere thanks to Mrs. Judy Hopfield for her meticulous editing of the manuscript. The authors also wish to thank Dr. Mathew Everly and Dr. Junchao Cai for their comments and criticisms during laboratory meetings and presentation.
Footnotes
The entire project is funded by Terasaki Family Foundation.
V.J. has no conflicts of interest to disclose. M.H..R. and the late P.I.T., as coinventors, have filed US and European patent applications on HLA-I polyreactive anti–HLA-E mAbs, which include mAb TFL-006.
P.I. initiated this project, discussed its plan and offered advice during the early phases of this investigation. V.J. designed and carried out all the experiments using 3 different monoclonal antibodies and 4 different single-antigen beads, analyzed the data in comparison with previous publications, prepared the tables and figures, wrote the first draft of the article meticulously. M.H.R., based on discussion with the third author, initiated the investigation on the use the 3 monoclonal antibodies on Luminex single antigen beads and on T cells to elucidate variations within a single HLA allele, examined the data, discussed periodically with the first author and contributed to the writing and editing of the article. The late P.I.T. initiated a discussion with the second author about the importance of defining the variations of individual HLA antigens on the Bead using the monoclonal antibody developed at Terasaki Foundation Laboratory (TFL-006) along with mAbs W6/32 and heavy chain (HC)-10. During early phases of data collection, he discussed with the second author highlighting the importance of iBeads for monitoring sera of pre and posttransplant patients’ sera.
Correspondence: Mepur H. Ravindranath, MSc, PhD, Terasaki Foundation Laboratory, Los Angeles, CA 90064. (ravimh@terasakilab.org).
In order to distinguish between relevant and irrelevant HLA antibodies, the authors study the role of antibodies to conformational variants of HLA-I and show that the presence of these antibodies should be tested in order to assess correctly the relevant strength a given antibody.
REFERENCES
- 1.Terasaki PI. Humoral theory of transplantation Am J Transplant 2003. 3665–673 [DOI] [PubMed] [Google Scholar]
- 2.Pei R, Lee JH, Shih NJ. Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities Transplantation 2003. 7543–49 [DOI] [PubMed] [Google Scholar]
- 3.Bray RA, Nolen JD, Larsen C. Transplanting the highly sensitized patient: the emory algorithm Am J Transplant 2006. 62307–2315 [DOI] [PubMed] [Google Scholar]
- 4.Bielmann D, Hönger G, Lutz D. Pretransplant risk assessment in renal allograft recipients using virtual crossmatching Am J Transplant 2007. 7626–632 [DOI] [PubMed] [Google Scholar]
- 5.Cecka JM, Kucheryavaya AY, Reinsmoen NL. Calculated PRA: initial results show benefits for sensitized patients and a reduction in positive crossmatches Am J Transplant 2011. 11719–724 [DOI] [PubMed] [Google Scholar]
- 6.Lefaucheur C, Loupy A, Hill GS. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation J Am Soc Nephrol 2010. 211398–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tait BD, Süsal C, Gebel HM. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation Transplantation 2013. 9519–47 [DOI] [PubMed] [Google Scholar]
- 8.El-Awar N, Terasaki PI, Nguyen A. Epitopes of human leukocyte antigen class I antibodies found in sera of normal healthy males and cord blood Hum Immunol 2009. 70844–853 [DOI] [PubMed] [Google Scholar]
- 9.Otten HG, Verhaar MC, Borst HPE. The significance of pretransplant donor-specific antibodies reactive with intact or denatured human leucocyte antigen in kidney transplantation Clin Exp Immunol 2013. 173536–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morales-Buenrostro LE, Terasaki PI, Marino-Vázquez LA. “Natural” human leukocyte antigen antibodies found in nonalloimmunized healthy males Transplantation 2008. 861111–1115 [DOI] [PubMed] [Google Scholar]
- 11.Visentin J, Guidicelli G, Bachelet T. Denatured class I human leukocyte antigen antibodies in sensitized kidney recipients: prevalence, relevance, and impact on organ allocation Transplantation 2014. 98738–744 [DOI] [PubMed] [Google Scholar]
- 12.Cai J, Terasaki PI, Anderson N. Intact HLA not beta2m-free heavy chain-specific HLA class I antibodies are predictive of graft failure Transplantation 2009. 88226–230 [DOI] [PubMed] [Google Scholar]
- 13.El-Awar N, Terasaki PI, Hajeer A. 12-P: A novel HLA class I single antigen bead preparation eliminates false positive reactions attributed to natural antibodies—in the sera of normal males and pre-transplant patients. Hum Immunol. 2010;71:S26. [Google Scholar]
- 14.Visentin J, Marroc M, Guidicelli G. Clinical impact of preformed donor-specific denatured class I HLA antibodies after kidney transplantation Clin Transplant 2015. 29393–402 [DOI] [PubMed] [Google Scholar]
- 15.Barnstable CJ, Bodmer WF, Brown G. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens—new tools for genetic analysis Cell 1978. 149–20 [DOI] [PubMed] [Google Scholar]
- 16.Parham P, Barnstable CJ, Bodmer WF. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens J Immunol 1979. 123342–349 [PubMed] [Google Scholar]
- 17.Smith KD, Mace BE, Valenzuela A. Probing HLA-B7 conformational shifts induced by peptide-binding groove mutations and bound peptide with anti-HLA monoclonal antibodies J Immunol 1996. 1572470–2478 [PubMed] [Google Scholar]
- 18.Vigna JL, Smith KD, Lutz CT. Invariant chain association with MHC class I: preference for HLA class I/beta 2-microglobulin heterodimers, specificity, and influence of the MHC peptide-binding groove J Immunol 1996. 1574503–4510 [PubMed] [Google Scholar]
- 19.Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products J Immunol 1986. 1372299–2306 [PubMed] [Google Scholar]
- 20.Perosa F, Luccarelli G, Prete M. Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide J Immunol 2003. 1711918–1926 [DOI] [PubMed] [Google Scholar]
- 21.Gillet AC, Pérarnau B, Mercier P. Serological analysis of the dissociation process of HLA-B and C class I molecules Eur J Immunol 1990. 20759–764 [DOI] [PubMed] [Google Scholar]
- 22.Carreno BM, Hansen TH. Exogenous peptide ligand influences the expression and half-life of free HLA class I heavy chains ubiquitously detected at the cell surface Eur J Immunol 1994. 241285–1292 [DOI] [PubMed] [Google Scholar]
- 23.Ravindranath MH, Terasaki PI, Pham T. Therapeutic preparations of IVIg contain naturally occurring anti-HLA-E antibodies that react with HLA-Ia (HLA-A/-B/-Cw) alleles Blood 2013. 1212013–2028 [DOI] [PubMed] [Google Scholar]
- 24.Ravindranath MH, Zhu D, Pham T, et al. Anti–HLA-E monoclonal antibodies reacting with HLA-la and lb alleles like IVIg as potential IVIg-immunomimetics: an evolving therapeutic concept. Clin Transpl. 2013:293–305. [PubMed] [Google Scholar]
- 25.Ravindranath MH, Terasaki PI, Pham T. Suppression of blastogenesis and proliferation of activated CD4(+) T cells: intravenous immunoglobulin (IVIg) versus novel anti-human leucocyte antigen (HLA)-E monoclonal antibodies mimicking HLA-I reactivity of IVIg Clin Exp Immunol 2014. 178154–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurimoto E, Kuroki K, Yamaguchi Y. Structural and functional mosaic nature of MHC class I molecules in their peptide-free form Mol Immunol 2013. 55393–399 [DOI] [PubMed] [Google Scholar]
- 27.Jacob EK, De Goey SR, Gandhi MJ. Positive virtual crossmatch with negative flow crossmatch results in two cases Transpl Immunol 2011. 2577–81 [DOI] [PubMed] [Google Scholar]
- 28.Dunn TB, Noreen H, Gillingham K. Revisiting traditional risk factors for rejection and graft loss after kidney transplantation Am J Transplant 2011. 112132–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otten HG, Verhaar MC, Borst HP. Pretransplant donor-specific HLA class-I and -II antibodies are associated with an increased risk for kidney graft failure Am J Transplant 2012. 121618–1623 [DOI] [PubMed] [Google Scholar]
- 30.Süsal C, Ovens J, Mahmoud K. No association of kidney graft loss with human leukocyte antigen antibodies detected exclusively by sensitive Luminex single-antigen testing: a Collaborative Transplant Study report Transplantation 2011. 91883–887 [DOI] [PubMed] [Google Scholar]
- 31.Gebel HM, Bray RA. HLA antibody detection with solid phase assays: great expectations or expectations too great? Am J Transplant 2014. 141964–1975 [DOI] [PubMed] [Google Scholar]
- 32.Gombos P, Opelz G, Scherer S. Influence of test technique on sensitization status of patients on the kidney transplant waiting list Am J Transplant 2013. 132075–2082 [DOI] [PubMed] [Google Scholar]
- 33.Nikaein A, El-Awar N, Hunt J. Clinically irrelevant circulating human leukocyte antigen antibodies in the presence of ventricular assist devices J Heart Lung Transplant 2012. 31443–447 [DOI] [PubMed] [Google Scholar]
- 34.Pereira S, Perkins S, Lee JH. Donor-specific antibody against denatured HLA-A1: clinically nonsignificant? Hum Immunol 2011. 72492–498 [DOI] [PubMed] [Google Scholar]
- 35.Wiebe C, Gibson IW, Blydt-Hansen TD. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant Am J Transplant 2012. 121157–1167 [DOI] [PubMed] [Google Scholar]
- 36.Wan L, Chen D. Quantitative relationship of Luminex single antigen bead assay (LSA), antibody titration and flow crossmatch in heart transplantation—a data analysis of 5 years clinical testing [abstract]. Am J Transplant. 2013;13(suppl 5) http://www.atcmeetingabstracts.com/abstract/quantitative-relationship-of-luminex-single-antigen-bead-assay-lsa-antibody-titration-and-flow-crossmatch-in-heart-transplantation-a-data-analysis-of-5-years-clinical-testing/. Accessed March 27, 2016. [Google Scholar]
- 37.Reed EF, Rao P, Zhang Z. Comprehensive assessment and standardization of solid phase multiplex-bead arrays for the detection of antibodies to HLA-drilling down on key sources of variation Am J Transplant 2013. 133050–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mata Molanes JJ, Burgos Rodríguez L, Delgado García JA. Relatively high levels of anti-HLA antibodies (measured by Luminex Single-Antigen bead assay) are required to mediate inhibition of lymphocyte proliferation nduced by sera from alloimmunized renal patients Ann Transplant 2014. 19652–659 [DOI] [PubMed] [Google Scholar]
- 39.Kosmoliaptsis V, Bradley JA, Peacock S. Detection of immunoglobulin G human leukocyte antigen-specific alloantibodies in renal transplant patients using single-antigen-beads is compromised by the presence of immunoglobulin M human leukocyte antigen-specific alloantibodies Transplantation 2009. 87813–820 [DOI] [PubMed] [Google Scholar]
- 40.Warner P. Nebulous humors: defining alloantibodies in the 21st century Hum Immunol 2009. 70623–626 [DOI] [PubMed] [Google Scholar]
- 41.Gao B, Rong C, Porcheray F. Evidence to support a contribution of polyreactive antibodies to HLA serum reactivity Transplantation 2016. 100217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Visentin J, Guidicelli G, Nong T. Evaluation of the iBeads assay as a tool for identifying class I HLA antibodies Hum Immunol 2015. 76651–656 [DOI] [PubMed] [Google Scholar]
- 43.Theodossis A. On the trail of empty MHC class-I Mol Immunol 2013. 55131–134 [DOI] [PubMed] [Google Scholar]
- 44.Nurzia E, Narzi D, Cauli A. Interaction pattern of Arg 62 in the A-pocket of differentially disease-associated HLA-B27 subtypes suggests distinct TCR binding modes. PLoS One. 2012;7:e32865. doi: 10.1371/journal.pone.0032865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulder A, Eijsink C, Kester MG. Impact of peptides on the recognition of HLA class I molecules by human HLA antibodies J Immunol 2005. 1755950–5957 [DOI] [PubMed] [Google Scholar]
- 46.Hawse WF, Gloor BE, Ayres CM. Peptide modulation of class I major histocompatibility complex protein molecular flexibility and the implications for immune recognition J Biol Chem 2013. 28824372–24381 [DOI] [PMC free article] [PubMed] [Google Scholar]