Abstract
There has recently been considerable progress in the results of pig organ transplantation in nonhuman primates, largely associated with the availability of (i) pigs genetically engineered to overcome coagulation dysregulation, and (ii) novel immunosuppressive agents. The barriers of thrombotic microangiopathy and/or consumptive coagulation were believed to be associated with (i) activation of the graft vascular endothelial cells by a low level of antipig antibody binding and/or complement deposition and/or innate immune cell activity, and (ii) molecular incompatibilities between the nonhuman primate and pig coagulation-anticoagulation systems. The introduction of a human coagulation-regulatory transgene, for example, thrombomodulin, endothelial protein C receptor, into the pig vascular endothelial cells has contributed to preventing a procoagulant state from developing, resulting in a considerable increase in graft survival. In the heterotopic (non–life-supporting) heart transplant model, graft survival has increased from a maximum of 179 days in 2005 to 945 days. After life-supporting kidney transplantation, survival has been extended from 90 days in 2004 to 499 days. In view of the more complex coagulation dysfunction seen after pig liver and, particularly, lung transplantation, progress has been less dramatic, but the maximum survival of a pig liver has been increased from 7 days in 2010 to 29 days, and of a pig lung from 4 days in 2007 to 9 days. There is a realistic prospect that the transplantation of a kidney or heart, in combination with a conventional immunosuppressive regimen, will enable long-term recipient survival.
The pig-to-nonhuman primate (NHP) model has become the standard experimental model in xenotransplantation research.1–5 Survival of wild-type (ie, genetically unmodified) pig kidneys in NHPs was generally no longer than a few minutes, and the longest life-supporting wild-type kidney graft survival was 22 days in 1998.4,6
Genetic modification of the organ-source pig was first reported by Fodor et al7 in 1994, and Cozzi and White8 in 1995. Both groups generated pigs expressing human complement-regulatory proteins, CD597 and CD55 (decay-accelerating factor),8 respectively. Transplantation in NHPs of organs from White’s CD55 transgenic pigs was associated with prolongation of graft survival (reviewed in Lambrigts et al6), with Baldan et al9 extending pig kidney graft survival to 90 days in 2004. The introduction of costimulation blockade by Bühler et al10 largely resolved the problem of the adaptive immune response, inhibiting the production of de novo anti-pig antibodies. The availability of α1,3-galactosyltransferase gene-knockout (GTKO) pigs, produced in 2003 by Phelps et al11 and in 2004 by Kolber-Simonds et al,12 extended pig heart graft survival in baboons to 179 days.13 The combination of GTKO and expression of a human complement-regulatory protein extended survival further.14
However, the barriers of thrombotic microangiopathy and/or consumptive coagulation then became more obvious.15,16 These complications were believed to be associated with (i) low-grade activation of the graft vascular endothelial cells (VECs) by a low level of anti-pig (non-Gal) antibody binding and/or complement deposition and/or innate immune cell activity, and (ii) molecular incompatibilities between the NHP and pig coagulation-anticoagulation systems.17,18 The activated VECs became procoagulant, and this could not be successfully controlled by the pig’s anticoagulant factors. The introduction of a human coagulation-regulatory transgene, for example, thrombomodulin, endothelial protein C receptor, into the NHP VECs, contributed greatly to preventing a procoagulant state from developing.
These genetic manipulations,19–25 coupled with improvements in the immunosuppressive regimens used, resulted in further progress in pig-to-NHP organ transplant models. It is this progress that we review here.
HEART
Considerable progress has been made in the pig-to-NHP heterotopic (non–life-supporting) heart xenotransplantation model but, before clinical trials can take place,26,27 this needs to be duplicated (at least to some extent) in the orthotopic (life-supporting) heart transplant model.
Heterotopic (Abdominal) Pig Heart Xenotransplantation
A benchmark was set by Kuwaki et al13 in 2005 when, by transplanting hearts from the first GTKO pigs that became available, graft survival of 179 days was achieved (Table 1A, Figure 1). Extensive thrombotic microangiopathy developed in the graft, leading to consumptive coagulopathy in the recipient. The experiment was also problematic because an anti-CD154 monoclonal antibody (mAb), which formed the basis of the immunosuppressive regimen, had been found to have thrombogenic properties.40,41 Whether the agent contributed to the thrombotic microangiopathy remains unknown, but probably did not because thrombotic microangiopathy can clearly occur when this agent is not being administered.
TABLE 1.
Recent progress in pig-to-NHP heart xenotransplantation
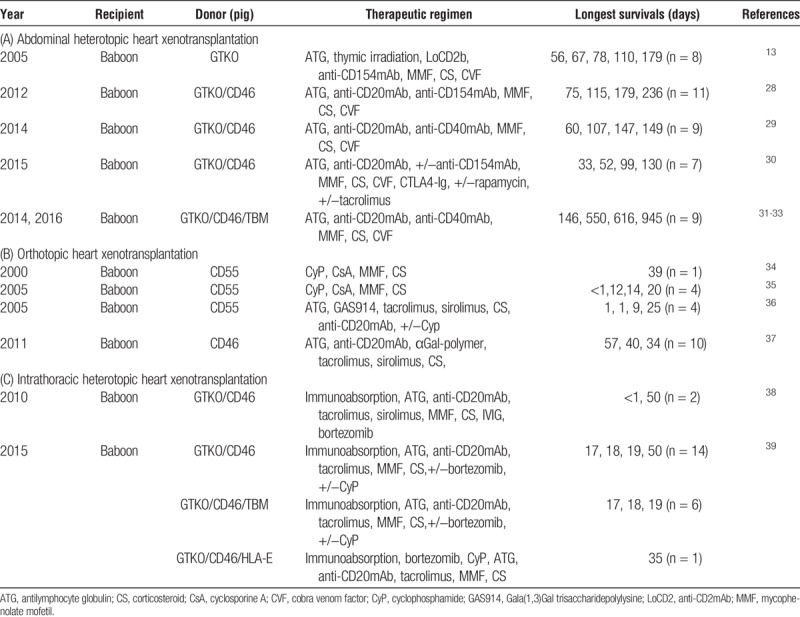
FIGURE 1.
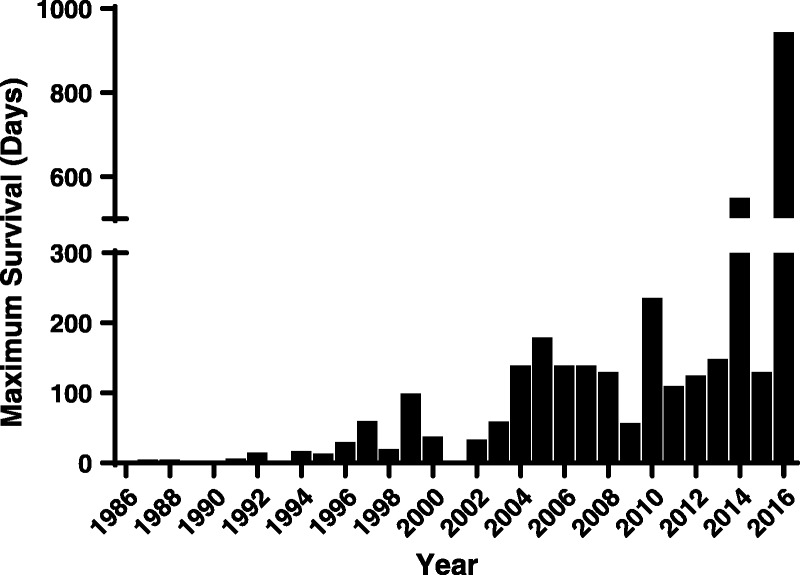
In heterotopic heart xenotransplantation, maximum survival has been improved from <8 hours in 1986 to 945 days.
In 2012, graft survival was increased to 236 days by adding a B cell–depleting agent (anti-CD20mAb) to the induction regimen (previously advocated by McGregor and his colleagues,42 but the maintenance regimen remained based on an anti-CD154mAb (Table 1).28 After replacing the anti-CD154mAb with an anti-CD40mAb, and, importantly, by adding a human coagulation-regulatory protein (thrombomodulin) on to the pig’s GTKO/CD46 background, the same group extended graft survival beyond 1 year (to 550 days), a milestone in the progress in xenotransplantation research (Table 1).31,32 In 2016, a further significant prolongation of graft survival to 945 days was achieved, using the same source pig and the same immunosuppressive regimen (Table 1, Figure 1).33
Encouraging results were also obtained by our own group,30 but without the extremely long graft survival reported by Mohiuddin and his colleagues. The discrepancy in these results may possibly be explained by the facts that (i) at that time, we did not include a B cell–depleting agent in our regimen, and (ii) Mohiuddin maintained a continuous intravenous infusion of heparin throughout the experiment, which would clearly not be ideal for clinical application. Mohiuddin’s regimen, therefore, requires some modifications before it would be applicable to clinical use. It will also be necessary to reproduce these encouraging results in an orthotopic, life-supporting model.
Life-supporting Orthotopic and Partial Life-supporting Intrathoracic Heterotopic Heart Xenotransplantation
There have been relatively few life-supporting pig orthotopic heart xenotransplants carried out in NHPs in recent years.35,36,43–50 A benchmark was set by Vial et al34 in 2000 when survival of 39 days was reported, using a CD55 transgenic pig heart (Table 1B). Byrne et al37 extended this survival to 57 days, using a pig transgenic for CD46 and, in the absence of GTKO, with the infusion of a Gal-polymer (to block anti-Gal antibody) (Table 1B).42 However, this group (and others) experienced a high early failure from a condition they have termed “perioperative cardiac xenograft dysfunction.”51 The exact reasons for this remain uncertain, but (i) technical problems, (ii) ischemia-reperfusion injury, and perhaps (ii) the impact of early antibody binding and complement activation (though insufficient to cause rejection) may be playing roles.51 However, a significant fall in thyroid hormones, particularly in free triiodothyronine (fT3), which occurs during cardiopulmonary bypass52–56 and also after any xenotransplant procedure,57,58 and is associated with a reduction in myocardial energy stores,59 may be a major causative factor. Exploration of this possibility is certainly indicated (recently, however, survival after orthotopic heart transplantation has been extended to 90 days [Reichart B, oral communication, September 2017]).
Survival after partial life-supporting intrathoracic heterotopic heart transplantation has also been limited, probably for the same reasons, with maximal survival of 50 days (Table 1C).38,39
In summary, the addition of a human coagulation-regulatory protein, for example, thrombomodulin,60 to the pig would appear to have largely overcome the combined barrier of thrombotic microangiopathy and consumptive coagulopathy, facilitating prolongation of graft survival. B cell depletion may also have contributed to the improved outcome. We suggest that studies should aim for consistent survival (eg, 5 of 6 experiments) of a NHP with a life-supporting pig heart for periods of at least 3 months (or possibly 6 months) with (i) a clinically applicable immunosuppressive regimen, and an absence of (ii) major life-threatening complications (eg, infections) and of (iii) histopathological features of antibody-mediated or cellular rejection and/or thrombotic microangiopathy.
KIDNEY
By 2004, CD55 kidney graft survival had been extended to 90 days in cynomolgus monkeys (Table 2, Figure 2).9 GTKO pig kidney graft survival reached 83 days in 2005 (with cotransplantation of donor-specific thymic tissue) (Table 2).61 Thereafter, pig kidney transplantation in NHPs showed only slow improvement until 2015 (Figure 2).
TABLE 2.
Recent progress in pig-to-NHP kidney xenotransplantation
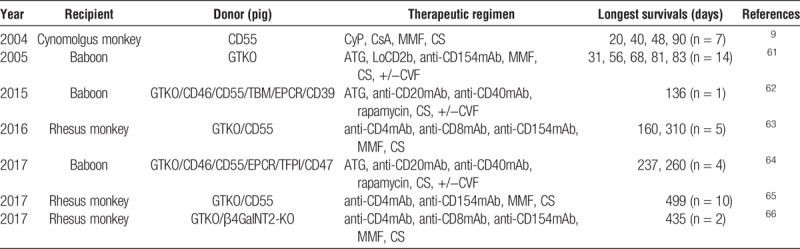
FIGURE 2.
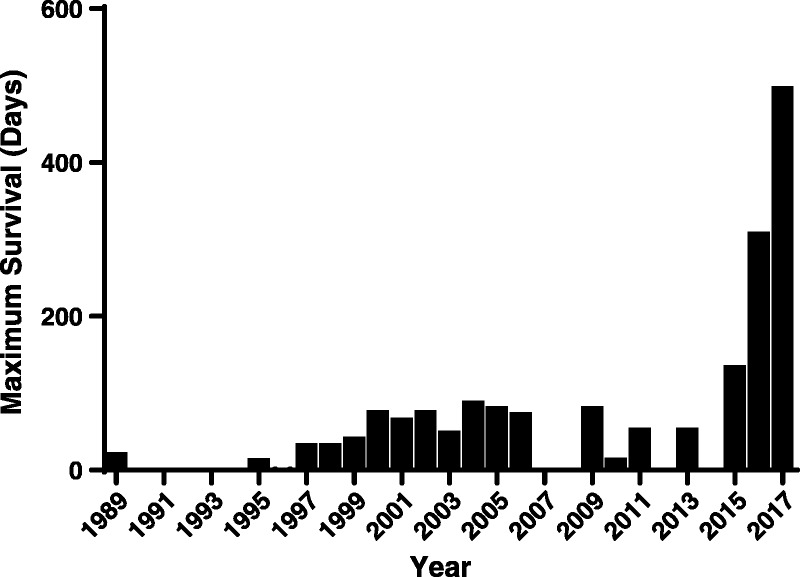
In kidney xenotransplantation, maximum survival has been significantly improved from 23 days in 1989 to >1 year. The longest survival was reported 499 days in 2017.
Higginbotham et al67 reported kidney recipient survival of greater than 122 and greater than 130 days (both monkeys ongoing at the time of the report) after the transplantation of GTKO/CD55 pig kidneys in rhesus monkeys selected for their low titers of antipig antibodies (Table 2). The immunosuppressive regimen differed to some extent from that used by most other groups, but was still based on costimulation blockade (with anti-CD154mAb). These 2 monkeys were followed further, the grafts failing at 160 and 310 days, respectively, with histopathological features of acute humoral xenograft rejection (Table 2, Figure 2).63
The Higginbotham study is important not only for achieving prolonged graft survival but also for drawing attention to the role played by anti-pig antibody in that survival. Using the same pig source and an identical immunosuppressive regimen, a monkey with a high level of anti-pig (non-Gal) antibodies rejected its graft within 6 days, indicating the importance of antibody to the outcome. This observation follows similar observations (though not followed up) by Kuwaki et al13 in 2005, who had selected NHPs with low antipig (non-Gal) antibody levels to offer an advantage.
From the same group as Higginbotham, Kim et al65 reported 499 days survival of a pig kidney graft (Table 2, Figure 2). Furthermore, using the same immunosuppressive regimen as Higginbotham, Martens et al66 reported kidney graft survival of >400 days in a rhesus monkey when the kidney was taken from a GTKO/β4GalNT2-KO pig (that did not express either Gal or Sda) (Table 2, Figure 2). No human complement- or coagulation-regulatory proteins were expressed in the pig.
Our own group has reported prolonged survival of a kidney grafts from pigs with multiple genetic modifications that included 2 human coagulation-regulatory transgenes (Table 2). One functioned in a baboon (with a high titer of anti-pig IgM) for 136 days, before having to be euthanized for a rare systemic infection.62 Two others remained healthy for 237 and 260 days, respectively, when again infectious complications led to termination of the experiments (Table 2).64 Biopsies in all 3 kidneys showed normal histology. Renal graft function remained normal with minimal proteinuria with no accompanying hypoalbuminemia. There were no or minimal features of a consumptive coagulopathy.
The importance of the expression of a human coagulation-regulatory protein is emphasized by the observation that 2 other baboons, treated identically to those above, but with very poor expression of a coagulation-regulatory protein (human thrombomodulin) in the kidneys, required euthanasia after 12 days for the development of a consumptive coagulopathy (Table 2).64 The difference in outcome between these 2 baboons (with pig kidneys not expressing an effective human coagulation-regulatory protein) and the 2 summarized above (with pig kidneys expressing 2 effective human coagulation-regulatory proteins) is remarkable, and provides support for the importance of expression of these proteins in this model.
However, it is of considerable interest to note that the pig kidneys transplanted into rhesus monkeys by Higginbotham and colleagues did not express a human coagulation-regulatory protein, and also the immunosuppressive regimen included the “thrombogenic” anti-CD154mAb. Furthermore, heparin was not administered to these monkeys. It is perhaps remarkable that neither a thrombotic microangiopathy nor a consumptive coagulopathy developed in either of these monkeys, suggesting that there may be biological differences between monkey and baboon. Whether a human recipient would be anticipated to be more comparable to the baboon or rhesus monkey in this regard is uncertain. However, other subtle differences in the immunosuppressive regimen may have influenced the outcome (Table 2).
The significant progress in pig kidney transplantation in baboons (though not in rhesus monkeys) has been achieved to a significant extent because of the protection of the pig tissues from the primate immune response and from coagulation dysregulation provided by the genetic manipulation of the pig.11,68,69 Effective immunosuppressive therapy and anti-inflammatory therapy in the form of tocilizumab (Table 2) almost certainly contributed to the good outcome (tocilizumab has significant effects on the immune system).70 In rhesus monkeys, however, the expression of either a human complement- or coagulation-regulatory protein would seem to be unnecessary. The differences between these 2 NHP species require further investigation.
LIVER
The rapid development (within minutes) of profound thrombocytopenia reported by Ekser et al71–74 after orthotopic GTKO or GTKO/CD46 pig liver transplantation in baboons was problematic and formed a basis for further studies (Table 3, Figure 3). Apart from this major complication, pig hepatic function appeared to be quite good (for up to 7 or 8 days),75,78,79 and so subsequent studies have been directed toward resolving this problem.80
TABLE 3.
Recent progress in pig-to-NHP liver xenotransplantation

FIGURE 3.
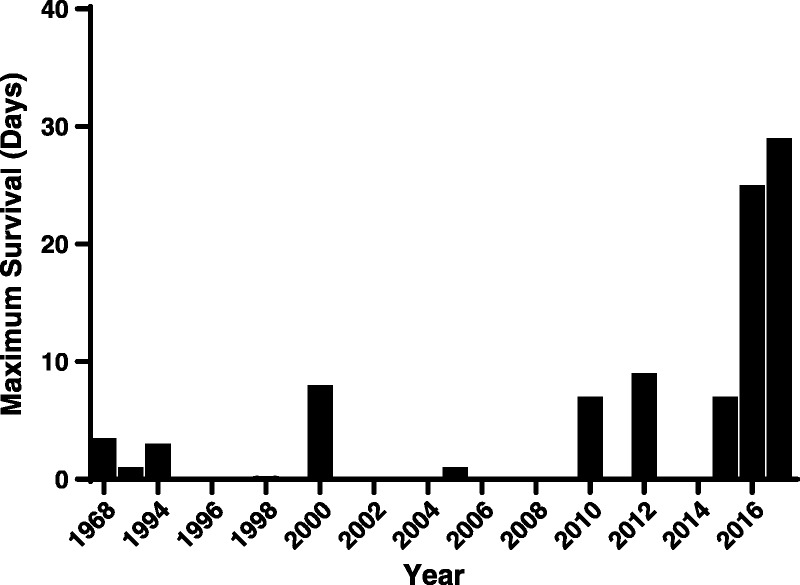
Only few data related to liver xenotransplantation have been reported, with the longest survival being 29 days.
A major factor would appear to be incompatibilities of cell surface receptor-ligand interactions between pig endothelial cells and primate platelets81,82 or the presence of natural antibodies to pig liver endothelial antigens, leading to endothelial cell activation.81,82
In vitro and in vivo studies suggested potential factors in the causation of thrombocytopenia and dysregulation of coagulation to be (i) asialoglycoprotein receptor 1 (ASGR1) involvement in xenogeneic platelet phagocytosis (seen in vitro and in a pig-to-human ex vivo perfusion model),83 and (ii) incompatibilities between species (eg, CD47/signal-regulatory protein alpha, CD18, macrophage antigen complex-1, von Willebrand Factor [vWF]/GPIb).82,84
Subsequently, Kim et al74 achieved 9 days of survival using a GTKO pig liver (Table 3, Figure 3). The administration of aminocaproic acid (amicar) resulted in the partial resolution of thrombocytopenia, maintaining platelet counts greater than 40 000/mm3 throughout the study. However, the recipient died from bleeding and enterococcal infection, though there was no evidence of rejection.
Most recently, Shah et al77 reported 25 and 29 days of life-supporting orthotopic GTKO pig liver xenograft survival with stable hepatic function (demonstrated by the production of porcine proteins and coagulation factors), using a continuous infusion of human prothrombin concentrate complex (exogenous human coagulation factor, human Factor VIIa) (Table 3). The baboons were euthanized because of plantar ulcerations and deteriorating liver function.
In summary, disturbances of coagulation were again at the core of survival of pig liver graft recipients, though these disturbances varied to some extent from those seen after heart and kidney xenotransplantation, requiring different genetic modifications of the source pig, for example, expression of human CD47, ASGR-KO. Treatment of the recipient with coagulation factors is less than ideal, and more effort needs to be directed to correct the deficiencies by genetic engineering. Nevertheless, an extension of graft survival to almost 1 month (in the absence of rejection) indicates that progress is being made.
Because there is no support available to patients in fulminant or severe hepatic failure (comparable to dialysis or a ventricular assist device), survival of a pig graft may not need to be for more than a few days or weeks to be considered for clinical application as a bridge to allotransplantation.85 Nevertheless, there has to be conclusive evidence from preclinical models that the treatment necessary to maintain the xenograft will not be detrimental to the outcome of the subsequent allograft.
LUNG
Donor shortage is problematic across all organ transplants, but lungs have the lowest harvest rates,86 increasing the need for an alternative source. Even lung allografts are highly sensitive to injury and to multiple immune rejection mechanisms,87,88 rendering the barrier to lung xenotransplantation, particularly high, and certainly higher than to other vital organs.89
Several experimental models have been established to study lung xenotransplantation.90,91 Most studies have employed ex vivo pig lung perfusion with human blood,92,93 but unilateral pig lung transplantation in baboons is also being carried out (Table 4). A complex interaction of inflammation, coagulation, and tissue injury leads to relatively rapid lung xenograft failure, both ex vivo during perfusion with human blood and after transplantation into a NHP. This is illustrated by the observation that, during the past 2 decades, life-supporting pig lung xenograft survival in NHPs has been extended only from 11 hours97 to 9 days (Table 4).95,96
TABLE 4.
Recent progress in pig-to-NHP lung xenotransplantation
In ex vivo perfusion models, using human blood, with monitoring for 4 to 8 hours, the following have been identified as being beneficial to lung function and survival: (i) expression of a human complement-regulatory protein, for example, hCD46,98 or (ii) HLA-E99 or (iii) knockout of N-glycolylneuraminic acid.68,100 A meta-analysis of multiple genetic modifications on pig lung xenografts101 found that expression of the inflammation-regulatory protein HO-1 or human endothelial protein C receptor, but not human thrombomodulin or hCD39 were associated with further beneficial effects on the outcome.
In xenotransplantation models, some reports indicate that certain genetic modifications prolong pig lung survival, for example, (i) GTKO,94 (ii) the addition of a human complement-regulatory protein, such as hCD4689,98,101,102 and hCD55/hCD5997,103 or (iii) expression of a human coagulation-regulatory protein, for example, human endothelial protein C receptor,95,104 human tissue factor pathway inhibitor95,105 or (iv) transgenic expression of hCD47.95,106,107
Pig vWF plays a role in “delayed” (24 hours) dysfunction in pulmonary xenotransplantation,106 and it has been suggested that the replacement of pig vWF with human vWF will help overcome hypercoagulability.108
As each individual genetic manipulation has been demonstrated to have only a limited effect, multigene-modified pigs will be necessary to achieve a clinically meaningful lung xenograft outcome. Pierson’s group have accomplished survival beyond 1 week with life-sustaining function using a “multigene” pig lung (Table 4). Normal lung function and histology was documented for several days.95
Pharmacological treatments (to recipient NHP and/or donor pig), targeting various known aspects of xenorejection mechanisms, including inflammation and coagulation pathways, have been shown to be effective and may prove to be essential to protect a xenogeneic lung graft after transplantation (Table 5).94,106,109–119
TABLE 5.
Pharmacological therapy tested in lung xenotransplantation
In summary, although significant progress has been made in pulmonary xenotransplantation, the barriers remain considerable. As with other solid organs, coagulation dysregulation has been identified as one of the major hurdles to be overcome, possibly representing, due to the structure and anatomy of the lung, an even greater problem in xeno lung transplantation than observed with other organs. Further exploration, involving new donor transgenes or transgene combinations and targeted drug treatments will be required to further advance pulmonary xenotransplantation toward clinical applications.
COMMENT
In the past several years, the survival of pig kidneys and hearts in NHPs has been significantly prolonged through the transplantation of organs from genetically engineered pigs and the administration of novel immunosuppressive therapy. Progress has been sufficiently encouraging for groups in the United States to begin to consider approaching the Food and Drug Administration to discuss potential clinical trials of pig kidney transplantation.120 The survival of pig livers and lungs has been limited and requires further genetic modification of the source pig.
Footnotes
Work on xenotransplantation at the University of Alabama at Birmingham is supported in part by NIH NIAID U19 grant AI090959.
The authors declare no conflicts of interest.
Data were collected by L.W., L.B., Y.W., H.I., and D.K.C.C. The article was prepared by L.W., L.B., Y.W., H.I., and D.K.C.C., and revised and approved by all authors.
Correspondence: Hayato Iwase, MD, PhD, Xenotransplantation Program, University of Alabama at Birmingham (UAB), ZRB 701, 1720, 2nd Ave South, Birmingham, AL 35294-0007. (hiwase@uabmc.edu).
The authors review the updated strategies using genetic manipulations combined with immunosuppressant for overcoming thrombotic microangiopathy in pig-to-nonhuman primate organ transplant models.
REFERENCES
- 1.Calne RY, White HJ, Herbertson BM. Pig-to-baboon liver xenografts Lancet 1968. 11176–1178 [DOI] [PubMed] [Google Scholar]
- 2.Lexer G, Cooper DK, Rose AG. Hyperacute rejection in a discordant (pig to baboon) cardiac xenograft model J Heart Transplant 1986. 5411–418 [PubMed] [Google Scholar]
- 3.Cooper DK, Human PA, Lexer G. Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon J Heart Transplant 1988. 7238–246 [PubMed] [Google Scholar]
- 4.Alexandre GP, Gianello P, Latinne D. Plasmapheresis and splenectomy in experimental renal xenotransplantation. In: Hardy M, editor. Xenograft 25. Amsterdam, New York, Oxford: Excerpta Medica; 1989:259–266. [Google Scholar]
- 5.Cooper DK, Satyananda V, Ekser B. Progress in pig-to-nonhuman primate transplantation models (1998-2013): a comprehensive review of the literature Xenotransplantation 2014. 21397–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambrigts D, Sachs DH, Cooper DK. Discordant organ xenotransplantation in primates: world experience and current status Transplantation 1998. 66547–561 [DOI] [PubMed] [Google Scholar]
- 7.Fodor WL, Williams BL, Matis LA. Expression of a functional human complement inhibitor in a transgenic pig as a model for the prevention of xenogeneic hyperacute organ rejection Proc Natl Acad Sci U S A 1994. 9111153–11157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cozzi E, White DJ. The generation of transgenic pigs as potential organ donors for humans Nat Med 1995. 1964–966 [DOI] [PubMed] [Google Scholar]
- 9.Baldan N, Rigotti P, Calabrese F. Ureteral stenosis in HDAF pig-to-primate renal xenotransplantation: a phenomenon related to immunological events? Am J Transplant 2004. 4475–481 [DOI] [PubMed] [Google Scholar]
- 10.Bühler L, Basker M, Alwayn IP. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates Transplantation 2000. 701323–1331 [DOI] [PubMed] [Google Scholar]
- 11.Phelps CJ, Koike C, Vaught TD. Production of alpha 1,3-galactosyltransferase-deficient pigs Science 2003. 299411–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolber-Simonds D, Lai L, Watt SR. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations Proc Natl Acad Sci U S A 2004. 1017335–7340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuwaki K, Tseng YL, Dor FJ. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience Nat Med 2005. 1129–31 [DOI] [PubMed] [Google Scholar]
- 14.Azimzadeh AM, Kelishadi SS, Ezzelarab MB. Early graft failure of GalTKO pig organs in baboons is reduced by expression of a human complement pathway-regulatory protein Xenotransplantation 2015. 22310–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houser SL, Kuwaki K, Knosalla C. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons Xenotransplantation 2004. 11416–425 [DOI] [PubMed] [Google Scholar]
- 16.Cooper DK, Ezzelarab MB, Hara H. The pathobiology of pig-to-primate xenotransplantation: a historical review Xenotransplantation 2016. 2383–105 [DOI] [PubMed] [Google Scholar]
- 17.Cowan PJ, d’Apice AJ. The coagulation barrier in xenotransplantation: incompatibilities and strategies to overcome them Curr Opin Organ Transplant 2008. 13178–183 [DOI] [PubMed] [Google Scholar]
- 18.Cowan PJ, Robson SC. Progress towards overcoming coagulopathy and hemostatic dysfunction associated with xenotransplantation Int J Surg 2015. 23296–300 [DOI] [PubMed] [Google Scholar]
- 19.Ayares D, Phelps C, Vaught T. Multi-transgenic pigs for vascularized pig organ xenografts. Xenotransplantation. 2011;18:269. [Google Scholar]
- 20.Ayares D. Genetic engineering of source pigs for xenotransplantation: progress and prospect. Xenotransplantation. 2013;20:361. [Google Scholar]
- 21.Cooper DK, Gollackner B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med 2002. 53133–147 [DOI] [PubMed] [Google Scholar]
- 22.Cooper DK, Bottino R. Recent advances in understanding xenotransplantation: implications for the clinic Expert Rev Clin Immunol 2015. 111379–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper DK, Ekser B, Ramsoondar J. The role of genetically engineered pigs in xenotransplantation research J Pathol 2016. 238288–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klymiuk N, Aigner B, Brem G. Genetic modification of pigs as organ donors for xenotransplantation Mol Reprod Dev 2010. 77209–221 [DOI] [PubMed] [Google Scholar]
- 25.Ekser B, Ezzelarab M, Hara H. Clinical xenotransplantation: the next medical revolution? Lancet 2012. 379672–683 [DOI] [PubMed] [Google Scholar]
- 26.Postrach J, Bauer A, Schmoeckel M. Heart xenotransplantation in primate models Methods Mol Biol 2012. 885155–168 [DOI] [PubMed] [Google Scholar]
- 27.Reichart B, Guethoff S, Mayr T. Discordant cardiac xenotransplantation: broadening the horizons Eur J Cardiothorac Surg 2014. 451–5 [DOI] [PubMed] [Google Scholar]
- 28.Mohiuddin MM, Corcoran PC, Singh AK. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months Am J Transplant 2012. 12763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohiuddin MM, Singh AK, Corcoran PC. Role of anti-CD40 antibody-mediated costimulation blockade on non-Gal antibody production and heterotopic cardiac xenograft survival in a GTKO.hCD46Tg pig-to-baboon model Xenotransplantation 2014. 2135–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwase H, Ekser B, Satyananda V. Pig-to-baboon heterotopic heart transplantation—exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens Xenotransplantation 2015. 22211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohiuddin MM, Singh AK, Corcoran PC. Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation J Thorac Cardiovasc Surg 2014. 1481106–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohiuddin MM, Singh AK, Corcoran PC. One-year heterotopic cardiac xenograft survival in a pig to baboon model Am J Transplant 2014. 14488–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohiuddin MM, Singh AK, Corcoran PC. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun. 2016;7:11138. doi: 10.1038/ncomms11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vial CM, Ostlie DJ, Bhatti FN. Life supporting function for over one month of a transgenic porcine heart in a baboon J Heart Lung Transplant 2000. 19224–229 [DOI] [PubMed] [Google Scholar]
- 35.Brenner P, Schmoeckel M, Wimmer C. Mean xenograft survival of 14.6 days in a small group of hDAF-transgenic pig hearts transplanted orthotopically into baboons Transplant Proc 2005. 37472–476 [DOI] [PubMed] [Google Scholar]
- 36.Brandl U, Michel S, Erhardt M. Administration of GAS914 in an orthotopic pig-to-baboon heart transplantation model Xenotransplantation 2005. 12134–141 [DOI] [PubMed] [Google Scholar]
- 37.Byrne GW, Du Z, Sun Z. Changes in cardiac gene expression after pig-to-primate orthotopic xenotransplantation Xenotransplantation 2011. 1814–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bauer A, Postrach J, Thormann M. First experience with heterotopic thoracic pig-to-baboon cardiac xenotransplantation Xenotransplantation 2010. 17243–249 [DOI] [PubMed] [Google Scholar]
- 39.Abicht JM, Mayr T, Reichart B. Pre-clinical heterotopic intrathoracic heart xenotransplantation: a possibly useful clinical technique Xenotransplantation 2015. 22427–442 [DOI] [PubMed] [Google Scholar]
- 40.Kawai T, Andrews D, Colvin RB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 41.Knosalla C, Gollackner B, Cooper DK. Anti-CD154 monoclonal antibody and thromboembolism revisted Transplantation 2002. 74416–417 [DOI] [PubMed] [Google Scholar]
- 42.McGregor CG, Davies WR, Oi K. Cardiac xenotransplantation: recent preclinical progress with 3-month median survival J Thorac Cardiovasc Surg 2005. 130844–851 [DOI] [PubMed] [Google Scholar]
- 43.Waterworth PD, Dunning J, Tolan M. Life-supporting pig-to-baboon heart xenotransplantation J Heart Lung Transplant 1998. 171201–1207 [PubMed] [Google Scholar]
- 44.Xu H, Gundry SR, Hancock WW. Prolonged discordant xenograft survival and delayed xenograft rejection in a pig-to-baboon orthotopic cardiac xenograft model J Thorac Cardiovasc Surg 1998. 1151342–1349 [DOI] [PubMed] [Google Scholar]
- 45.Brandl U, Michel S, Erhardt M. Transgenic animals in experimental xenotransplantation models: orthotopic heart transplantation in the pig-to-baboon model Transplant Proc 2007. 39577–578 [DOI] [PubMed] [Google Scholar]
- 46.Bauer A, Baschnegger H, Abicht JM. hDAF porcine cardiac xenograft maintains cardiac output after orthotopic transplantation into baboon—a perioperative study Xenotransplantation 2005. 12444–449 [DOI] [PubMed] [Google Scholar]
- 47.Bauer A, Renz V, Baschnegger H. Microcirculatory alterations after orthotopic pig-to-baboon heart transplantation Xenotransplantation 2011. 18232–238 [DOI] [PubMed] [Google Scholar]
- 48.McGregor CGA, Byrne GW, Vlasin M. Cardiac function after preclinical orthotopic cardiac xenotransplanation. Am J Transplant. 2009;9:380. [Google Scholar]
- 49.Mohiuddin MM, Reichart B, Byrne GW. Current status of pig heart xenotransplantation Int J Surg 2015. 23234–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayr T, Bauer A, Reichart B, et al. Hemodynamic and perioperative management in two different preclinical pig-to-baboon cardiac xenotransplantation models. Xenotransplantation. 2017;24. 10.1111/xen.12295. [DOI] [PubMed] [Google Scholar]
- 51.Byrne GW, McGregor CG. Cardiac xenotransplantation: progress and challenges Curr Opin Organ Transplant 2012. 17148–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bremner WF, Taylor KM, Baird S. Hypothalamo-pituitary-thyroid axis function during cardiopulmonary bypass J Thorac Cardiovasc Surg 1978. 75392–399 [PubMed] [Google Scholar]
- 53.Robuschi G, Medici D, Fesani F. Cardiopulmonary bypass: a low T4 and T3 syndrome with blunted thyrotropin (TSH) response to thyrotropin-releasing hormone (TRH) Horm Res 1986. 23151–158 [DOI] [PubMed] [Google Scholar]
- 54.Novitzky D, Human PA, Cooper DK. Effect of triiodothyronine (T3) on myocardial high energy phosphates and lactate after ischemia and cardiopulmonary bypass. An experimental study in baboons J Thorac Cardiovasc Surg 1986. 96600–607 [PubMed] [Google Scholar]
- 55.Novitzky D, Human PA, Cooper DK. Inotropic effect of triiodothyronine following myocardial ischemia and cardiopulmonary bypass: an experimental study in pigs Ann Thorac Surg 1988. 4550–55 [DOI] [PubMed] [Google Scholar]
- 56.Novitzky D, Cooper DK, Swanepoel A. Inotropic effect of triiodothyronine (T3) in low cardiac output following cardioplegic arrest and cardiopulmonary bypass: an initial experience in patients undergoing open heart surgery Eur J Cardiothorac Surg 1989. 3140–145 [DOI] [PubMed] [Google Scholar]
- 57.Iwase H, Ekser B, Satyananda V. Plasma free triiodothyronine (fT3) levels in baboons undergoing pig organ transplantation: relevance to early recovery of organ function Xenotransplantation 2014. 21582–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iwase H, Ekser B, Hara H. Thyroid hormone: relevance to xenotransplantation Xenotransplantation 2016. 23293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Novitzky D, Cooper DK, Morrell D. Change from aerobic to anaerobic metabolism after brain death, and reversal following triiodothyronine therapy Transplantation 1988. 4532–36 [DOI] [PubMed] [Google Scholar]
- 60.Wuensch A, Baehr A, Bongoni AK. Regulatory sequences of the porcine THBD gene facilitate endothelial-specific expression of bioactive human thrombomodulin in single- and multitransgenic pigs Transplantation 2014. 97138–147 [DOI] [PubMed] [Google Scholar]
- 61.Yamada K, Yazawa K, Shimizu A. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue Nat Med 2005. 1132–34 [DOI] [PubMed] [Google Scholar]
- 62.Iwase H, Liu H, Wijkstrom M. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date Xenotransplantation 2015. 22302–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Higginbotham L, Kim S, Mathews D, et al. Late renal xenograft failure is antibody-mediated: description of the longest-reported survival in pig-to-primate renal xenotransplantation. ATC. 2016; (Abstract #A6). [Google Scholar]
- 64.Iwase H, Hara H, Ezzelarab M, et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation. 2017;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim S, Higginbotham LB, Mathews DV. CD4 depletion is necessary and sufficient for long-term pig-to-nonhuman primate renal xenotransplant survival. Xenotransplantation. 2017;24:e12328. [Google Scholar]
- 66.Martens G, Estrada J, Sidner RA. Porcine GGTA1/B4GalNT2 gene knockout reduces antibody binding and achieves one year life-supporting renal xenograft in pig-to-rhesus model. Xenotransplantation. 2017;24:e12328. [Google Scholar]
- 67.Higginbotham L, Mathews D, Breeden CA. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model Xenotransplantation 2015. 22221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lutz AJ, Li P, Estrada JL. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose α-1,3-galactose reduce the humoral barrier to xenotransplantation Xenotransplantation 2013. 2027–35 [DOI] [PubMed] [Google Scholar]
- 69.Estrada JL, Martens G, Li P. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes Xenotransplantation 2015. 22194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi J, Aubert O, Vo A. Assessment of tocilizumab (anti-Interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients Am J Transplant 2017. 172381–2389 [DOI] [PubMed] [Google Scholar]
- 71.Ekser B, Long C, Echeverri GJ. Impact of thrombocytopenia on survival of baboons with genetically-modified pig liver transplants: clinical relevance Am J Transplant 2010. 10273–285 [DOI] [PubMed] [Google Scholar]
- 72.Ekser B, Klein E, He J. Genetically-engineered pig-to-baboon liver xenotransplantation: histopathology of xenografts and native organs. PLoS One. 2012;7:e29720. doi: 10.1371/journal.pone.0029720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ezzelarab M, Ekser B, Gridelli B. Thrombocytopenia after pig-to-baboon liver xenotransplantation: where do platelets go? Xenotransplantation 2011. 18320–327 [DOI] [PubMed] [Google Scholar]
- 74.Kim K, Schuetz C, Elias N. Up to 9-day survival and control of thrombocytopenia following alpha1,3-galactosyl transferase knockout swine liver xenotransplantation in baboons Xenotransplantation 2012. 19256–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ekser B, Echeverri GJ, Hassett AC. Hepatic function after genetically engineered pig liver transplantation in baboons Transplantation 2010. 90483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shah JA, Navarro-Alvarez N, DeFazio M. A bridge to somewhere: 25-day survival after pig-to-baboon liver xenotransplantation Ann Surg 2016. 2631069–1071 [DOI] [PubMed] [Google Scholar]
- 77.Shah JA, Patel MS, Elias N. Prolonged survival following pig-to-primate liver xenotransplantation utilizing exogenous coagulation factors and costimulation blockade Am J Transplant 2017. 172178–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramirez P, Chavez R, Majado M. Life-supporting human complement regulator decay accelerating factor transgenic pig liver xenograft maintains the metabolic function and coagulation in the nonhuman primate for up to 8 days Transplantation 2000. 70989–998 [DOI] [PubMed] [Google Scholar]
- 79.Ekser B, Gridelli B, Cooper DK. Porcine alanine transaminase after liver allo-and xenotransplantation Xenotransplantation 2012. 1952–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cooper DK, Dou KF, Tao KS. Pig liver xenotransplantation: a review of progress toward the clinic Transplantation 2016. 1002039–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cooper DK, Ekser B, Tector AJ. Immunobiological barriers to xenotransplantation Int J Surg 2015. 23211–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ekser B, Markmann JF, Tector AJ. Current status of pig liver xenotransplantation Int J Surg 2015. 23240–246 [DOI] [PubMed] [Google Scholar]
- 83.Bongoni AK, Kiermeir D, Denoyelle J. Porcine extrahepatic vascular endothelial asialoglycoprotein receptor 1 mediates xenogeneic platelet phagocytosis in vitro and in human-to-pig ex vivo xenoperfusion Transplantation 2015. 99693–701 [DOI] [PubMed] [Google Scholar]
- 84.Chihara RK, Paris LL, Reyes LM. Primary porcine Kupffer cell phagocytosis of human platelets involves the CD18 receptor Transplantation 2011. 92739–744 [DOI] [PubMed] [Google Scholar]
- 85.Ekser B, Gridelli B, Tector AJ. Pig liver xenotransplantation as a bridge to allotransplantation: which patients might benefit? Transplantation 2009. 881041–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cypel M, Yeung JC, Liu M. Normothermic ex vivo lung perfusion in clinical lung transplantation N Engl J Med 2011. 3641431–1440 [DOI] [PubMed] [Google Scholar]
- 87.Ranieri VM, Suter PM, De Tullio R. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial JAMA 1999. 28254–61 [DOI] [PubMed] [Google Scholar]
- 88.den Hengst WA, Gielis JF, Lin JY. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process Am J Physiol Heart Circ Physiol 2010. 299H1283–1299 [DOI] [PubMed] [Google Scholar]
- 89.Gonzalez-Stawinski GV, Daggett CW, Lau CL. Non-anti-Gal alpha1-3Gal antibody mechanisms are sufficient to cause hyperacute lung dysfunction in pulmonary xenotransplantation J Am Coll Surg 2002. 194765–773 [DOI] [PubMed] [Google Scholar]
- 90.Pierson RN, Dorling A, Ayares D. Current status of xenotransplantation and prospects for clinical application Xenotransplantation 2009. 16263–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pierson RN. Antibody-mediated xenograft injury: mechanisms and protective strategies Transpl Immunol 2009. 2165–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nguyen BN, Azimzadeh AM, Schroeder C. Absence of Gal epitope prolongs survival of swine lungs in an ex vivo model of hyperacute rejection Xenotransplantation 2011. 1894–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burdorf L, Azimzadeh A, Pierson RN. Xenogeneic lung transplantation models Methods Mol Biol 2012. 885169–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nguyen BN, Azimzadeh AM, Zhang T. Life-supporting function of genetically modified swine lungs in baboons J Thorac Cardiovasc Surg 2007. 1331354–1363 [DOI] [PubMed] [Google Scholar]
- 95.Kubicki N, Laird C, Burdorf L. Current status of pig lung xenotransplantation Int J Surg 2015. 23247–254 [DOI] [PubMed] [Google Scholar]
- 96.Burdorf L, Laird C, O’Neill N, et al. Progress and remaining challenges in xenogeneic lung transplantation [abstract]. IXA. 2017. (O.7.6). [Google Scholar]
- 97.Daggett CW, Yeatman M, Lodge AJ. Swine lungs expressing human complement-regulatory proteins are protected against acute pulmonary dysfunction in a human plasma perfusion model J Thorac Cardiovasc Surg 1997. 113390–398 [DOI] [PubMed] [Google Scholar]
- 98.Burdorf L, Stoddard T, Zhang T. Expression of human CD46 modulates inflammation associated with GalTKO lung xenograft injury Am J Transplant 2014. 141084–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Laird CT, Burdorf L, French BM, et al. Transgenic expression of human leukocyte antigen-E attenuates GalKO.hCD46 porcine lung xenograft injury. Xenotransplantation. 2017;24. Epub 2017 Mar 3. [DOI] [PubMed] [Google Scholar]
- 100.Burdorf L, Ali F, Ramsoondar J, et al. N-Glycolylneuraminic acid (Neu5GC) knock-out in GalTKO.HCD46 pig lungs improves pulmonary function in a xenogeneic pig-to-human lung perfusion model [abstract]. Xenotransplantation. 2015(S84):552. [Google Scholar]
- 101.Harris DG, Quinn KJ, French BM. Metaanalysis of the independent and cumulative effects of multiple genetic modifications on pig lung xenograft performance during ex vivo perfusion with human blood Xenotransplantation 2015. 22102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diamond LE, Quinn CM, Martin MJ. A human CD46 transgenic pig model system for the study of discordant xenotransplantation Transplantation 2001. 71132–142 [DOI] [PubMed] [Google Scholar]
- 103.Yeatman M, Daggett CW, Parker W. Complement-mediated pulmonary xenograft injury: studies in swine-to-primate orthotopic single lung transplant models Transplantation 1998. 651084–1093 [DOI] [PubMed] [Google Scholar]
- 104.Petersen B, Ramackers W, Tiede A. Pigs transgenic for human thrombomodulin have elevated production of activated protein C Xenotransplantation 2009. 16486–495 [DOI] [PubMed] [Google Scholar]
- 105.Chen D, Riesbeck K, McVey J. Regulated inhibition of coagulation by porcine endothelial cells expressing P-selectin-tagged hirudin and tissue factor pathway inhibitor fusion proteins Transplantation 1999. 68832–839 [DOI] [PubMed] [Google Scholar]
- 106.Cantu E, Gaca JG, Palestrant D. Depletion of pulmonary intravascular macrophages prevents hyperacute pulmonary xenograft dysfunction Transplantation 2006. 811157–1164 [DOI] [PubMed] [Google Scholar]
- 107.Ide K, Wang H, Tahara H. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages Proc Natl Acad Sci U S A 2007. 1045062–5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gaca JG, Lesher A, Aksoy O. The role of the porcine von Willebrand factor: baboon platelet interactions in pulmonary xenotransplantation Transplantation 2002. 741596–1603 [DOI] [PubMed] [Google Scholar]
- 109.Collins BJ, Blum MG, Parker RE. Thromboxane mediates pulmonary hypertension and lung inflammation during hyperacute lung rejection J Appl Physiol (1985 2001. 902257–2268 [DOI] [PubMed] [Google Scholar]
- 110.Schwartz E, Burdorf L, Parsell D. Effects of a thromboxane synthase inhibitor, 1-BIA, on eicosanoid metabolism in a GalTKO.hCD46 xenogeneic lung perfusion model [abstract]. Xenotransplantation. 2015;(S184):934. [Google Scholar]
- 111.Laird C, Burdorf L, Pierson RN. Lung xenotransplantation: a review Curr Opin Organ Transplant 2016. 21272–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim YT, Lee HJ, Lee SW. Pre-treatment of porcine pulmonary xenograft with desmopressin: a novel strategy to attenuate platelet activation and systemic intravascular coagulation in an ex-vivo model of swine-to-human pulmonary xenotransplantation Xenotransplantation 2008. 1527–35 [DOI] [PubMed] [Google Scholar]
- 113.Burdorf L, Riner A, Rybak E. Platelet sequestration and activation during GalTKO.hCD46 pig lung perfusion by human blood is primarily mediated by GPIb, GPIIb/IIIa, and von Willebrand Factor Xenotransplantation 2016. 23222–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Burdorf L, Zhang T, Rybak E. Combined GPIb and GPIIb/IIIa blockade prevents sequestration of platelets in a pig-to-human lung perfusion model. Xenotransplantation. 2011;18:287. [Google Scholar]
- 115.Burdorf L, Zhang T, Rybak E, et al. Blocking GP1b-vWF interaction by anti-GP1b fab reduces activation and sequestration of platelets in a xenogeneic pig lung perfusion model. J Heart Lung Transplant. 2011(S103):30. [Google Scholar]
- 116.Azimzadeh A, Cheng N, Cheng X, et al. Multiplex profiling of plasma proteins after xenogeneic lung exposure [abstract]. Xenotransplantation. 2015(S181):1056. [Google Scholar]
- 117.Gaca JG, Palestrant D, Lukes DJ. Prevention of acute lung injury in swine: depletion of pulmonary intravascular macrophages using liposomal clodronate J Surg Res 2003. 11219–25 [DOI] [PubMed] [Google Scholar]
- 118.Schröder C, Pfeiffer S, Wu G. Effect of complement fragment 1 esterase inhibition on survival of human decay-accelerating factor pig lungs perfused with human blood J Heart Lung Transplant 2003. 221365–1375 [DOI] [PubMed] [Google Scholar]
- 119.Harris D, Benipal P, Cheng X. Four-dimensional in-vitro modeling of xenogeneic thrombosis under shear flow: a novel technique. Xenotransplantation. 2013;20:356. [Google Scholar]
- 120.Cooper DK, Pierson RN, Hering BJ. Regulation of clinical xenotransplantation—time for a reappraisal? Transplantation 2017. 1011766–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]


