Abstract
Background
Understanding ischemia reperfusion injury (IRI) is essential to further improve outcomes after liver transplantation (LT). Porcine isolated liver perfusion (ILP) is increasingly used to reproduce LT-associated IRI in a strictly controlled environment. However, whether ILP is a reliable substitute of LT was never validated.
Methods
We systematically reviewed the current experimental setups for ILP and parameters of interest reflecting IRI.
Results
Isolated liver perfusion was never compared with transplantation in animals. Considerable variability exists between setups, and comparative data are unavailable. Experience so far suggests that centrifugal pump(s) with continuous flow are preferred to reduce the risk of embolism. Hepatic outflow can be established by cannulation of the inferior vena cava or freely drained in an open bath. Whole blood at approximately 38°C, hematocrit of 20% or greater, and the presence of leukocytes to trigger inflammation is considered the optimal perfusate. A number of parameters related to the 4 liver compartments (hepatocyte, cholangiocyte, endothelium, immune cells) are available; however, their significance and relation to clinical outcomes is not well described.
Conclusions
Porcine ILP provides a reproducible model to study early IRI events. As all models, it has its limitations. A standardization of the setup would allow comparison of data and progress in the field.
Isolated liver perfusion (ILP) is a procedure in which a liver is normothermically perfused ex situ with an oxygenated and nutrient-enriched perfusate. Isolated liver perfusion has been used to investigate hepatic pathophysiology and metabolism,1,2 test drug toxicity,3 or to develop bridge therapy for patients suffering from acute liver failure.4,5
In the liver transplantation (LT) setting, ILP has been used to investigate ischemia reperfusion injury (IRI) as a substitute for LT and recently also implemented as a technology to preserve liver grafts, generally referred to as machine perfusion preservation or dynamic preservation.6 Although ILP was initially used to replace LT in rodents,7 the last decades have seen a rise in porcine models, especially in studies investigating protective strategies by means of liver dynamic preservation. The pig seems an appropriate animal to establish ILP and LT models, because the porcine liver is anatomically and physiologically close to humans, with comparable organ dimension and similar bile composition.8,9 The study of IRI during LT is important as IRI invariably takes place in every transplant with injuries that may vary from minimal to complete graft destruction and may either go clinically unnoticed or present as dysfunction or absence of life-sustaining hepatic metabolic activity. Ischemia leads to the disruption of hepatocyte aerobic respiration and a cascade of cellular and metabolic disturbances.10,11 Static cold storage, the standard preservation technique involving cooling and storing of the graft at 4°C to 6°C, reduces but does not abolish oxygen dependent metabolism. Subsequent graft reperfusion with warm oxygenated blood triggers the formation of reactive oxygen species and sterile inflammation, amplifying ischemia-induced damages.12
Large animal models of LT are labor-intensive, technically challenging, and carry a high financial burden.13 In contrast, ILP allows the reduction of the number of experimental animals since a recipient is not needed. Indeed, all steps preceding the transplantation of the graft can be replicated during ILP and the liver reperfused mimicking the sequence of events of LT.13 Therefore, ILP can be used to investigate the impact of (i) warm/cold ischemia, or (ii) protective strategies (ie, drugs, dynamic preservation) since the reperfusion phase takes place in a controlled environment. Additionally, ILP offers the possibility to serially sample perfusate, bile, and liver tissue, monitoring various parameters to evaluate IRI. Therefore, ILP constitutes a valuable alternative for LT research in large animals and is particularly attractive as a preclinical tool to test new approaches to protect organs from IRI.
Experience with porcine ILP is nowadays considerable; however, this model was never validated before and comparative interpretation of published studies is difficult due to a wide variation in model design, parameters of interest, and endpoints. The aim of our study was (i) to review the methodology of ILP, (ii) to evaluate it as a model to replace LT experiments in animals, and (iii) to summarize the most promising parameters to evaluate IRI during ILP. We also highlight benefits and limitations of the model and discuss possible future developments.
MATERIALS AND METHODS
Experimental studies reporting on methodology and results of porcine ILP as a substitute for LT were considered regardless of the type of graft preservation (ie, cold storage, dynamic preservation) or protective strategy investigated (SDC, Methods, http://links.lww.com/TP/B545).
Data Source, Search Strategy, and Eligibility Criteria
Details are given in SDC, Methods, http://links.lww.com/TP/B545.
Data Extraction
Details of the criteria for data acquisition are given in SDC, Methods, http://links.lww.com/TP/B545. The extracted data were summarized in a descriptive review of the methodology of ILP and possible (bio)markers of IRI.
Quality Assessment
Details of the quality assessment are given in SDC, Methods, http://links.lww.com/TP/B545.
RESULTS
The search identified 257 articles of which 32 were eligible for further full text screening. Twenty-three papers were included in the quality assessment (SDC, Results and Figure S1, http://links.lww.com/TP/B545) and 11 different setups were identified (Figure 1).
FIGURE 1.
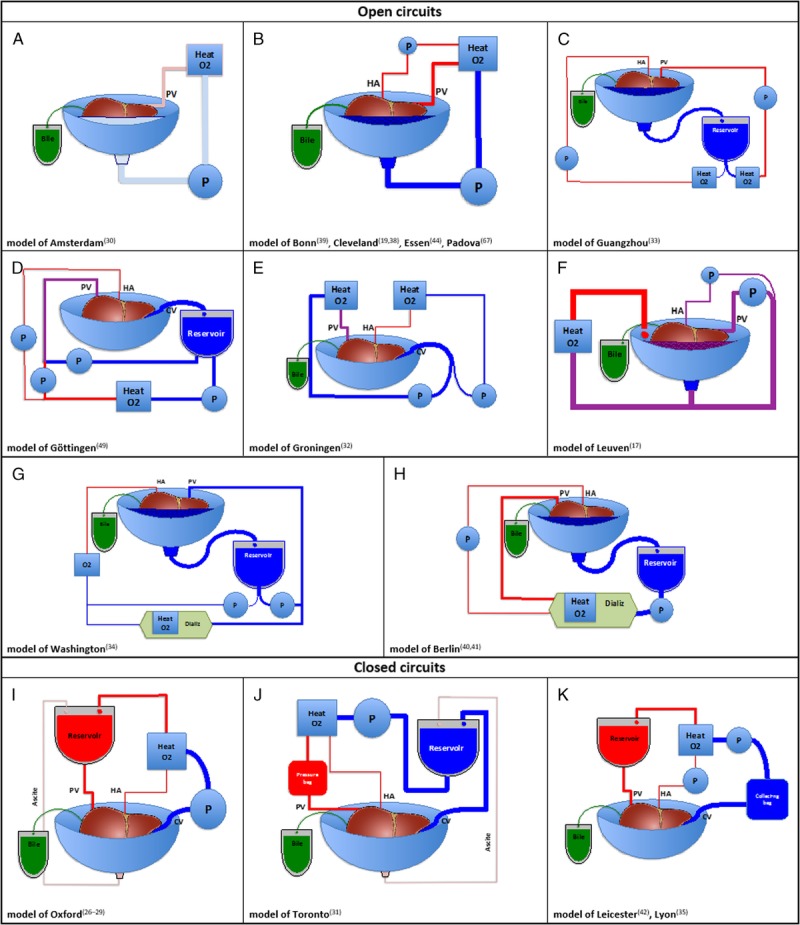
Schematic illustration of the porcine ILP models identified after systematic literature review. Wide variation in the design exists among the 11 setups considered and the drainage of the IVC constitutes the major difference. Open circuits (from A to H) passively drain the IVC outflow either in the liver receptacle or in a venous reservoir, whereas in closed circuits (from I to K) the IVC is cannulated and directly connected to the pump. In all setups, the perfusion of the liver is performed via both the PV and the HA, except for the model of Amsterdam (A) where only the PV is perfused. The majority of the groups divert the flow generated by a single pump into both PV and HA circulation, with the exception of the model from Guangzhou (C), Groningen (E), and Washington (G) in which 2 distinct circuits separately perfuse PV and HA. In the model proposed by the groups from Oxford (I), Toronto (J), Leicester and Lyon (K), the PV is connected to a reservoir and perfused by gravity only. A dialysis unit was included in the circuits described by the group of Berlin (B), and Washington (G). The concentration of administered oxygen was identical in the PV compared to the HA circulation in most of the circuits, with the exception of the models of Guangzhou (C), Göttingen (D), Groningen (E), and Washington (G), in which the partial oxygen tension was kept lower in the PV. Note that the model of Pittsburgh14 could not be included in the figure due to insufficient details on circuit design reported in the original paper. Heat, heat exchanger; CV, caval vein; O2, oxygenator; P, pump.
All setups performed ILP as a substitute for LT to test various approaches to prevent/reduce the severity of IRI: 17 of 23 articles investigated the effects of dynamic preservation, whereas the remaining studied different types of preservation solutions. No study included a LT group as control. All studies largely underreported various characteristics of the experimental setting, configuring an overall unclear risk of biases. Additional details are given in SDC, Results, http://links.lww.com/TP/B545.
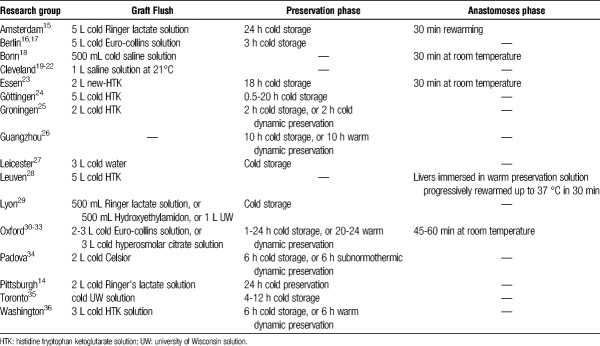
Mimicking LT
Liver transplantation involves 4 surgical phases: (i) organ procurement, including flushing, cooling, and hepatectomy, (ii) preservation, (iii) anastomosis with progressive rewarming of the graft, and (iv) reperfusion with whole blood in the recipient. Ideally, these prereperfusion steps should be reproduced during ILP. As shown in Table 1, various methods have been published, the suitability of which may depend on the specific experimental setting. After procurement, porcine grafts are generally flushed and cooled with different solutions at variable temperatures. To mimic the graft rewarming during the anastomosis, livers are slowly rewarmed to 25°C to 37°C by submerging in warm fluid,37 or kept at room temperature for a brief period.38 Finally, the reperfusion phase is simulated by connecting the liver to the ILP circuit with recirculation of warm oxygenated perfusate.
TABLE 1.
Summary of different approaches to reproduce the steps occurring before graft reperfusion described in porcine ILP setups identified after systematic review of the literature
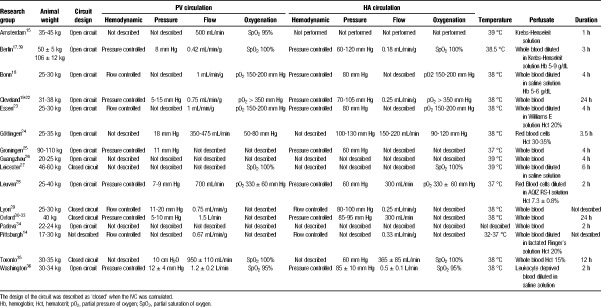
Table 2 summarizes details of the experimental settings used in the different models of porcine ILP reported to date. A wide variation is seen in key parameters, such as (a) circuit design, (b) perfusion pressure and flow, (c) nature of perfusate, (d) perfusate temperature, (e) oxygenation, and (f) duration of perfusion.
TABLE 2.
Summary of the experimental settings of different setups of porcine ILP identified after systematic review of the literature
Circuit Design
Both the hepatic artery (HA) and portal vein (PV) provide blood flow to the liver integrating at the sinusoids with efferent blood collected in the inferior vena cava (IVC). Physiological hepatic flow in pigs is approximately 1 mL/min per gram of liver, with 75% of total blood flow delivered by the PV and 25% by the HA.40 Any ILP circuit provides this dual inflow but models vary with respect to the type and number of pumps, closed versus open system, heater-coolers, and oxygenators used (Figure 1).
Flow during ILP is generated either by roller or by centrifugal pumps. Both exert mechanical stress with destruction of red blood cells and platelets,19,28,41,42 although this risk is lower with centrifugal pumps.43 Which pump to choose might be determined by the anticipated ILP duration; for example, for a short-lasting ILP, hemolysis and platelet destruction by roller pumps might be less relevant. Both roller and centrifugal pumps can generate laminar or pulsatile flow, although clinical experience with cardiopulmonary bypass has not yet revealed significant advantage of pulsatile flow.44
Hepatic inflow is generated by a single pump30–32,45–47 for both portal and arterial circulation, or by 2 separate pumps.15,33,35 Alternatively, portal perfusion can be achieved by gravity25,26,30,45–47 (Figure 1). Typically, ILP using blood as perfusate is performed at 1 mL/min per gram,25,26,36,40 whereas the flow rate is generally increased with acellular solution perfusates to achieve sufficient oxygenation.32
The design of the hepatic outflow circuit varies between ILP settings. In most models, the IVC freely drains in the organ receptacle which functions as a reservoir (open circuit),14,15,20,29,33,36,37,40,48 whereas in a fully closed system, the IVC is cannulated and connected directly to the pump or a venous reservoir18,25,30,45–47 (Figure 1). Such a fully closed system allows thorough hemodynamic monitoring including measuring IVC flow and pressure. In a closed system, the IVC wall can collapse by negative pressure generated by the pump causing congestion in the sinusoids.
Pressure and Flow
Pressure and flow are intertwined hemodynamic parameters, and adequate regulation is essential. Elevated pressure may provoke excessive shear-stress, barotrauma, and sinusoid endothelial damage, whereas low pressure might lead to sinusoidal space collapse and inhomogeneous perfusion.7,40 Dual pressure-controlled perfusion aiming at near-to-physiological upper pressure limits (5-15 mm Hg for PV, 60-130 mm Hg for HA) is used by most groups (Table 2). Some use a flow-controlled perfusion25,26 though this carries a risk of barotrauma if intrahepatic pressures rise, or heterogeneous perfusion if lower flows are generated. In the models of Cleveland and Göttingen, the flow is adjusted within a physiologic range by increasing pump speed upon reaching the target pressure.20,29,40,48 However, it is unclear if further adjustments of flow are needed since “auto-regulation” of hepatic circulation has been described.31,49 In the Oxford model, portal perfusion only relies on gravity, and its pressure is determined by the height of the column of perfusate in the reservoir. As such, portal flow is “autoregulated” by the intrahepatic resistance and IVC pressure,31,49 although the physiological ratio between portal and arterial flow might not be respected constantly.
Finally, a hybrid system with flow-controlled perfusion for the PV and pressure-controlled perfusion for the HA has also been described by few groups.14,16
Perfusate
The perfusate for ILP can be either an acellular solution or a solution containing erythrocytes. However, acellular perfusates are unlikely to reproduce the full IRI cascade as leukocytes, platelets, and other soluble factors are missing. The majority of authors (12 of 16 research groups) therefore use whole blood14,17,25–27,29,32,33,36,40,45,46,48,49 aiming to reproduce the complex cascade of events during IRI.23,49
Porcine blood can be collected during organ procurement via cannulation of cervical or abdominal vessels or a blood donor pig can be used.17,26,29,40,45,46 Blood is typically heparinized or mesh-filtered to prevent clotting.27 It can be used immediately or stored in citrate, phosphate, or dextrose for up to 1 week.14,17,29,36,40,45,46,48,50 The use of heterologous blood would include the alloimmune reactivity and inflammation typically observed during IRI and LT.51 Using large-size pigs (70-80 kg) allows for sufficient blood collection for 2 ILP runs.29,40 In case the total volume retrieved is insufficient for multiple perfusions, some authors reported the dilution of whole blood provided that the hematocrit is kept sufficiently high to ensure oxygen delivery. Indeed, the hematocrit is typically maintained around 20%,26,29,36,40,45 although lower and higher targets are reported.23,32,37
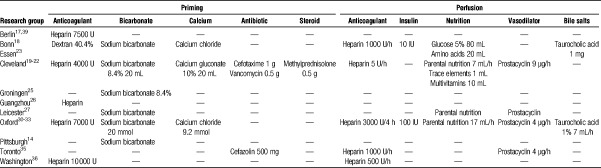
Whole blood perfusate is usually supplemented (Table 3). To maintain physiological pH and ion balance, most groups add sodium bicarbonate and calcium chloride before starting ILP.14,16,18,29,30,33,39,40,45–47,52 Antibiotics were added by some groups29,32,39,40,52 to prevent bacterial contamination. Heparin is also almost universally infused to prevent clotting.15,20,29,30,32,35,39,45–48,52
TABLE 3.
Summary of additives infused during priming and perfusion in porcine ILP setups described by some of the studies identified after systematic literature review. When described in the original paper, the dosage of the compounds is given
After starting ILP, most groups regard the supplementation of nutrients, such as glucose and amino acids, critical to optimally sustain liver metabolism.14,16,18,29,30,39,40,45–47,52 Insulin is added by some because it is believed essential to support the glucose metabolism.14,16,30,45–47
During ILP, neural, hormonal, and metabolic feedbacks regulating the intrahepatic vascular resistance are absent. Therefore, prostacyclin is infused to ensure arterial vasodilation.18,29,30,32,39,40,45–47,52 A potential drawback is that prostacyclin may inhibit platelet activity and blunt IRI,24 but a minimal concentration seems needed to keep flows within acceptable ranges.39
In the absence of the enterohepatic recirculation, a continuous infusion of bile salts can be given to counteract declining bile production typically observed after 10 hours of ILP.14,16,30,46,47,53 However, during ILP of human livers for shorter periods (eg, 6 hours) without bile salts infusion, bile flow was not altered.54 Whether bile salt depletion may only become relevant during longer periods of ILP (eg, ≥ 6 hours) is not known.
To prevent acidosis or hyperkalemia during ILP, a dialysis unit was added to the circuit by some groups20,35,48 but regarded unnecessary by others who observed a stable acid-base equilibrium and ion balance during ILP without dialysis when adequate supply of glucose, insulin, and other metabolic substrates is provided.17,50
Temperature
Minor temperature changes may affect the process of IRI.55,56 It is therefore imperative to use heat exchangers, usually coupled to the oxygenator, to keep the perfusate temperature within the average range of body temperature in pigs (37-39.6°C).57
Oxygenation
Physiological partial oxygen pressure (pO2) in pigs ranges between 72 to 95 mm Hg in the HA57 and 44 to 58 mm Hg in the PV.21 Yet, higher pO2 targets are commonly used to compensate for the low perfusate hematocrit, especially in acellular perfusates lacking oxygen-carrying capacity. Hollow fiber oxygenators are typically used delivering identical pO2 for the HA and PV.14,29,36,37,40,50 A lower portal pO2 can be generated with 2 separate circuits and oxygenators33 or by mixing oxygen-saturated arterial and desaturated IVC blood to perfuse the PV.49 In the clinical setting, there is evidence that hyperoxygenation (>600 mm Hg) might be harmful.22
Duration of Perfusion
The duration of perfusion has been variously reported from 1 up to 24 hours, with the exception of 1 report in which nonischemic livers underwent ex situ dynamic preservation for 72 hours.50
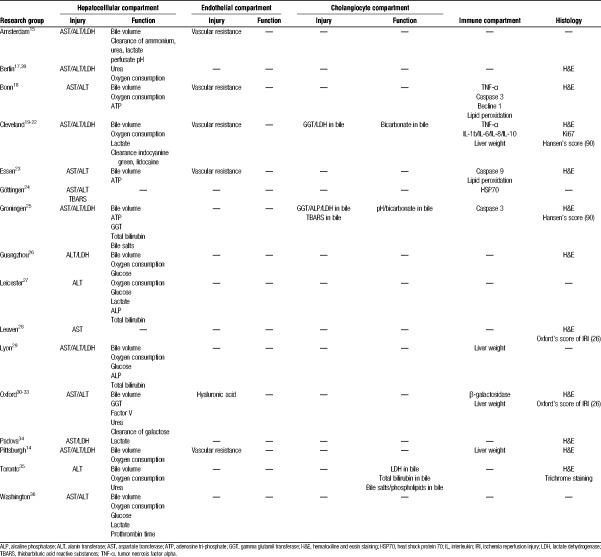
Parameters of Interest During Isolated Liver Perfusion
Table 4 summarizes all parameters of interest related to the 4 liver compartments (hepatocytes, cholangiocytes, endothelium, immune).
TABLE 4.
Summary of parameters of interest monitored during ILP in the different setups identified after systematic literature review
Hepatocellular Compartment
Hepatocellular Injury
Cytoplasmic hepatic enzymes, such as aspartate aminotransferase (AST), alanine aminotransferase (ALT) and lactate dehydrogenase (LDH), are released when hepatocyte damage occurs. AST, ALT, and LDH levels are well known to be related to the severity of graft injury after ischemia. Similarly, a rise in the concentration of these markers was consistently observed in all models of ILP.14–18,20,25–27,29,31–33,35–37,39,40,45,46,49,52,58
Hepatocellular Function
The concentration of adenosine triphosphate (ATP) can be used as a measure of recovery of energy balance during IRI and ILP.14,16 Ischemia causes the adenine nucleotide metabolism in the liver to shut down.59 Good functioning grafts showed a faster recovery of ATP after IRI, whereas graft failure was associated with adenine nucleotide production.60
The consumption of oxygen, the last effector for ATP synthesis, can approximate hepatic metabolism, and can be inferred using the formula of Tolboom et al.61 Changes in oxygen consumption might be related to changes in metabolic activity, although their interpretation in the isolated setting has not been elucidated yet.
Metabolically active livers self-regulate glucose concentration in the perfusate.45 Thus, a stable glucose concentration with a progressive decline of lactic acid indirectly reflects aerobic metabolism. The perfusate pH and bicarbonate concentration may be used as indirect markers of hepatic metabolism, although these data need to be interpreted at the net of the oxygen and carbon dioxide delivery during ILP.17
Protein catabolism generates ammonium and bicarbonate, which buffers the acid-base equilibrium.31 Ammonium and urea can both be added to the perfusate and its clearance monitored as a parameter of functional recovery during ILP.31 Similarly, metabolism of both indocyanine green, which depends on functional hepatocyte mass, and lidocaine, which is metabolized in the liver via cytochrome P450, is used to assess overall hepatic function.52,62
Factor V, a procoagulant protein produced by hepatocytes, is clinically known as a sensitive marker of synthetic function. Factor V can be monitored in the perfusate during ILP, keeping into account that pigs can produce fivefold the amount of factor V produced by humans.63
The production of bile is directly related to hepatocyte viability, metabolism, and synthesis27,50 and can be monitored during ILP by cannulation of the bile duct.50
Histology
A uniformly accepted histological scoring system to evaluate IRI severity is currently unavailable. The Oxford group designed a semiquantitative score considering the degree of sinusoidal dilatation and congestion, hepatocellular vacuolization, mitosis, apoptosis, and percentage of nonviable tissue.30,47 Addressing specific features of donation after circulatory death, our group developed a similar score evaluating sinusoids dilatation, anoxic vacuoles formation, enlargement of the space of Disse, loss of parenchymal cells and cellular cohesion, and neutrophils infiltration.37,64
Endothelial Compartment
Endothelial Injury
Vascular resistance, which can be assessed real-time, increases during ILP in damaged livers.45 A vascular resistance increase results from disruption of the endothelial cell lining and so-called no flow phenomenon and can therefore be regarded as an indirect marker of IRI.14,16,26,29–31,39,40,45–47,52
Hyaluronic acid, a glycosaminoglycan metabolized by sinusoidal endothelial cells65,66 accumulates in the hepatic microcirculation with ischemic endothelial injury and graft dysfunction.60 Likewise, increased levels of hyaluronic acid have been observed during ILP and considered a marker of endothelium viability.30,47
Cholangiocyte Compartment
Cholangiocyte Injury
Biliary epithelial injury can be monitored by measuring the biliary concentration of cytoplasmic cholangiocyte enzymes including gamma-glutamyl transferases (GGT), alkaline phosphatase (ALP), and LDH.33,40
Biliary concentrations of thiobarbituric acid reactive substances as a marker of oxidative stress of cholangiocytes33 have been also reported.
A composite score assesses the amount of bile duct wall necrosis, peribiliary vascular lesions, intramural bleeding, and arteriolonecrosis of the peribiliary vascular plexus.67 Integrity and proliferation of peribiliary glands were later integrated in this score34 and used to asses bile duct viability after IRI during ILP.40
Cholangiocyte Function
Bile production requires energy-dependent secretion of bicarbonate by cholangiocytes as a buffer to the potentially detrimental bile acids. Some groups proposed assessment of cholangiocyte function by measuring biliary concentration of glucose, bicarbonates, and pH.33,40
Analysis of the concentration of bile acids in bile fluid (along with their clearance in the perfusate),46 and the bile salt-to-phospholipid ratio68 may provide additional information on hepatic synthesis.
Immune Compartment
Ischemia reperfusion injury typically triggers inflammation with recruitment of leukocytes into the liver parenchyma, proinflammatory cytokine production, sinusoidal congestion, and edema. An increase in liver weight at the end of ILP may be related to the presence and severity of inflammation.25,26,29,30,39,40,45–47,52
The appearance of the cytokines tumor necrosis factor-α (TNF-α), interleukin (IL)-8, and IL-10 in the perfusate directly indicates activation of proinflammatory pathways.52
Oxidative stress during IRI can also be evaluated by quantifying the concentration of lipid peroxides.14,16
Inflammation in the liver may lead to hepatocyte necrosis, apoptosis or autophagy: Beclin 1 and Caspase 3 may be measured as indicators of hepatocyte autophagy and apoptosis, respectively.14,33
DISCUSSION
Our systematic review identified 23 articles in which pig ILP replaced LT to investigate IRI or to explore novel approaches to optimize liver preservation (the majority of which involved dynamic preservation). All the studies carried an unclear risk of bias as they generally underreport different aspects of the experimental environment and outcomes interpretation. Importantly, none of the studies compared ILP with actual LT in pigs and some of the steps and methodology undertaken by the different groups rely on assumed best practice rather than acquired evidences. Porcine ILP is not a standardized model and the variability in the methodology inhibits any direct comparative analysis between different settings. Indeed, pending uncertainties on circuit design, hemodynamic settings, and perfusate should be addressed to facilitate the comparison of results reflecting injury and function of the 4 main liver compartments.
The studies identified reported largely different circuit design and hemodynamic settings equally ensuring adequate perfusion of the liver and mimicking of IRI. However, none of the studies compared diverse ILP settings (ie, open vs close system, flow-controlled vs pressure-controlled perfusion, 1 pump vs 2 pumps), hence it is not possible to comment on the superiority of a specific circuit design or hemodynamic to reproduce IRI and mimic LT.
Our knowledge of the nutritional needs of a liver isolated from a metabolically demanding organism is limited, and it is difficult to establish whether the liver displays active metabolism during ILP, for example, elevated oxygen consumption has been associated to both functioning and failing livers.26,50 Some groups infused insulin and glucose to support hepatic metabolism during perfusion; however, the glucose transporter 2 expressed on the surface of hepatocytes is not regulated by insulin.69 Additionally, insulin does not stimulate glycolysis in the liver, but triggers glycogen synthesis and inhibits gluconeogenesis. Therefore, insulin and glucose infusion might replete the hepatocytes with glycogen rather than fueling the chain of aerobic oxidation to support the physiological needs of the liver.
Heparin is also commonly infused; however, the (anti)coagulative status of the liver during ILP is not well studied. Some authors reported stable synthesis of coagulative factor V during perfusion,30,45,47,50 whereas others observed prolonged prothrombin time.35 Currently, data on the effective production of fibrinogen (last effector of the coagulation cascade) and antithrombin (heparin-cofactor) during pig ILP are lacking.
Despite the existing uncertainties, ILP can be used to study events occurring in the early phases of reperfusion. Indeed, parameters of interest and biomarkers released during ILP tend to reflect mainly hepatocellular, endothelial, and—to a lesser extent—cholangiocyte injury, showing a pattern that fairly resembles our current knowledge on the sequence of events occurring in early stages of IRI in both animal experiments and clinical transplantation. Because ILP was never compared with LT, how to interpret parameters and biomarkers released during ILP or how to correlate them to relevant clinical outcomes, including recipient survival, graft function, and posttransplant complications remains unknown.
Obviously, ILP has its advantages and limitations, especially regarding the reproduction and the evaluation of late phases of reperfusion injury. Indeed, at present a maximum of 72 hours, with most studies not extending perfusions over 24 hours, has been reported. Short ILP might not capture all the events occurring during IRI, whereas during extended ILP, the absence of hormonal, neural, and metabolic feedback might become more relevant. Although those who described ILP lasting 24 hours did not observe such disturbances, it is yet unclear whether perfusion mimicking the first day after LT would be enough to simulate the most relevant changes occurring at graft reperfusion, as the exact kinetics of IRI are still unknown.70 Second, monocytes and other immune cells are usually mobilized in the late reperfusion phase from primary and secondary lymphoid organs (ie, lymph-nodes, spleen, and bone marrow) actively contributing to the pathophysiology of IRI,40 and the lack of this immune interaction might blunt IRI.
Finally, remote injuries related to IRI or long-term drawbacks after LT, such as renal impairment, cannot be evaluated. Nonanastomotic biliary strictures represent a clinically relevant problem, typically occurring within 1 month up to 1 year after transplantation. However, our understanding of the pathogenesis is still limited, and reproducible animal models of nonanastomotic strictures are currently missing.71
The limitations on investigating the reperfusion injury during ILP might also be related to the lack of reliable biomarkers. Indeed, markers of cholangiocytes injury, function, and regeneration are only reported sporadically and early surrogate of biliary complications have never been described during porcine ILP. Similarly, our capacity to monitor injury and functional recovery of sinusoidal endothelial cells, known to be the least resistant to ischemia and to play a pivotal role in IRI,72 is also very limited.
In summary, porcine ILP can be considered a valuable model to study events of the early reperfusion phase of the ischemia reperfusion cascade that occurs during organ transplantation and a particularly convenient method to explore novel approaches to protect or optimize liver grafts. The advent of dynamic preservation holds potentials to revolutionize the present LT practice. However, a lot of our current knowledge on the effects and benefits of liver dynamic preservation are deduced from ILP models. As all models, ILP has limitations of which researchers need to be aware while interpreting and comparing results. Animal transplantation models should still be considered to further confirm ILP findings, to explore longer-term outcomes, and to evaluate graft and recipient survival.
A standardization of the ILP model is desired and would allow direct comparison of results. The scarce available knowledge on the physiology of an isolated liver (ie, metabolism, coagulative status, endothelial and cholangiocyte function) limits progress in both our understanding of hepatic IRI and development of alternative preservation methods by means of dynamic preservation. To advance the field, further studies are needed to compare ILP to LT and to deepen our knowledge of mechanisms by which technical aspects of perfusion might influence IRI.
Footnotes
F.M. and N.G. contributed equally.
J.P. holds a named chair at the KU Leuven from the Institut Georges Lopez. J.P., D.M., and I.J. hold a named chair at the KU Leuven from the “Centrale Afdeling voor Fractionering.” D.M. is a senior clinical investigator of the research foundation Flanders, Belgium (FWO 18B1916N). No specific funding was sought for the present study.
The authors declare no conflicts of interest.
F.M., N.G., I.J. and D.M. designed the study, acquired, analyzed and interpreted the data, wrote the manuscript and take responsibility for the integrity of the data and the accuracy of the data analysis. S.L. acquired, analyzed and interpreted the data. G.C., R.R., J.P., and P.F. contributed to the interpretation of data, critically revised and approved the manuscript and take responsibility for the integrity of the data and the accuracy of the data analysis.
Correspondence: Diethard Monbaliu, MD, PhD, Abdominal Transplant Surgery and Coordination, University Hospitals Leuven, Herestraat 49, B-3000 Leuven, Belgium. (diethard.monbaliu@uzleuven.be).
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
The authors summarize the methods of isolated liver perfusion (ILP) together with the parameters for evaluation of ischemia reperfusion injury. ILP is also evaluated as a model to replace liver transplantation in animals with the benefits and limitations. Supplemental digital content is available in the text.
REFERENCES
- 1.Tygstrup N. Aspects of hepatic hypoxia: observations on the isolated, perfused pig liver Bull N Y Acad Med 1975. 51551–556 [PMC free article] [PubMed] [Google Scholar]
- 2.Sestoft L, Tonnesen K, Hansen FV. Fructose and D-glyceraldehyde metabolism in the isolated perfused pig liver Eur J Biochem 1972. 30542–552 [DOI] [PubMed] [Google Scholar]
- 3.Damgaard SE, Lundquist F, Tonnesen K. Metabolism of ethanol and fructose in the isolated perfused pig liver Eur J Biochem 1973. 3387–97 [DOI] [PubMed] [Google Scholar]
- 4.Abouna GM. Extracorporeal liver perfusion for hepatic coma. Lancet. 1971;1:1185. doi: 10.1016/s0140-6736(71)91695-3. [DOI] [PubMed] [Google Scholar]
- 5.Abouna GM, Boehmig HG, Serrou B. Long-term hepatic support by intermittent multi-species liver perfusions Lancet 1970. 2391–396 [DOI] [PubMed] [Google Scholar]
- 6.Jochmans I, Akhtar MZ, Nasralla D. Past, present, and future of dynamic kidney and liver preservation and resuscitation Am J Transplant 2016. 162545–2555 [DOI] [PubMed] [Google Scholar]
- 7.Bessems M, ’t Hart NA, Tolba R. The isolated perfused rat liver: standardization of a time-honoured model Lab Anim 2006. 40236–246 [DOI] [PubMed] [Google Scholar]
- 8.Vilei MT, Granato A, Ferraresso C. Comparison of pig, human and rat hepatocytes as a source of liver specific metabolic functions in culture systems—implications for use in bioartificial liver devices Int J Artif Organs 2001. 24392–396 [PubMed] [Google Scholar]
- 9.Kobayashi T, Taniguchi S, Ye Y. Comparison of bile chemistry between humans, baboons, and pigs: implications for clinical and experimental liver xenotransplantation Lab Anim Sci 1998. 48197–200 [PubMed] [Google Scholar]
- 10.Peralta C, Jiménez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu J Hepatol 2013. 591094–1106 [DOI] [PubMed] [Google Scholar]
- 11.Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation Nat Med 2011. 171391–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Groot H, Rauen U. Ischemia-reperfusion injury: processes in pathogenetic networks: a review Transplant Proc 2007. 39481–484 [DOI] [PubMed] [Google Scholar]
- 13.Yanaga K, Makowka L, Lebeau G. A new liver perfusion and preservation system for transplantation research in large animals J Investig Surg 1990. 365–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minor T, Efferz P, Fox M. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion Am J Transplant 2013. 131450–1460 [DOI] [PubMed] [Google Scholar]
- 15.Gong J, Lao XJ, Wang XM. Preservation of non-heart-beating donor livers in extracorporeal liver perfusion and histidine-trytophan-ketoglutarate solution World J Gastroenterol 2008. 142338–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoyer DP, Mathé Z, Gallinat A. Controlled oxygenated rewarming of cold stored livers prior to transplantation: first clinical application of a new concept Transplantation 2016. 100147–152 [DOI] [PubMed] [Google Scholar]
- 17.St Peter SD, Imber CJ, Kay J. Hepatic control of perfusate homeostasis during normothermic extrocorporeal preservation Transplant Proc 2003. 351587–1590 [DOI] [PubMed] [Google Scholar]
- 18.Gravante G, Ong SL, Metcalfe MS. Effects of hypoxia due to isovolemic hemodilution on an ex vivo normothermic perfused liver model J Surg Res 2010. 16073–80 [DOI] [PubMed] [Google Scholar]
- 19.Jakob H, Kutschera Y, Palzer B. In-vitro assessment of centrifugal pumps for ventricular assist Artif Organs 1990. 14278–283 [DOI] [PubMed] [Google Scholar]
- 20.Grosse-Siestrup C, Pfeffer J, Unger V. Isolated hemoperfused slaughterhouse livers as a valid model to study hepatotoxicity Toxicol Pathol 2002. 30749–754 [DOI] [PubMed] [Google Scholar]
- 21.El-Desoky AE, Jiao LR, Havlik R. Measurement of hepatic tissue hypoxia using near infrared spectroscopy: comparison with hepatic vein oxygen partial pressure Eur Surg Res 2000. 32207–214 [DOI] [PubMed] [Google Scholar]
- 22.Watson CJE, Kosmoliaptsis V, Randle LV. Normothermic perfusion in the assessment and preservation of declined livers before transplantation: hyperoxia and vasoplegia-important lessons from the first 12 cases Transplantation 2017. 1011084–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schütz E, Wieland E, Hensel A. Suppression of leukocyte-enhanced cold ischemia/reperfusion injury of liver endothelium with the benzoquinone antioxidant idebenone Clin Biochem 1997. 30619–624 [DOI] [PubMed] [Google Scholar]
- 24.Ghonem N, Yoshida J, Stolz DB. Treprostinil, a prostacyclin analog, ameliorates ischemia-reperfusion injury in rat orthotopic liver transplantation Am J Transplant 2011. 112508–2516 [DOI] [PubMed] [Google Scholar]
- 25.Adham M, Peyrol S, Chevallier M. The isolated perfused porcine liver: assessment of viability during and after six hours of perfusion Transpl Int 1997. 10299–311 [DOI] [PubMed] [Google Scholar]
- 26.Ikeda T, Yanaga K, Lebeau G. Hemodynamic and biochemical changes during normothermic and hypothermic sanguinous perfusion of the porcine hepatic graft Transplantation 1990. 50564–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagel S, Hegemann O, Groneberg DA. An improved model of isolated hemoperfused porcine livers using pneumatically driven pulsating blood pumps Toxicol Pathol 2005. 33434–440 [DOI] [PubMed] [Google Scholar]
- 28.Murphy GS, Hessel EA, Groom RC. Optimal perfusion during cardiopulmonary bypass: an evidence-based approach Anesth Analg 2009. 1081394–1417 [DOI] [PubMed] [Google Scholar]
- 29.Nassar A, Liu Q, Farias K. Ex vivo normothermic machine perfusion is safe, simple, and reliable: results from a large animal model Surg Innov 2015. 2261–69 [DOI] [PubMed] [Google Scholar]
- 30.Reddy S, Zilvetti M, Brockmann J. Liver transplantation from non-heart-beating donors: current status and future prospects Liver Transplant 2004. 101223–1232 [DOI] [PubMed] [Google Scholar]
- 31.Bessems M, Doorschodt BM, Dinant S. Machine perfusion preservation of the pig liver using a new preservation solution, polysol Transplant Proc 2006. 381238–1242 [DOI] [PubMed] [Google Scholar]
- 32.Boehnert MU, Yeung JC, Bazerbachi F. Normothermic acellular ex vivo liver perfusion reduces liver and bile duct injury of pig livers retrieved after cardiac death Am J Transplant 2013. 131441–1449 [DOI] [PubMed] [Google Scholar]
- 33.Op den Dries S, Sutton ME, Karimian N. Hypothermic oxygenated machine perfusion prevents arteriolonecrosis of the peribiliary plexus in pig livers donated after circulatory death. PLoS One. 2014;9:e88521. doi: 10.1371/journal.pone.0088521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.op den Dries S, Karimian N, Weeder PD. Normothermic acellular machine perfusion and bile duct injury in pig livers retrieved after cardiac death. Am J Transplant. 2013;13:3289. doi: 10.1111/ajt.12496. [DOI] [PubMed] [Google Scholar]
- 35.Banan B, Chung H, Xiao Z. Normothermic extracorporeal liver perfusion for donation after cardiac death (DCD) livers Surgery 2015. 1581642–1650 [DOI] [PubMed] [Google Scholar]
- 36.Koetting M, Lüer B, Efferz P. Optimal time for hypothermic reconditioning of liver grafts by venous systemic oxygen persufflation in a large animal model Transplantation 2011. 9142–47 [DOI] [PubMed] [Google Scholar]
- 37.Liu Q, Vekemans K, Iania L. Assessing warm ischemic injury of pig livers at hypothermic machine perfusion J Surg Res 2014. 186379–389 [DOI] [PubMed] [Google Scholar]
- 38.Minor T, Paul A. Hypothermic reconditioning in organ transplantation Curr Opin Organ Transplant 2013. 18161–167 [DOI] [PubMed] [Google Scholar]
- 39.Nassar A, Liu Q, Farias K. Role of vasodilation during normothermic machine perfusion of DCD porcine livers Int J Artif Organs 2014. 37165–172 [DOI] [PubMed] [Google Scholar]
- 40.Liu Q, Nassar A, Farias K. Sanguineous normothermic machine perfusion improves hemodynamics and biliary epithelial regeneration in donation after cardiac death porcine livers Liver Transpl 2014. 20987–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oku T, Harasaki H, Smith W. Hemolysis. A comparative study of four nonpulsatile pumps ASAIO Trans 1988. 34500–504 [PubMed] [Google Scholar]
- 42.Rawn DJ, Harris HK, Riley JB. An under-occluded roller pump is less hemolytic than a centrifugal pump J Extra Corpor Technol 1997. 2915–18 [PubMed] [Google Scholar]
- 43.Wheeldon DR, Bethune DW, Gill RD. Vortex pumping for routine cardiac surgery: a comparative study Perfusion 1990. 5135–143 [DOI] [PubMed] [Google Scholar]
- 44.Hornick P, Taylor K. Pulsatile and nonpulsatile perfusion: the continuing controversy J Cardiothorac Vasc Anesth 1997. 11310–315 [DOI] [PubMed] [Google Scholar]
- 45.Imber CJ, St Peter SD, Lopez de Cenarruzabeitia I. Advantages of normothermic perfusion over cold storage in liver preservation Transplantation 2002. 73701–709 [DOI] [PubMed] [Google Scholar]
- 46.St Peter SD, Imber CJ, Lopez I. Extended preservation of non-heart-beating donor livers with normothermic machine perfusion Br J Surg 2002. 89609–616 [DOI] [PubMed] [Google Scholar]
- 47.Reddy SP, Bhattacharjya S, Maniakin N. Preservation of porcine non-heart-beating donor livers by sequential cold storage and warm perfusion Transplantation 2004. 771328–1332 [DOI] [PubMed] [Google Scholar]
- 48.Grosse-Siestrup C, Nagel S, Unger V. The isolated perfused liver. A new model using autologous blood and porcine slaughterhouse organs J Pharmacol Toxicol Methods 2001. 46163–168 [DOI] [PubMed] [Google Scholar]
- 49.Schütz E, Wieland E, Heine L. Acceleration of hepatocellular energy by idebenone during early reperfusion after cold preservation ameliorates heat shock protein 70 gene expression in a pig liver model Transplantation 1997. 64901–907 [DOI] [PubMed] [Google Scholar]
- 50.Butler AJ, Rees MA, Wight DG. Successful extracorporeal porcine liver perfusion for 72 hr Transplantation 2002. 731212–1218 [DOI] [PubMed] [Google Scholar]
- 51.Minguela A, Marín L, Torío A. CD28/CTLA-4 and CD80/CD86 costimulatory molecules are mainly involved in acceptance or rejection of human liver transplant Hum Immunol 2000. 61658–669 [DOI] [PubMed] [Google Scholar]
- 52.Liu Q, Nassar A, Farias K. Comparing normothermic machine perfusion preservation with different perfusates on porcine livers from donors after circulatory death Am J Transplant 2016. 16794–807 [DOI] [PubMed] [Google Scholar]
- 53.Imber CJ, St Peter SD, de Cenarruzabeitia IL. Optimisation of bile production during normothermic preservation of porcine livers Am J Transplant 2002. 2593–599 [DOI] [PubMed] [Google Scholar]
- 54.Sutton ME, op den Dries S, Karimian N. Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion. PLoS One. 2014;9:e110642. doi: 10.1371/journal.pone.0110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heijnen BH, van Veen SQ, Straatsburg IH. Pronounced effect of minor changes in body temperature on ischemia and reperfusion injury in rat liver J Appl Physiol (1985 2001. 91265–268 [DOI] [PubMed] [Google Scholar]
- 56.Sahin S, Rowland M. Development of an optimal method for the dual perfusion of the isolated rat liver J Pharmacol Toxicol Methods 1998. 3935–43 [DOI] [PubMed] [Google Scholar]
- 57.Hannon JP, Bossone CA, Wade CE. Normal physiological values for conscious pigs used in biomedical research Lab Anim Sci 1990. 40293–298 [PubMed] [Google Scholar]
- 58.Gringeri E, Bonsignore P, Bassi D. Subnormothermic machine perfusion for non-heart-beating donor liver grafts preservation in a Swine model: a new strategy to increase the donor pool? Transplant Proc 2012. 442026–2028 [DOI] [PubMed] [Google Scholar]
- 59.Churchill TA, Green CJ, Fuller BJ. Protective properties of amino acids in liver preservation: effects of glycine and a combination of amino acids on anaerobic metabolism and energetics J Hepatol 1995. 23720–726 [DOI] [PubMed] [Google Scholar]
- 60.Verhoeven CJ, Farid WR, de Jonge J. Biomarkers to assess graft quality during conventional and machine preservation in liver transplantation J Hepatol 2014. 61672–684 [DOI] [PubMed] [Google Scholar]
- 61.Tolboom H, Pouw RE, Izamis ML. Recovery of warm ischemic rat liver grafts by normothermic extracorporeal perfusion Transplantation 2009. 87170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Janssen MW, Druckrey-Fiskaaen KT, Omidi L. Indocyanine green R15 ratio depends directly on liver perfusion flow rate J Hepatobiliary Pancreat Sci 2010. 17180–185 [DOI] [PubMed] [Google Scholar]
- 63.Reverdiau-Moalic P, Watier H, Vallée I. Comparative study of porcine and human blood coagulation systems: possible relevance in xenotransplantation Transplant Proc 1996. 28643–644 [PubMed] [Google Scholar]
- 64.Monbaliu D, Libbrecht L, De Vos R. The extent of vacuolation in non-heart-beating porcine donor liver grafts prior to transplantation predicts their viability Liver Transpl 2008. 141256–1265 [DOI] [PubMed] [Google Scholar]
- 65.Eriksson S, Fraser JR, Laurent TC. Endothelial cells are a site of uptake and degradation of hyaluronic acid in the liver Exp Cell Res 1983. 144223–228 [DOI] [PubMed] [Google Scholar]
- 66.Itasaka H, Suehiro T, Wakiyama S. Significance of hyaluronic acid for evaluation of hepatic endothelial cell damage after cold preservation/reperfusion J Surg Res 1995. 59589–595 [DOI] [PubMed] [Google Scholar]
- 67.Hansen T, Hollemann D, Pitton MB. Histological examination and evaluation of donor bile ducts received during orthotopic liver transplantation—a morphological clue to ischemic-type biliary lesion? Virchows Arch 2012. 46141–48 [DOI] [PubMed] [Google Scholar]
- 68.Yska MJ, Buis CI, Monbaliu D. The role of bile salt toxicity in the pathogenesis of bile duct injury after non-heart-beating porcine liver transplantation Transplantation 2008. 851625–1631 [DOI] [PubMed] [Google Scholar]
- 69.Thorens B. Glucose transporters in the regulation of intestinal, renal, and liver glucose fluxes Am J Physiol 1996. 270G541–G553 [DOI] [PubMed] [Google Scholar]
- 70.Jochmans I, Monbaliu D, Pirenne J. The beginning of an end point: peak AST in liver transplantation J Hepatol 2014. 611186–1187 [DOI] [PubMed] [Google Scholar]
- 71.Karimian N, Op den Dries S, Porte RJ. The origin of biliary strictures after liver transplantation: is it the amount of epithelial injury or insufficient regeneration that counts? J Hepatol 2013. 581065–1067 [DOI] [PubMed] [Google Scholar]
- 72.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury—a fresh look Exp Mol Pathol 2003. 7486–93 [DOI] [PubMed] [Google Scholar]