Abstract
Background
As the population in the United States continues to age, an increase in the number of potential donation after circulatory death (DCD) donors with advanced chronological age can be expected. The aim of this study was to analyze a multi-institutional experience in liver transplantation using DCD donors 50 years or older.
Methods
All DCD liver transplant (LT) performed at Mayo Clinic Florida, Mayo Clinic Rochester, and Mayo Clinic Arizona from 2002 to 2016 were included. Recipients of DCD LT were divided into 2 groups: those with donors 50 years or older (N = 155) and those with donors younger than 50 years(N = 316).
Results
Graft survival was similar between the DCD donors 50 years or older group and DCD donors younger than 50 group(P = 0.99). Graft survival at 1, 3, and 5 years was 87.0%, 75.6%, and 71.8% in the DCD donors 50 years or older group and 85.8%, 76.0%, and 70.4% in the DCD donors younger than 50 group.
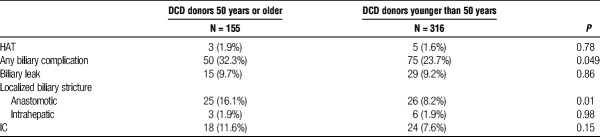
The rate of total biliary complications (32.3% vs 23.7%; P = 0.049) and of anastomotic strictures (16.1% vs 8.2%; P = 0.01) were higher in the DCD donors 50 years or older compared with the DCD donors younger than 50 group. No statistical significant difference in the rate of ischemic cholangiopathy (11.6% vs 7.6%; P = 0.15) was seen between the 2 groups. Due to homogeneous practice patterns at the involved institutions, additional Cox regression analysis using national data obtained from Scientific Registry of Transplant Recipients was used to evaluate predictors of graft failure in DCD donors 50 years or older. Significant predictors of graft failure included: a calculated Model for End-Stage Liver Disease score of 30 or higher (P < 0.001), mechanical ventilation at the time of transplant (P < 0.001), medical condition (in intensive care unit) (P = 0.002), and cold ischemia time (P < 0.001).
Conclusions
The present study demonstrates that acceptable graft and patient survival can be achieved with the usage of DCD LT with donors 50 years or older. Optimizing recipient selection criteria and minimizing cold ischemia time may further improve outcomes.
To help address the increasing discrepancy between the number of liver transplant (LT) candidates and the availability of liver grafts, the utilization of donation after circulatory death (DCD) donors for LT has been postulated as one potential approach.1 Initial reports examining the use of liver grafts from DCD donors described inferior long term outcomes when compared to donation after brain death (DBD) donors as the result of high rates of biliary complications, increased rates of primary nonfunction and hepatic artery thrombosis (HAT).2–6 More recently, individual center reports have demonstrated similar outcomes between DCD and DBD LT, with appropriate donor and recipient selection.7,8 In addition, national trends have shown improved outcomes with DCD LT.9
As the population in the United States continues to age, there will undoubtedly be an increase in the number of potential donors with advanced chronological age. In addition, there has been a shift toward DCD donors as a result of aggressive neurological and neurosurgical care of patients.10–12 This combination will likely increase the rates of DCD donors with advanced age. Multiple previous reports have suggested that donors 50 years or older are a risk factor for graft loss after DCD LT.13,14 This has resulted in hesitancy by many transplant programs to pursue DCD donors 50 years or older for LT. The 3 institutions included in the present study have frequently pursued and transplanted patients with liver grafts from DCD donors 50 years or older. With the growing potential of liver graft availability from DCD donors with advanced chronological age, it is important to further investigate long term outcomes in recipients of these grafts.
The present study aimed to compare the results of DCD LT between donors 50 years or older and donors younger than 50 years at 3 centers performing a high volume of DCD LT. We aimed to provide granular data, particular related to biliary complications. Analysis of national data using Scientific Registry of Transplant Recipients (SRTR) data set was performed to determine risk factors for graft loss which may be associated with older DCD livers.
MATERIALS AND METHODS
Approval from the Mayo Clinic Institutional Review Board was obtained. The study population included all DCD and DBD LT performed at Mayo Clinic Florida, Mayo Clinic Rochester, and Mayo Clinic Arizona from January 1, 2002, to September 30, 2016. Center data were obtained from prospectively maintained databases. National outcome data on patient and graft loss were also obtained and extracted from the United Network of Organ Sharing (UNOS) Standard Analysis and Research file over the same period.
Recipient factors were examined, including recipient age, body mass index (BMI), gender, etiology of original liver disease, secondary diagnosis, such as hepatocellular carcinoma (HCC), medical status at transplant, calculated Model for End-Stage Liver Disease (MELD) at the time of transplant, and allocation MELD scores. Donor factors examined included donor warm ischemic time (DWIT), donor gender, donor BMI and all components of the donor risk index (DRI).15
Graft survival was calculated from the time of LT until death, graft loss, or date of last follow-up. The occurrence and the date of death were verified from internal databases, data reported to the SRTR, and were completed by data from the US Social Security Administration and from the Organ Procurement Transplant Network.
The techniques of organ retrieval and LT at the involved centers have previously been described in detail.16–18 All transplants were performed with the piggyback procedure without a portocaval shunt or caval clamping. Thrombolytic agents were not used in any of the grafts during organ procurement or at the time of LT. Duct-to-duct biliary reconstruction was used in both DCD and DBD graft recipients except in with primary sclerosing cholangitis or when deemed unfeasible by the recipient surgeon. In 2 of 3 institutions, a transcystic duct biliary catheter was placed through the donor cystic duct and protocol cholangiograms through the biliary catheter were performed on postoperative day (POD) 3 and POD 21 on patients with biliary catheters.19 If no abnormalities were identified, the biliary catheter was then removed. Cholangiograms were performed before POD 21 if clinically indicated. All intrahepatic and extrahepatic strictures were documented even if not clinically significant at the time of cholangiogram. At the third institution, biliary catheters were not routinely placed and cholangiograms, either as endoscopic retrograde cholangiogram or as percutaneous transhepatic cholangiogram or magnetic resonance cholangiopancreatogram were performed based on clinical presentation. Strictures were classified according to previously described systems as localized (anastomotic or nonanastomotic) or diffuse ischemic cholangiopathy (IC).19,20 IC was defined as diffuse, intrahepatic bile duct strictures, in the absence of HAT, diagnosed with either cholangiogram using an intraoperatively placed transcystic duct biliary tube, by endoscopic retrograde cholangiogram or by percutaneous transhepatic cholangiogram.
Selection criteria for using DCD donors 50 years or older were relatively uniform in the 3 institutions involved in the study; therefore, insignificant heterogeneity of variables existed to perform a Cox regression predicting graft failure. As such, national data from the SRTR was used in investigating predictors of graft failure in the recipients of liver grafts from DCD donors 50 years or older. This cohort consisted of all LT using DCD donors 50 years or older performed during the study period.
All statistical analyses were performed using STATA 12 (Stata Corp., College Station, TX). Differences between groups were analyzed using the unpaired t test for continuous variables and by the χ2 test or continuity correction method for categorical variables. Wilcoxon rank-sum was used for variables that did not display a normal distribution. Survival curves for patient or graft survival were generated using the Kaplan-Meier method and compared by the log-rank test. Cox proportional hazard univariate regression for graft survival was performed. All statistical tests were 2-sided, and differences were considered significant when P is less than 0.05.
RESULTS
Between January 1, 2002, and September 30, 2016, a total of 5042 LT were performed in the 3 participating centers (Mayo Clinic Florida, Mayo Clinic Rochester, Mayo Clinic Arizona). Of these, 471 were from DCD donors and 4571 were from DBD donors. The DCD donors were divided into the 2 study groups, those 50 years or older (N = 155) and those younger than 50 years (N = 316).
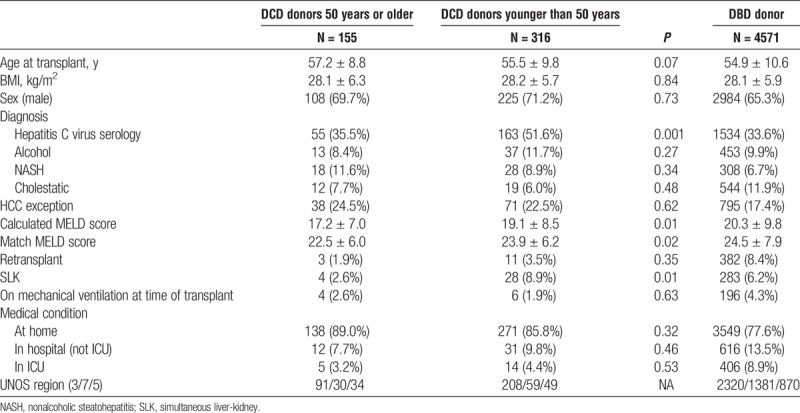
Follow-up of at least 12 months was complete in all patients included in the study. Recipient characteristics for the 2 DCD groups can be seen in Table 1A. Mean recipient age at LT was 57.2 ± 8.8 years in the DCD donor 50 years or older and 55.5 ± 9.8 years in the DCD donors younger than 50 years groups (P = 0.07). Calculated MELD at transplant was 17.2 ± 7.0 and 19.1 ± 8.5 (P = 0.01), whereas allocation MELD score was 22.5 ± 6.0 and 23.9 ± 6.2 (P = 0.02) in the DCD donors 50 years or older and DCD donors younger than 50 years groups, respectively. The number of patients with a diagnosis of HCC was 38 (24.5%) in the DCD donors 50 years or older and 71 (22.5%) in the DCD donors younger than 50 years groups (P = 0.62). A higher proportion of patients had hepatitis C as the etiology of liver disease in the DCD donors younger than 50 years group (51.6%) compared with the DCD donors 50 years of older group (P = 0.001). Alcohol, nonalcoholic steahepatitis, and cholestatic liver disease as the etiology of liver disease were similar between the 2 groups. A higher proportion of patients in the DCD donors younger than 50 years had an SLK (8.9%) compared with the DCD donors 50 years or older group (2.6%) (P = 0.01). Both DCD donors 50 years or older and DCD donors younger than 50 years groups had a similar number of recipients on mechanical ventilation (2.6% vs 1.9%; P = 0.63) and located in the intensive care unit (ICU) (3.2% vs 4.4%; P = 0.53) at the time of transplant. Recipient characteristics of patients undergoing DBD LT during the same period can be seen in Table 1A.
TABLE 1A.
Characteristics of LT recipients in the DCD donors 50 years or older, DCD donor younger than 50 years and DBD groups at 3 institutions
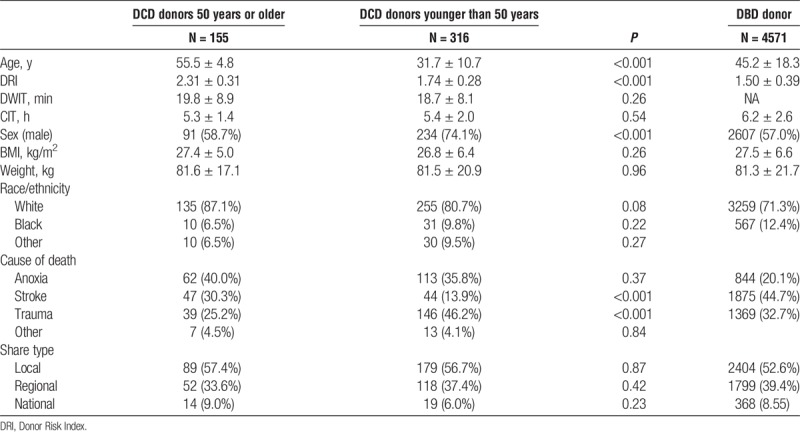
Donor and graft characteristics for the 2 groups can be seen in Table 1B. DCD donors were, by study design, older in the DCD donors 50 years or older group (55.5 ± 4.8) (age range, 50-81 years) compared to the DCD donors younger than 50 years group (31.7 ± 10.7) (age range, 7-49 years)(P < 0.001). In the DCD donors 50 years or older group, 130 donors were aged 50 to 59 years, 14 donors were aged 60 to 65 years and 11 donors were older than 65 years. No significant difference in DWIT, cold ischemia time (CIT), BMI, weight, race or share type was seen between the 2 groups. Donor characteristics of patients undergoing DBD LT during the same period can also be seen in Table 1B.
TABLE 1B.
Donor characteristics in the DCD donors 50 years or older, DCD donors younger than 50 years and DBD groups
Postoperative biliary and vascular outcomes for the 2 groups can be seen in Table 2. Rate of HAT (1.9% vs 1.6%; P = 0.78) was similar between the DCD donors 50 years or older and DCD donors younger than 50 years groups. The rate of total biliary complications (32.3% vs 23.7%; P =0.049) and of anastomotic strictures (16.1% vs 8.2%; P = 0.01) were higher in the DCD donors 50 years or older compared with the DCD donors younger than 50 years group. No significant difference in the rate of IC (11.6% vs 7.6%; P = 0.15) was seen between the 2 groups.
TABLE 2.
Postoperative complications in the DCD donors 50 years or older and DCD donors younger than 50 years groups
The median follow-up was 42 months in the DCD donors 50 years or older group and 48 months in the DCD donors younger than 50 years group. A minimum follow-up of at least 12 months was complete in all patients included in the study. Graft survival was similar between the DCD donors 50 years or older group and DCD donors younger than 50 years group (P = 0.99): graft survival at 1, 3, and 5 years was 87.0%, 75.6%, and 71.8% in the DCD donors 50 years or older group and 85.8%, 76.0%, and 70.4% in the DCD donors younger than 50 years group (Figure 1). No difference in graft survival was seen when the DCD donors 50 years or older group, and the DCD donors younger than 50 years group were compared with patients undergoing DBD LT during the same period (P = 0.40 and P = 0.21, respectively). Patient survival was also similar between the DCD donors 50 years or older group and DCD donors younger than 50 years group (P = 0.67): patient survival at 1, 3, and 5 years was 91.1%, 84.2%, and 81.6% in the DCD donors 50 years or older group and 92.1%, 82.1% and 76.1% in the DCD donors younger than 50 years group (Figure 2). No difference in patient survival was seen when the DCD donors 50 years or older group and the DCD donors younger than 50 years group were compared with patients undergoing DBD LT during the same period (P = 0.80 and P = 0.66 respectively).
FIGURE 1.
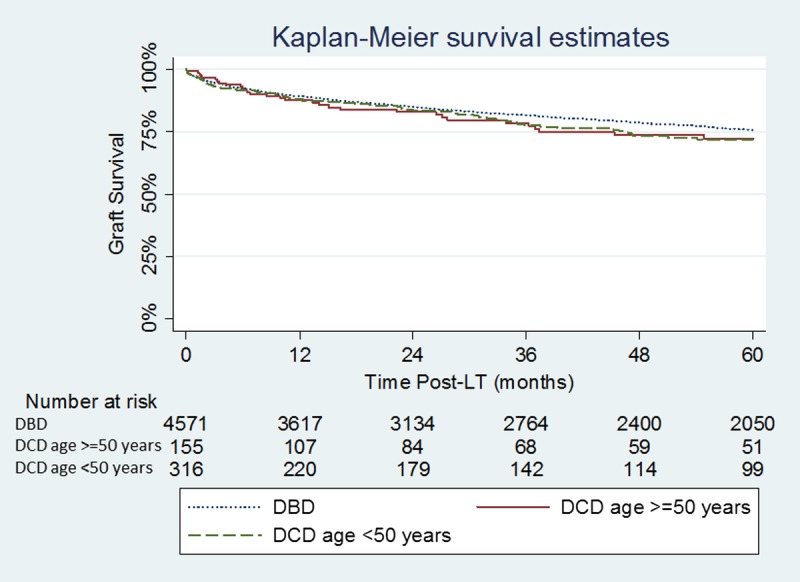
Kaplan-Meier graft survival estimates in the DCD donors 50 years or older, DCD donor younger than 50 years and DBD groups. DCD donors 50 years or older vs DCD donor younger than 50 years (P = 0.99); DCD donors 50 years or older vs DBD (P = 0.40); DCD donors younger than 50 years vs DBD (P = 0.21).
FIGURE 2.
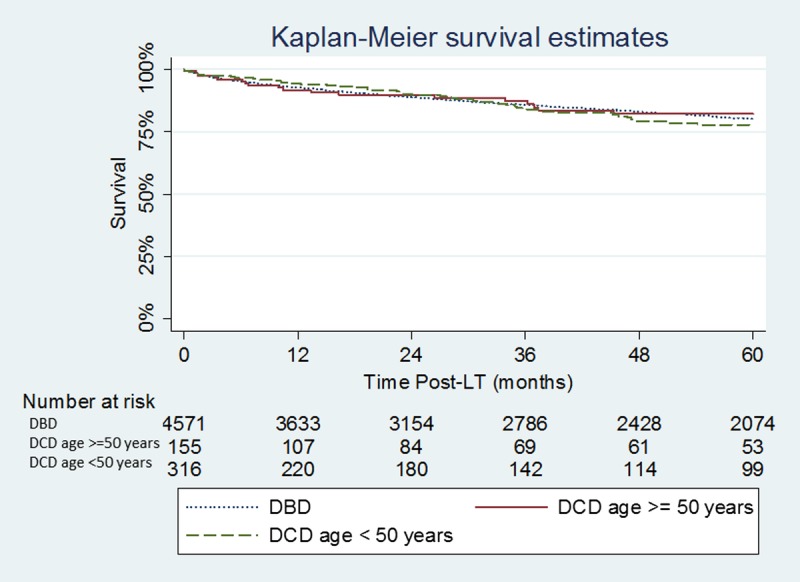
Kaplan-Meier patient survival estimates in the DCD donors 50 years or older, DCD donor younger than 50 years and DBD groups. DCD donors 50 years or older vs DCD donor younger than 50 years (P = 0.67); DCD donors 50 years or older vs DBD (P =0.80); DCD donor younger than 50 years vs DBD (P = 0.66).
DCD LT were further divided into groups based on donor age (<40, 40-49, 50-59, and ≥60 years). No significant difference in graft survival was seen between the groups divided based on donor age (P = 0.71) (Figure 3).
FIGURE 3.
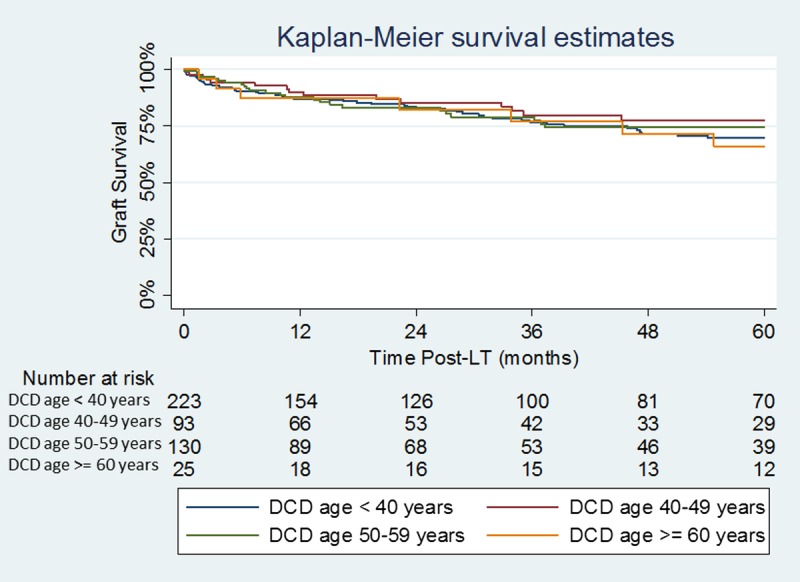
Kaplan-Meier Graft survival estimates by DCD donor age.
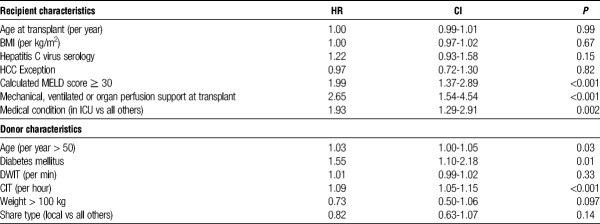
Using national data obtained from SRTR, a Cox regression evaluating predictors of graft failure from DCD donors 50 years or older was performed (Table 3). A total of 661 DCD LT from donors 50 years or older were performed nationally during the study period. Significant recipient predictors of graft failure included a calculated MELD score ≥ 30 (P < 0.001), mechanical ventilation at the time of transplant (P < 0.001) and medical condition (in ICU) (P = 0.002). Significant donor predictors of graft failure included incremental increasing donors older than 50 years (P = 0.03), diabetes mellitus (P = 0.01) and CIT (P < 0.001).
TABLE 3.
Cox regression using national LT with DCD donors 50 years or older (N = 661)
DISCUSSION
Although initial reports investigating DCD LT demonstrated inferior results, more recently reported single institution and national experience have suggested that outcomes using DCD LT are improving.8,9,21 In this multicentered study of 3 institutions using a large number of DCD LT, we demonstrate that with careful donor and recipient selection, similar results can be achieved between DCD donors 50 years or older and DCD donors younger than 50 years. Advanced donor age has previously been shown to be a predictor of inferior outcomes in DCD LT, resulting in many transplant programs having upper age thresholds of 45 to 50 years.13,14 Early single-center studies demonstrated trends of inferior patient and graft survival as well as higher rates of IC in donors older than 40 years compared with donors 40 years or older.3 In a study looking at US National registry data, Mathur et al13 demonstrated that donors older than 50 years and donors older than 60 years were associated with a higher adjusted risk of graft failure compared with donors aged 18 to 50 years (HR 1.39 and HR 1.88 respectively). A more recent single center report comparing DCD donors 45 years or older (N =37) to DCD donors younger than 45 years demonstrated no difference in graft survival rates or IC.22 In the current study, we demonstrate no difference in graft or patient survival between DCD donors 50 years or older compared with DCD donors younger than 50 years. These results should not be interpreted as implying that advanced DCD donor age is insignificant. Although this result is encouraging, underlying donor selection bias and matching with appropriate recipients should be emphasized; specifically, most DCD donors used that were 50 years or older were between 50 and 60 years in this study. Regardless of these recognized and unrecognized shortcomings, the results of the present study demonstrate that advanced DCD donor age in itself should not be an absolute contraindication to the usage of these livers. Instead, with increasing and successful utilization of DCD liver grafts by transplant programs, donor selection criteria, including donor age, will likely be widened.
The overall rate of biliary complications was higher in DCD donors 50 years or older group when compared with DCD donors younger than 50 years group. Although no difference in biliary leak rate was seen between the 2 groups, a higher rate of anastomotic strictures was seen in the DCD donors 50 years or older group. Since the majority of anastomotic strictures can be dealt with endoscopically, this finding alone should not exclude the use of these livers. The rate of IC, which often results in graft failure and retransplantation, was not statistically different between the 2 groups; however, a trend of higher rate was seen in the DCD donor 50 years or older group. A recently published study by the Birmingham, UK group investigated the outcomes of DCD donors older than 60 years compared with those 60 years or older and demonstrated no differences in graft or patient survival between the groups.23 In this study, the rate of vascular, biliary and overall complications was similar between the groups. It therefore remains to be determined if a slightly higher rate of IC can be expected when using DCD donors with advanced age.
The data from this study represent the largest and first multicenter study to demonstrate acceptable results with the utilization of DCD LT donors 50 years or older. In addition, the transplant centers included in this study are located in 3 different UNOS Regions with different waitlist dynamics (regions 3, 7, and 5). This highlights that DCD LT donors 50 years or older can be used effectively even in UNOS Regions with high median match MELD scores at transplantation. Difference in the utilization of DCD livers, represents one of the major contributors to differences in noneligible donor utilization rates across the Unites States.24 The low calculated MELD score in recipients of elderly DCD LT from this study demonstrates that all 3 programs were preferentially using these livers for patients with HCC or patients lower on their waitlists who would not otherwise receive liver offers. Using these donors not only provides immediate benefit to those patients transplanted with a DCD organ, but results in a halo effect to all patients on the waiting list. Effective use of these organs can therefore decrease overall waitlist mortality and is part of an effective waitlist management strategy.
The selection criteria used for DCD donor 50 years or older were relatively uniform between the 3 institutions involved in this study. These criteria have previously been described.8 Due to this lack of heterogeneity, we used national data from SRTR to investigate predictors of graft failure with the use of advanced age DCD LT. This analysis demonstrated inferior graft survival when advanced age DCD donors were used for recipients with a calculated MELD ≥ 30, on mechanical ventilation or in the ICU at the time of transplant. Incremental increasing donors older than 50 years and increasing CIT also negatively affected graft survival. The presence of diabetes mellitus in DCD donors 50 years or older was also a negative predictor of graft survival. Although the SRTR data are limited on the extent and duration of DM in these donors, it is possible that these donors may have underlying microvascular disease that may increase the risk of biliary complications posttransplant. All of the above findings corroborate the selection criteria used by the 3 centers in the present multicentered study. These liver grafts should preferentially be used for recipients who are disadvantaged on the waitlist, namely, those patients with complications of liver disease, yet with lower biologic MELD scores. From logistic standpoint, an attempt to keep CIT shorter than 6 hours should be made.
Limitations of the present study are largely related to over-interpretation of the findings. Advanced age DCD LT donors in this study represent a highly selected cohort. The number of advanced donor age DCD donors declined by the involved institutions during the study period is not available. Results from this study should not be interpreted as suggesting donor age is not a factor in utilization of DCD LT. Rather it should suggest that in appropriately selected donors and recipients advanced donor age unto itself should not be used as an absolute contraindication to the usage of DCD LT.
In conclusion, the present study demonstrates that acceptable graft and patient survival can be achieved with utilization of DCD donors 50 years or older if appropriate recipient and donor selection is employed. An increased rate of biliary complications was observed in this group; however, the majority of these were anastomotic strictures. If pursued, DCD donors 50 years or older may be best used for recipients with a biologic MELD score less than 30 who are not requiring ICU support, and CIT should be minimized.
Footnotes
The authors declare no funding or conflicts of interest.
K.P.C., A.K.M., A.A.M., C.B.R., J.K.H., C.B.T. participated in research design. K.P.C. and C.B.T.participated in data analysis. K.P.C., C.B.T. participated in the performance of the research. K.P.C., A.K.M., A.A.M., C.B.R., J.K.H., C.B.T. participated in the writing of the article.
Correspondence: C. Burcin Taner, MD, Department of Transplant, Mayo Clinic, 4500, San Pablo Rd, Jacksonville, FL 32224. (taner.burcin@mayo.edu).
This multicenter study demonstrates acceptable graft and patients’ survival with utilization of donation after circulatory death (DCD) donors age ≥ 50 if appropriate measures are employed, particularly regarding recipient characteristics at transplantation.
REFERENCES
- 1.Washburn K, Pomfret E, Roberts J. Liver allocation and distribution: possible next steps Liver Transpl 2011. 171005–1012 [DOI] [PubMed] [Google Scholar]
- 2.Mateo R, Cho Y, Singh G. Risk factors for graft survival after liver transplantation from donation after cardiac death donors: an analysis of OPTN/UNOS data Am J Transplant 2006. 6791–796 [DOI] [PubMed] [Google Scholar]
- 3.Foley DP, Fernandez LA, Leverson G. Donation after cardiac death: the University of Wisconsin experience with liver transplantation Ann Surg 2005. 242724–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skaro AI, Jay CL, Baker TB. The impact of ischemic cholangiopathy in liver transplantation using donors after cardiac death: the untold story Surgery 2009. 146543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Vera ME, Lopez-Solis R, Dvorchik I. Liver transplantation using donation after cardiac death donors: long-term follow-up from a single center Am J Transplant 2009. 9773–781 [DOI] [PubMed] [Google Scholar]
- 6.Jay C, Ladner D, Wang E. A comprehensive risk assessment of mortality following donation after cardiac death liver transplant—an analysis of the national registry J Hepatol 2011. 55808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laing RW, Scalera I, Isaac J. Liver transplantation using grafts from donors after circulatory death: a propensity score-matched study from a single center Am J Transplant 2016. 161795–1804 [DOI] [PubMed] [Google Scholar]
- 8.Croome KP, Lee DD, Perry DK. Comparison of longterm outcomes and quality of life in recipients of donation after cardiac death liver grafts with a propensity-matched cohort Liver Transpl 2017. 23342–351 [DOI] [PubMed] [Google Scholar]
- 9.Croome KP, Lee DD, Keaveny AP. Improving national results in liver transplantation using grafts from donation after cardiac death donors Transplantation 2016. 1002640–2647 [DOI] [PubMed] [Google Scholar]
- 10.Saidi RF, Bradley J, Greer D. Changing pattern of organ donation at a single center: are potential brain dead donors being lost to donation after cardiac death? Am J Transplant 2010. 102536–2540 [DOI] [PubMed] [Google Scholar]
- 11.Kramer AH, Zygun DA, Doig CJ. Incidence of neurologic death among patients with brain injury: a cohort study in a Canadian health region CMAJ 2013. 185E838–E845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orman ES, Mayorga ME, Wheeler SB. Declining liver graft quality threatens the future of liver transplantation in the United States Liver Transpl 2015. 211040–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathur AK, Heimbach J, Steffick DE. Donation after cardiac death liver transplantation: predictors of outcome Am J Transplant 2010. 102512–2519 [DOI] [PubMed] [Google Scholar]
- 14.Harring TR, Nguyen NT, Cotton RT. Liver transplantation with donation after cardiac death donors: a comprehensive update J Surg Res 2012. 178502–511 [DOI] [PubMed] [Google Scholar]
- 15.Feng S, Goodrich NP, Bragg-Gresham JL. Characteristics associated with liver graft failure: the concept of a donor risk index Am J Transplant 2006. 6783–790 [DOI] [PubMed] [Google Scholar]
- 16.Taner CB, Bulatao IG, Perry DK. Asystole to cross-clamp period predicts development of biliary complications in liver transplantation using donation after cardiac death donors Transpl Int 2012. 25838–846 [DOI] [PubMed] [Google Scholar]
- 17.Taner CB, Bulatao IG, Willingham DL. Events in procurement as risk factors for ischemic cholangiopathy in liver transplantation using donation after cardiac death donors Liver Transpl 2012. 18100–111 [DOI] [PubMed] [Google Scholar]
- 18.Grewal HP, Willingham DL, Nguyen J. Liver transplantation using controlled donation after cardiac death donors: an analysis of a large single-center experience Liver Transpl 2009. 151028–1035 [DOI] [PubMed] [Google Scholar]
- 19.Giesbrandt KJ, Bulatao IG, Keaveny AP. Radiologic characterization of ischemic cholangiopathy in donation-after-cardiac-death liver transplants and correlation with clinical outcomes AJR Am J Roentgenol 2015. 205976–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Croome KP, McAlister V, Adams P. Endoscopic management of biliary complications following liver transplantation after donation from cardiac death donors Can J Gastroenterol 2012. 26607–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bohorquez H, Seal JB, Cohen AJ. Safety and outcomes in 100 consecutive donation after circulatory death liver transplants using a protocol that includes thrombolytic therapy Am J Transplant 2017. 172155–2164 [DOI] [PubMed] [Google Scholar]
- 22.Firl DJ, Hashimoto K, O’Rourke C. Impact of donor age in liver transplantation from donation after circulatory death donors: a decade of experience at Cleveland Clinic Liver Transpl 2015. 211494–1503 [DOI] [PubMed] [Google Scholar]
- 23.Schlegel A, Scalera I, Thamara M, et al. Impact of donor age in donation after cardiac death liver transplantation: is the cut-off “60” still of relevance? Liver Transpl. 2017. [Epub ahead of print]. [Google Scholar]
- 24.Croome KP, Lee DD, Keaveny AP. Noneligible donors as a strategy to decrease the organ shortage Am J Transplant 2017. 171649–1655 [DOI] [PubMed] [Google Scholar]