Abstract
Background
Carbapenem-resistant Enterobacteriaceae (CRE) is an emergent microorganism of infections after liver transplant (LT). The aim of this study was to analyze the risk factors for CRE acquisition and infection after LT.
Methods
This was a prospective cohort study involving patients who underwent LT in the 2010 to 2014 period. Surveillance cultures for CRE were collected immediately before LT and weekly thereafter until hospital discharge.
Results
We analyzed 386 patients undergoing a total of 407 LTs. Before LT, 68 (17.6%) patients tested positive for CRE, 11 (16.2%) of those patients having CRE infection, whereas 119 (30.8%) patients acquired CRE after LT. Post-LT CRE infection was identified in 59 (15.7%) patients: Klebsiella pneumoniae was isolated in 83.2%; surgical site infection was the most common type of infection (46.7%). Multivariate analysis showed that post-LT dialysis was the only risk factor for post-LT CRE acquisition. Eighty-two percent of patients who underwent 3 or more post-LT dialysis sessions and acquired CRE before LT evolved with post-LT CRE infection. Other risk factors for CRE infection were acquisition of CRE post-LT, Model for End-Stage Liver Disease score greater than 32, combined transplantation, and reoperation. Patients who acquired CRE before LT had a high risk of developing CRE infection (P < 0.001).
Conclusions
Measures for minimizing that risk, including altering the antibiotic prophylaxis, should be investigated and implemented.
The prevalence of multidrug-resistant gram-negative bacteria (MDR-GNB) as agents of infection after solid organ transplantation (SOT) is increasing. It is estimated that 10% to 20% of SOT recipients become infected with MDR-GNB.1–3 Such infections occur most often in the early posttransplant period, and the site of infection is usually related to the transplant process. Various studies have reported decreased survival in SOT recipients infected with MDR-GNB.2–6 Among the emergent MDR-GNB are carbapenem-resistant Enterobacteriaceae (CRE), and the incidence of infection with CRE has increased worldwide.7
In liver transplantation (LT) recipients, CRE infections occur in the early posttransplant period, the median time from LT to CRE infection ranging from12 to 24 days.8–10 Such infections usually present with bacteremia, and intra-abdominal infection is identified in 55% to 79% of the cases.8,9 Because of the high associated mortality, CRE infection has a significant impact on first-year survival.8,10 Knowledge of the risk factors for developing CRE infection in LT recipients is crucial to the development of effective prevention strategies. In the general population, previous colonization by CRE is one of the major risks for subsequent infection.11 However, there have been few studies involving active surveillance of LT recipients. Prospective studies designed to analyze risk factors for CRE infection should include such screening, especially in areas where the incidence of such infection is high.
The aim of this study was to analyze the risk factors for CRE acquisition and infection after LT in a population submitted to weekly screenings for CRE. We also analyze the impact that pre-LT acquisition of CRE has on the risk of post-LT CRE infection and early mortality.
MATERIALS AND METHODS
Study Design and Patient Sample
This was a prospective cohort study involving all patients who underwent LT between January 2010 and December 2014 at the University of São Paulo School of Medicine, in Brazil. Patients were followed up from hospital admission until the end of the second month after transplantation. We excluded patients who died within the first 48 hours after transplantation.
Infections were identified through active surveillance on the LT ward and through the review of outpatient records. The criteria used in order to identify and classify healthcare-associated infections (HAIs) were those outlined by the National Healthcare Safety Network,12 the only exception being that surgical site infections (SSIs) meeting the criteria were reported for up to 60 days after LT. All patients with CRE detected were isolated in a separate room and maintained in contact precaution during all hospital stay and subsequent admissions. Colonization was defined as CRE being isolated in cultures of surveillance or clinical specimens, with no evidence of infection. We defined CRE acquisition as having a surveillance or clinical specimen that was positive for CRE, with no evidence of CRE isolated in any culture within the last 2 years. We defined CRE as strains of Enterobacteriaceae resistant to at least one carbapenem. We defined effective treatment as having been treated for 48 hours or longer with at least 1 antimicrobial agent proven to display in vitro activity against CRE.
The standard surgical prophylaxis in use during the study period was a 48-hour course of ampicillin with cefotaxime. For cases in which patients underwent LT during treatment for an infection, as well as for those in which the donor had a suspected or confirmed infection, the prophylaxis protocol was modified. In such cases, the antibiotic used for prophylaxis was the same as that used for the treatment of the infection in question. The standard immunosuppression regimen was tacrolimus plus a corticosteroid, and immunosuppression was induced by administration of methylprednisolone.
During the follow-up period, clinical samples were collected for culture when any infection was suspected. To identify organisms and perform antimicrobial susceptibility testing, we used an automated system (VITEK 2; bioMérieux, Marcy l’Étoile, France). Minimum inhibitory concentrations (MICs) were interpreted according to the breakpoints established by the Clinical and Laboratory Standards Institute.13 Enterobacteriaceae for which carbapenem resistance were classified using CLSI criteria older than those published in June 2010 were reclassified using the newer breakpoints. All CRE strains were submitted to polymerase chain reaction for the blaKPC and blaCTXM gene.
Surveillance Cultures
Surveillance cultures (SC) were collected immediately before LT (independently of results of previous cultures) and weekly thereafter, until hospital discharge. Samples were obtained with perirectal swabs and stored in Stuart’s transport medium. The samples were directly inoculated into brain heart infusion broth containing imipenem (1 μg/mL) and were cultured overnight, after which they were plated on MacConkey agar and all suspect. As in the case of the clinical samples, we used the VITEK 2 system (bioMérieux) to identify organisms and perform antimicrobial susceptibility testing, and we interpreted MICs according to the breakpoints established by the Clinical and Laboratory Standards Institute.13 For all strains isolated in clinical or SCs, polymyxin resistance was defined as an MIC > 2 mg/L in broth microdilution.13
Pulsed-Field Gel Electrophoresis Analysis
After extraction and digestion of whole bacterial DNA with XbaI restriction enzyme (50 units; New England Biolabs, UK), we performed pulsed-field gel electrophoresis (PFGE), as described by Gautom et al,14 using an electric field system (CHEF-DR III; Bio-Rad Laboratories, Hercules, CA), in accordance with the CDC protocol.15 The running parameters were as follows: initial switch time, 2.2 seconds; final switch time, 54.2 seconds; total run time, 19 hours; temperature, 14°C; and voltage, 6 V/cm. The PFGE images were processed and analyzed with BioNumerics software, version 7.1 (Applied Maths, Sint-Martens-Latem, Belgium). The images were normalized through the use of standard molecular markers, and banding patterns were compared. A similarity analysis was performed using Dice coefficients, with a band position tolerance of 1.5% and an optimization of 1.5%. Isolates were separated into similarity clusters by the unweighted pair group method with arithmetic mean. Only bands larger than 45.5 kb were included in the analysis.
Statistical Analysis
In our analysis of risk factors for CRE acquisition, infection, and mortality, we included each patient only once, regardless of the number of transplants they underwent, analyzing only the first LT performed during the study period. We evaluated the following variables related to the LT process: cold ischemia time, number of units of blood transfused intraoperatively, reoperation, retransplantation in the first 60 days after LT, level of experience of the lead surgeon (more than 5 years), American Society of Anesthesiologists physical status classification, surgical time, donor type (living or deceased), extensiveness of the procedure (liver-only or combined liver-kidney transplantation), the type of surgical prophylaxis, biliary complication (stricture, leak, sphincter of Oddi dysfunction), and type of biliary anastomosis. We also evaluated variables related to the LT recipient: age; sex; presence or absence of fulminant hepatitis, hepatocellular carcinoma; Model for End-Stage Liver Disease (MELD) score; and pretransplant serum creatinine level. In addition, we evaluated variables related to hospitalization: length of hospital stay before LT; post-LT need for dialysis; duration of abdominal drainage; and infection in the last week before transplantation. For the patients who evolved to CRE acquisition or infection, the variables related to exposure time were recorded from hospital admission to the first CRE-positive culture or from hospital admission to infection, respectively. Dichotomous variables were considered positive if the event occurred before the first CRE-positive culture (for acquisition) or before the patient developed CRE infection. For the remaining patients, those variables were recorded for the total time at risk during the first 60 days after transplantation.
The main outcome measure was CRE infection during the first 60 days after transplantation (post-LT CRE infection). A secondary outcome measure was CRE acquisition during that same period (post-LT CRE acquisition). In our analysis of the risk of post-LT CRE acquisition, we excluded patients in whom CRE was detected before LT. In analyzing the risk of post-LT CRE infection, we excluded patients who underwent LT with active CRE infection, although we included patients in whom CRE had previously been identified but who showed no evidence of infection.
For dichotomous variables, we performed univariate analysis using the χ2 test or Fisher exact test, as appropriate. For continuous variables, we used the Mann-Whitney test. Continuous variables were transformed into dichotomous variables through cluster analysis. Multivariate analysis was performed by stepwise binary logistic regression. The criterion for inclusion in the multivariate analysis was P value less than 0.2 in the univariate analysis. Variables that then reduced the −2 log likelihood or showed P value less than 0.05 were retained in the model. We also used decision tree analysis—specifically the classification and regression tree (CART) analysis method—to identify risk factors for infection with CRE. The CART analysis was performed on the entire dataset, with chi-square automatic interaction detection. We performed internal validation using tenfold cross-validation. Each predictor variable was tested within the overall cohort, and the statistically significant predictor with the largest odds ratio (OR) was selected manually to form the split. Each split created 2 nodes; ORs were recalculated, and the process was repeated within each node. This process proceeded iteratively until either or both of the following the conditions were satisfied: there were no remaining variables with a statistically significant OR (P < 0.05); and a further split yielded a subgroup with fewer than 15 total patients. For recursive partitioning, we used CART in the program R (http://www.R-project.org/).
For the survival analysis, the outcome measure used was death within the first 60 days after the first LT. Univariate and multivariate analysis were performed by Cox regression. Data were processed and analyzed with the SPSS Statistics software package, version 22.0 (IBM Corporation, Armonk, NY).
This study was approved by ethical review board of University of São Paulo School of Medicine.
RESULTS
Clinical and Epidemiological Characteristics
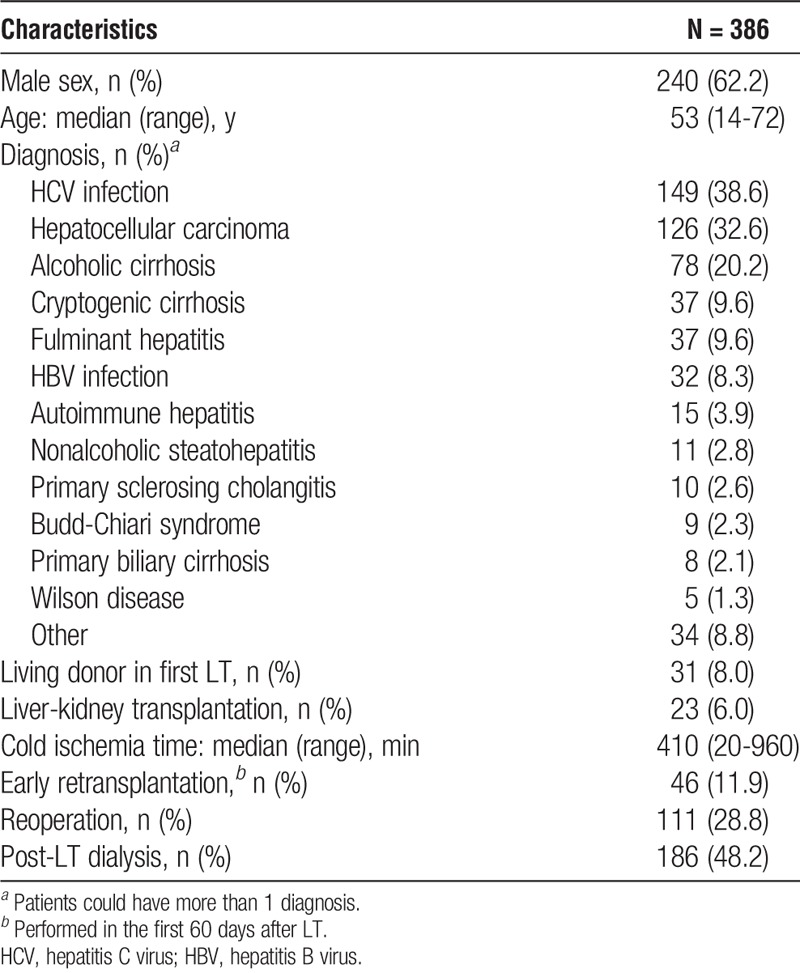
During the study period, 401 patients underwent LT. Of those 401 patients, 15 (3.7%) died within the first 48 hours after LT. Therefore, we analyzed 386 patients, who collectively underwent a total of 407 LTs. In the first LT, 355 (92.0%) of the patients received a deceased donor organ. Of the 386 LT recipients, 240 (62.2%) were male. The median age was 53 years (range, 14-72 years). The most common underlying disease, observed in 149 (38.6%) of the recipients, was HCV-related cirrhosis (Table 1).
TABLE 1.
Demographic characteristic of patients undergoing LT
Pretransplant CRE Acquisition
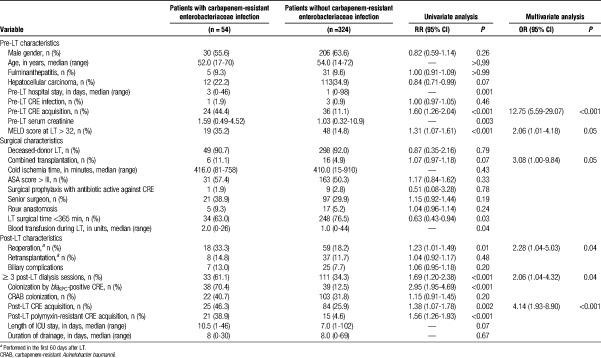
In 68 (17.6%) patients, CRE species were identified before LT, 2 different species being isolated in 4 (5.9%) patients. Therefore, there were a total of 72 isolates. The median time between the last positive culture and LT was 1 day (range, 0-42 days). Of the 72 isolates, 70 (97.2%) were tested for blaKPC, of which 33 (47.1%) tested positive. Eleven (16.2%) of the 68 patients had CRE infection in the pre-LT period: 8 (73.0%) of those patients had CRE isolated in blood; and the most common type of infection was spontaneous peritonitis, in 4 (36.5%). Among those 11 patients, the median time between CRE infection and LT was 3 days. Of those, 8 patients underwent LT with active infection. However, even patients who had no evidence of infection were given CRE treatment until LT. Of 57 patients who did not develop infection in pre-LT period, only 2 (3.5%) received antibiotic prophylaxis that included an antimicrobial agent with proven activity against CRE. Of the 68 patients in whom CRE were identified in the pre-LT period, 28 (36.8%) evolved to post-LT CRE infection.
Post-LT CRE Acquisition
During the post-LT period, 119 (30.8%) of the 386 patients acquired CRE after LT. In 7 (5.8%) cases, the patient tested positive for 1 CRE species in the pre-LT period and acquired a different species in the post-LT period (Figure 1). The most common species isolated in the post-LT period was Klebsiella pneumoniae, in 99 patients (83.2%). Of those 99 isolates, 26 (26.3%) were resistant to polymyxin. The SCs were positive in 111 (93.3%) of the 119 patients who acquired CRE after LT. In 106 (89.1%) patients, the first CRE-positive culture was a SC, and 39 (36.8%) of those patients developed post-LT CRE infection.
FIGURE 1.
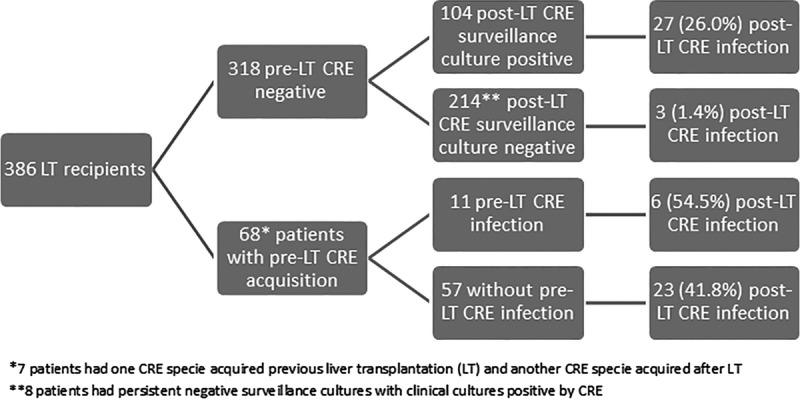
Distribution of CRE infection according time of CRE acquisition.
Of the 119 CRE species identified in the post-LT period, 112 (92.6%) were tested for blaKPC, of which 52 (46.4%) tested positive, among blaKPC negative strains, 47 (78.3%) strains were positive by for CTX-M group 8 to 25 and 10 strains (16.7%) were positive for CTX-M group 1-2-9.
As can be seen in Figure 2, the proportion of CRE strains that were tested positive for blaKPC increased over the study period (P = 0.07). No other carbapenemase was identified in study period.
FIGURE 2.
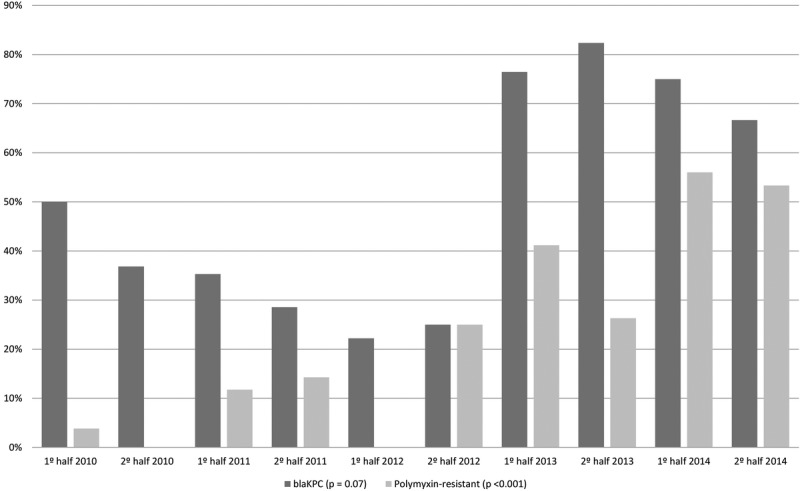
Proportion of isolated strains that were blaKPC-producing, as well as the proportion that showed an elevated MIC for polymyxin, among 99 patients found to be infected with carbapenem-resistant Klebsiella pneumoniae between January 2010 and December 2014.
Post-LT CRE Infection
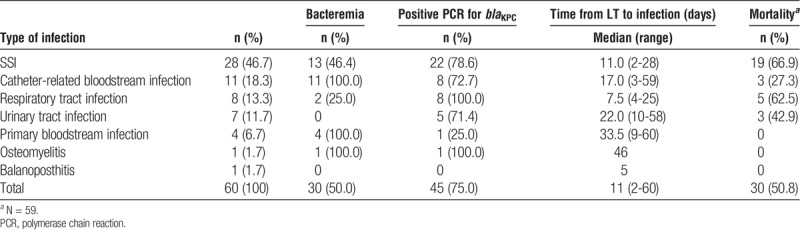
In the post-LT period, 60 CRE infections were identified in 59 (15.7%) of the 386 patients. The most common type of infection was SSI, all classified as organ space, which was seen in 28 (46.7%) of those 59 patients, followed by catheter-related bloodstream infection, in 11 (18.3%). The median time between LT and the first post-LT CRE infection was 11 days (range, 2-60 days), the median time between identified CRE acquisition and infection was 8 days (range, 0-219 days). Ten (16.9%) of the 59 patients developed a CRE infection despite showing no evidence of previous positive SC. Bacteremia was detected in 30 (50.0%) of the 60 post-LT CRE infections. Six patients evolved to death before starting the antibiotic therapy for CRE infection. Among those who received treatment, 15 (27.8%) received monotherapy and 72.2% (39) received combined therapy. The most common treatments used were carbapenem plus colistin (11-20.4%), and carbapenem + colistin + amikacin (11-20.4%) followed by carbapenem + colistin + tigecycline + amikacin (6-11.1%). Of the 59 patients with 60 post-LT CRE infection, 30 (50.8%) died within the first 60 days after LT (Table 2), no specific type of treatment was associated with a lower mortality.
TABLE 2.
Type of infection, concomitant bacteremia, time to infection after LT, and 60-day mortality in 59 patients with post-LT Carbapenem-resistant Enterobacteriaceae infection
Risk Factors
In the multivariate analysis, post-LT dialysis was identified as the only risk factor for post-LT CRE acquisition (Table 3). Risk factors for developing a CRE infection were pre-LT CRE acquisition, MELD score greater than 32 at LT, combined transplant, reoperation, 3 or more post-LT dialysis sessions, and post-LT CRE acquisition (Table 4). In the CART analysis, the most important variable for discriminating between LT recipients who would and would not develop CRE infection was pre-LT CRE acquisition. The other major risk factor for post-LT CRE infection was requiring 3 or more post-LT dialysis sessions, which was found to increase that risk in regardless of the moment of CRE acquisition (Figure 3). When only CRE colonized patients were analyzed, the risk factors for developing CRE infection were length of time of abdominal drainage (P = 0.02; OR, 1.05 [1.01-1.09, 95%CI]), whether dialysis was performed after LT (P = 0.01; OR, 2.80 [1.28-6.15, 95%CI]), acquisition of CRE before LT (P = 0.03; OR, 2.20 [1.11-4.39, 95%CI]); combined transplant was almost statistically significant (P = 0.06; OR, 3.56 [0.94-13.53, 95%CI]).
TABLE 3.
Univariate and multivariate analyses of risk factors for post-liver transplantation carbapenem resistant Enterobacteriaceae acquisition in 325 LT recipients
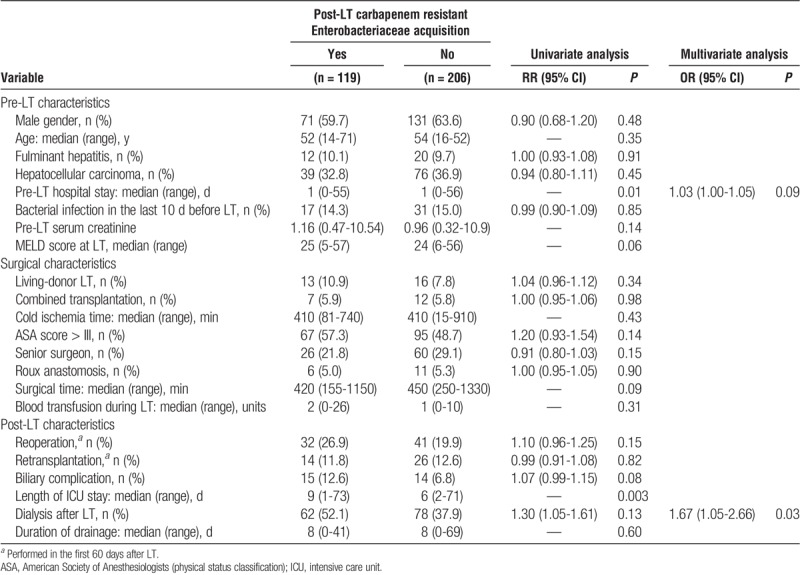
TABLE 4.
Univariate and multivariate analyses of risk factors for post-LT carbapenem-resistant enterobacteriaceae infection in 378 LT recipients
FIGURE 3.
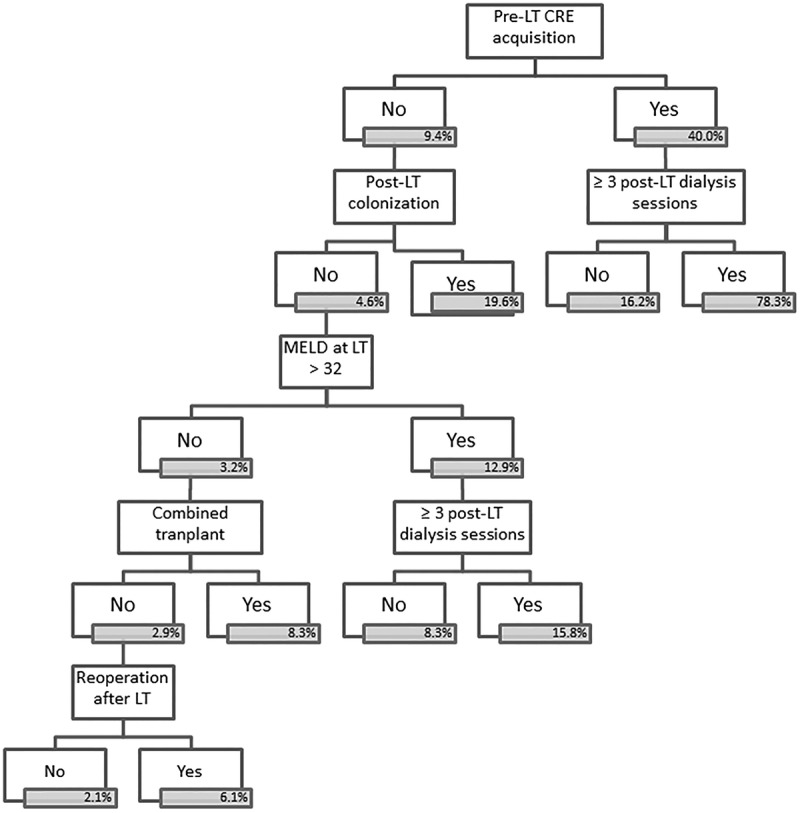
CART analysis of risk factors for infection with CRE among 386 patients undergoing LT. In blue boxes the incidence of CRE infection in that subgroup, in node square de P value for of the prediction of the outcome in this particular subgroup.
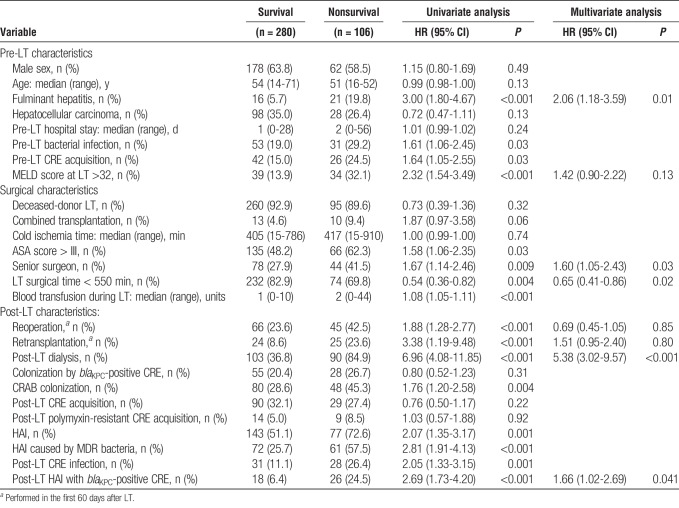
The overall 60-day mortality rate was 27.4%. The most common cause of death was bacterial or fungal infection (50.9%), followed by primary graft dysfunction (13.2%),14 and surgical complications (8.5%).9 Among the patients who developed a CRE infection, 60-day mortality was 50.0%. Risk factors for 60-day mortality were fulminant hepatitis as the cause of LT, surgical time longer than 550 minutes, post-LT dialysis, and HAI with a blaKPC-positive CRE species. The LT being performed by a senior surgeon was identified as a protective factor (Table 5).
TABLE 5.
Univariate and multivariate analyses of risk factors for 60-day mortality in 386 LT recipients
PFGE
We analyzed 62 strains of carbapenem-resistant K. pneumoniae from 47 patients and identified 25 different PFGE clusters. A pulsotype A1 cluster was observed in the first quarter of 2010, and a pulsotype B1 cluster, in the second quarter of 2010. Of the 62 strains isolated, 26 were KPC-producing. In 12 patients who developed post-LT CRE infection, paired strains were analyzed (from SCs—from clinical cultures related to infection). In 5 patients, pre-LT and post-LT paired strains were analyzed. Among those, 4 clusters were identified, and all paired strains belonged to the same cluster. In 7 patients, paired post-LT SCs and clinical cultures related to infection were analyzed. Seven PFGE clusters were identified, and all paired strains belonged to the same cluster (Figure S1, SDC, http://links.lww.com/TP/B384).
DISCUSSION
The incidence of post-LT CRE infection varies widely among transplant centers, ranging from 3% to 23%, and recent studies have reported higher rates of CRE colonization and infection.9,16,17 At our facility, the incidence of CRE colonization is high, and a progressive increase in the proportion of KPC-producing strains was noticed over the study period.18 Because of the high prevalence of CRE colonization, 15% of our patients developed post-LT CRE infection. However, the proportion of colonized patients who developed infection was lower than that reported in previous studies10,17—36.8% versus 48.8% to 88.9%.
There have been few studies analyzing the risk of CRE acquisition and infection in LT patients in a context of weekly screening. Giannella et al17 analyzed 237 LT recipients who were screened weekly for CRE starting at LT and identified CRE acquisition at any time as the variable most strongly correlated with post-LT CRE infection. In our study, previous colonization was also the most important risk factor for developing CRE infection, although we found pre-LT acquisition to be associated with a higher risk than was post-LT acquisition. One possible explanation for this finding is that pre-LT CRE acquisition could be a marker of poorer clinical conditions. Another is that the standard antibiotic prophylaxis might have been less effective in the patients colonized by CRE.
There are few data regarding outcomes in SOT recipients colonized by MDR-GNB before transplantation, and the impact of modifying the surgical prophylaxis has not been established.17,19,20 One study of infection with carbapenem-resistant Acinetobacter baumannii also reported that pre-LT acquisition is a risk factor for post-LT infection.21 However, the authors of that study found that adjusting the antibiotic prophylaxis was not preventive of infection. Our institutional protocol did not recommend adjusting prophylaxis regarding patient colonization. As a result, the number of patients in whom the antibiotic prophylaxis included an antimicrobial agent with proven activity against CRE was very small, which prevents us from drawing any conclusions regarding that at this time.
Although pre-LT CRE acquisition had a significant impact on the risk of developing CRE infection in our study, post-LT acquisition was the second most important risk factor. This finding is similar to that reported by Giannella et al,17 supporting the recommendation to perform systematic post-LT SC for CRE at facilities where the prevalence of CRE infection is high. Early identification can increase the precision of empirical therapy in patients with infection and could reduce the mortality.22
The need for dialysis was also identified as a risk factor for post-LT CRE infection in our study. Requiring dialysis is a well-documented risk factor for bacterial infection in LT and has previously been shown to increase the risk of CRE infection, as well as for infection with other MDR-GNB, in LT recipients.17,21,23 It is of note that, in the present study, dialysis increased the risk of CRE infection to a greater degree for the patients who had acquired CRE in the pre-LT period than for those who had not. The possible explanation for that finding is that patients requiring dialysis are more often subjected to the use of invasive devices and are more often admitted to the intensive care unit, both of which increase the risk of bacterial infection.1,6 Renal failure itself can be immunosuppressive and an indirect marker of graft malfunction. Acute kidney injury can also be seen in LT recipients with severe preoperative hepatic impairment. All of these factors can increase the of MDR-GNB invasive infection.
We found that the risk of CRE infection was also increased among the patients with severely impaired hepatic function at LT, especially among those requiring dialysis. A high MELD score at LT has been associated with a greater risk of bacterial infection, specifically post-LT bacteremia caused by Enterobacteriaceae.24 One study evaluating the risk of developing infection with carbapenem-resistant or carbapenem-susceptible K. pneumoniae also found that a high pre-LT MELD score is a risk factor for post-LT infection.9
In the present study, the CART analysis identified a group at intermediate risk for post-LT CRE infection. That group comprised patients undergoing combined transplant or reoperation. It is reasonable to hypothesize that the risk of becoming infected with MDR bacteria is higher for patients undergoing surgical procedures that are more complex. Another study identified combined transplant as a risk factor for post-SOT infection with carbapenem-resistant K. pneumoniae.5 Reoperation has also been previously identified as a risk factor for post-LT SSI,25 the most common type of CRE infection in the present study.
The 2 most common types of CRE infection in our sample were SSI and catheter-related bloodstream infection, similar to what has been reported in previous studies of CRE in LT.8–10,17 Although SSI was the most common site, CRE infection was not associated to biliary complication, probably because such infections occurred in the early post-LT period. We also identified a high rate of bacteremia associated with such infections. The 60-day mortality rate in patients with CRE infection was 50%, and mortality was highest among the patients with SSI or respiratory infection. In the literature, the mortality associated with CRE infection in LT recipients ranges from 41% to 72%.8,10,16,17,26 In our mortality analysis, we found that only infection with KPC-producing strains was associated with a worse outcome. Among the patients infected with KPC-producing CRE in our sample, the 60-day mortality rate after LT was 59.1%, compared with 13.3% among those infected with KPC-negative CRE and 23.9% among those not infected with CRE. Bogan et al27 reported that mortality was higher among patients infected with KPC-producing K. pneumoniae than among those infected with extended-spectrum β-lactamase–producing K. pneumoniae, suggesting that the former is more virulent. That difference could be attributable to the fact that the initial antibiotic therapy was inappropriate in a high proportion of the patients infected with CRE.27 Also, strains of KPC-negative CRE usually have lower MIC for carbapenem, which contributes to a better response to antibiotic therapy, specifically in the cases where carbapenem was used.28
This study included a large number of patients in a scenario with high incidence of CRE, allowing statistical power. However, this was a single study center, and the results have to be extrapolated with caution, especially in institutions with low incidence of CRE. Another limitation was that only in half of the cases of CRE the carbapenem resistance was due to blakpc production, which is currently the most frequent mechanism of carbapenem resistance.
We found that the incidence of post-LT CRE infections was high among patients previously colonized by CRE, especially among those who had acquired CRE in the pre-LT period. Various combinations of risk factors have different effects on the risk of CRE infection, and knowledge of those interactions is crucial to designing strategies to prevent CRE infection and to introduce empirical therapy for such infection. Patients who were colonized by CRE when they underwent LT were found to be at a high risk for developing infection, and measures for minimizing that risk, such as adjusting the antibiotic prophylaxis, should be investigated and implemented.
ACKNOWLEDGMENTS
The authors are grateful to laboratory assistant Juliana Januario Gautereto, for the technical support and assistance provided.
Footnotes
This study received financial support from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation; Grant no. 2010/02267-6).
The authors declare no conflicts of interest.
M.P.F. participated in the performance of the research, in the writing of the article and in data analysis. I.C.V.SO participated in the performance of the research and in the data collection. L.C.P. participated in the performance of the research and in the writing of the article. P.R.B. participated in the performance of the research. L.M.D.O. participated in the performance of the research. A.W.S. participated in the performance of the research. C.H.C participated in the performance of the research. I.M.v.d.H. participated in the performance of the research. F.R. participated in the performance of the research. S.F. C. participated in the performance of the research and in the revised the article. L.A.C.D’A. participated as general supervision of transplant unit and in the performance of the research. E.A. participated in research design, in the performance of the research and in the writing of the article.
Correspondence: Maristela Pinheiro Freire, Infection Control Team, University of São Paulo School of Medicine Hospital das Clínicas, São Paulo, Brazil. (maristelapf@uol.com.br).
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
The authors of this prospective study establish risk factors for carbapenem-resistant Enterobacteriaceae, an emergent microorganism of infections after liver transplantation, highlighting that colonization before transplantation is one of the main risk factors that could potentially be targetted with adequate antibiotic prophylaxis. Supplemental digital content is available in the text.
REFERENCES
- 1.Zhong L, Men TY, Li H. Multidrug-resistant gram-negative bacterial infections after liver transplantation—spectrum and risk factors J Infect 2012. 64299–310 [DOI] [PubMed] [Google Scholar]
- 2.Bodro M, Sabé N, Tubau F. Risk factors and outcomes of bacteremia caused by drug-resistant ESKAPE pathogens in solid-organ transplant recipients Transplantation 2013. 96843–849 [DOI] [PubMed] [Google Scholar]
- 3.Ye QF, Zhao J, Wan QQ. Frequency and clinical outcomes of ESKAPE bacteremia in solid organ transplantation and the risk factors for mortality Transpl Infect Dis 2014. 16767–774 [DOI] [PubMed] [Google Scholar]
- 4.van Duin D, van Delden C. Multidrug-resistant gram-negative bacteria infections in solid organ transplantation Am J Transpl 2013. 1331–41 [DOI] [PubMed] [Google Scholar]
- 5.Freire MP, Abdala E, Moura ML. Risk factors and outcome of infections with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in kidney transplant recipients Infection 2015. 43315–323 [DOI] [PubMed] [Google Scholar]
- 6.Shi SH, Kong HS, Xu J. Multidrug resistant gram-negative bacilli as predominant bacteremic pathogens in liver transplant recipients Transpl Infect Dis 2009. 11405–412 [DOI] [PubMed] [Google Scholar]
- 7.Gyung Y, Choi SH, Choo EJ. Risk factors for the acquisition of carbapenem-resistant Klebsiella pneumonia among hospitalized patients Microb Drug Resist 2005. 11165–168 [DOI] [PubMed] [Google Scholar]
- 8.Kalpoe JS, Sonnenberg E, Factor SH. Mortality associated with carbapenem-resistant Klebsiella pneumoniae infections in liver transplant recipients Liver Transpl 2012. 18468–474 [DOI] [PubMed] [Google Scholar]
- 9.Pereira MR, Scully BF, Pouch SM. Risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae infections in liver transplant recipients Liver Transpl 2015. 211511–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lübbert C, Becker-Rux D, Rodloff AC. Colonization of liver transplant recipients with KPC-producing Klebsiella pneumoniae is associated with high infection rates and excess mortality: a case-control analysis Infection 2014. 42309–316 [DOI] [PubMed] [Google Scholar]
- 11.Munoz-Price LS, Poirel L, Bonomo RA. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases Lancet Infect Dis 2013. 13785–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC/NHSN Surveillance Definitions for Specific Types of Infections. CDC/NHSN. [Online] January de 2016. http://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf. [Google Scholar]
- 13.M100-S22: Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. Institute, Clinical and Laboratory Standards. 2012;Vol 32 No. 3. [Google Scholar]
- 14.Gautom RK. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day J Clin Microbiol 1997. 352977–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.PulseNet/CDC. Pathogens & Protocols. Pulsenet. [Online] April de 2013. http://www.cdc.gov/pulsenet/PDF/ecoli-shigella-salmonella-pfge-protocol-508c.pdf. [Google Scholar]
- 16.Satlin MJ, Jenkins SG, Walsh TJ. The global challenge of carbapenem-resistant Enterobacteriaceae in transplant recipients and patients with hematologic malignancies Clin Infect Dis 2014. 581274–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giannella M, Bartoletti M, Morelli MC. Risk factors for infection with carbapenem-resistant Klebsiella pneumoniae after liver transplantation: the importance of pre- and posttransplant colonization Am J Transplant 2015. 151708–1715 [DOI] [PubMed] [Google Scholar]
- 18.de Oliveira MS, de Assis DB, Freire MP. Treatment of KPC-producing Enterobacteriaceae: suboptimal efficacy of polymyxins Clin Microbiol Infect 2015. 21179.e1–179.e7 [DOI] [PubMed] [Google Scholar]
- 19.Bert F, Larroque B, Dondero F. Risk factors associated with preoperative fecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae in liver transplant recipients Transpl Infect Dis 2014. 1684–89 [DOI] [PubMed] [Google Scholar]
- 20.Kim YJ, Kim SI, Jun YH. Clinical significance of surveillance culture in liver transplant recipients Transplant Proc 2014. 46828–831 [DOI] [PubMed] [Google Scholar]
- 21.Freire MP, Pierrotti LC, Oshiro IC. Carbapenem resistant Acinetobacter baumannii acquired before liver transplantation: impact on recipient outcomes Liver Transpl 2016. 22615–626 [DOI] [PubMed] [Google Scholar]
- 22.Dellinger RP, Levy MM, Rhodes A. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012 Crit Care Med 2013. 41580–637 [DOI] [PubMed] [Google Scholar]
- 23.Singh N, Gayowski T, Wagener MM. Posttransplantation dialysis-associated infections: morbidity and impact on outcome in liver transplant recipients Liver Transpl 2001. 7100–105 [DOI] [PubMed] [Google Scholar]
- 24.Bellier C, Bert F, Durand F. Risk factors for Enterobacteriaceae bacteremia after liver transplantation Transpl Int 2008. 21755–763 [DOI] [PubMed] [Google Scholar]
- 25.García Prado ME, Matia EC, Ciuro FP. Surgical site infection in liver transplant recipients: impact of the type of perioperative prophylaxis Transplantation 2008. 851849–1854 [DOI] [PubMed] [Google Scholar]
- 26.Clancy CJ, Chen L, Shields RK. Epidemiology and molecular characterization of bacteremia due to carbapenem-resistant Klebsiella pneumoniae in transplant recipients Am J Transplant 2013. 132619–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogan C, Kaye KS, Chopra T. Outcomes of carbapenem-resistant Enterobacteriaceae isolation: matched analysis Am J Infect Control 2014. 42612–620 [DOI] [PubMed] [Google Scholar]
- 28.Tumbarello M, Trecarichi EM, De Rosa FG. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study J Antimicrob Chemother 2015. 702133–2143 [DOI] [PubMed] [Google Scholar]