Abstract
Since 2012, the Organ Procurement and Transplantation Network (OPTN)/United Network for Organ Sharing (UNOS) has required transplant centers to record the citizenship residency status of patients undergoing transplantation in the United States. This policy replaced the 5% threshold of the non–US citizen/nonresidents (NC/NR) undergoing organ transplantation that could result in an audit of transplant center activity. Since April 1, 2015, the country of residence for the NC/NR on the waitlist has also been recorded. We analyzed the frequency of NC/NR deceased donor organ transplants and waitlist registrations at all US transplant centers by data provided by UNOS for that purpose to the UNOS Ad Hoc International Relations Committee. During the period of 2013 to 2016, 1176 deceased donor transplants (of all organs) were performed in non–US citizen/non–US resident (NC/NR) candidates (0.54% of the total number of transplants). We focused on high-volume NC/NR transplant centers that performed more than 5% of the deceased donor kidney or liver transplants in NC/NR or whose waitlist registrants exceeded 5% NC/NR. This report was prepared to fulfill the transparency policy of UNOS to assure a public trust in the distribution of organs. When viewed with a public awareness of deceased donor organ shortages, it suggests the need for a more comprehensive understanding of current NC/NR activity in the United States. Patterns of organ specific NC/NR registrations and transplantations at high-volume centers should prompt a review of transplant center practices to determine whether the deceased donor and center resources may be compromised for their US patients.
Since 2012, the Organ Procurement and Transplantation Network (OPTN)/United Network for Organ Sharing (UNOS) has required transplant centers to record the citizenship and residency status of a patient undergoing organ transplantation in the United States. For the precision of recording, categories of national identity were established to ascertain whether the candidate listed for organ transplantation was a citizen of the United States, a non–US citizen residing in the US, or a non–US citizen not residing in the United States (NC/NR). Since 2014, the collection of transplant data was also revised to record whether the NC/NR foreign patient had traveled to the United States for the purpose of transplantation. These policies were developed to provide a transparency of practice for societal/public trust in the distribution of deceased donor organs, and it replaced the policy of a 5% threshold of NC/NR undergoing transplantation at a US center that could result in an audit of transplant center activity.1
The following report analyzes the NC/NR transplants performed in the United States since the 2012 policy change: by the number of NC/NR candidates added annually to the waitlist and by the number of NC/NR who traveled to the United States for the purpose of organ transplantation (TFT) or reported to have undergone organ transplantation in the United States having traveled to the United States for a reason other than the intention of organ transplantation (TFO).
The aim of this analysis was to assess the frequency of NC/NR deceased donor organ transplants and waitlist registrations at all US transplant centers. The data have a limitation of validity because they are self-reported by transplant centers with an arbitrary categorization as to TFT and TFO.
At specific centers with the highest number of NC/NR transplants, we sought to determine whether there was a measurable impact of an increased number NC/NR traveling to the United States for transplantation. For high-volume centers, the time to transplantation after registration and waitlist mortality for NC/NR patients were compared with all other patients at centers in their UNOS region. The quality of organs transplanted for NC/NR patients at high-volume centers was also compared with all other patients at the same center.
MATERIALS AND METHODS
The citizenship data of the candidate listed for transplantation was obtained from the Transplant Center Registrations forms. Categories for NC/NR changed in 2012. Before March 2012, the data of candidate listing was recorded as “nonresident alien.” The terms “nonresident alien” (NRA) or “resident alien” (RA) are formal US immigration terms used by the Immigration and Naturalization Service with very specific visa requirements. Neither of these terms captures “illegal” or “undocumented” aliens or residents, who have no visas or have overstayed the duration of the visa, estimated to be more than 11 million people.2 Before the introduction of the new categories into UNOS regulations no attempt was made by transplant programs to confirm whether a candidate was NRA/RA/or a citizen, and there was no category for the undocumented.
After March 2012, the data were collected as follows:
non–US citizen/non–US resident, TFT
non–US citizen/non–US resident, TFO.
After March 2015, the country of residence was also collected for NC/NR patients undergoing organ transplantation. The new categories have universal relevance and ask two basic question: (1) are you a citizen (of the United States); and (2) are you a resident (of the United States, meaning “is the United States your permanent abode”?). No attempt is made to ascertain if a noncitizen is “undocumented” since this determination is not in the expertise or purview of transplant programs. For example, a noncitizen/nonresident could be a foreign student or businessperson traveling to the United States, whereas an undocumented individual living in the United States would also be a noncitizen/resident. The staff of transplant programs are not trained nor qualified to make these categorical determinations.
The transplant center waitlist data were derived from the Transplant Center Registration Forms of patients added to the waitlist between January 1, 2013, and December 31, 2016. These data are self-reported. Access to these data was provided to the OPTN/UNOS Ad Hoc International Relations Committee; authors S.G., K.I., and J.R. were members of the committee. Authors F.D., G.D., T.P., and N.A. contributed to the analysis of the data by correspondence and phone conference calls.
The transplant data were derived from deceased donor transplants performed in the United States from January 1, 2013, to December 31, 2016. Since April 1, 2015, the country of residence for the NC/NR on the waitlist has also been recorded. No living donor NC/NR transplants were evaluated by this report.
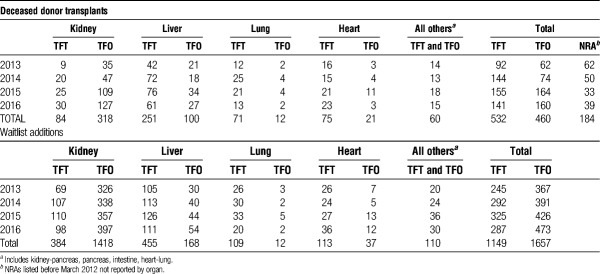
The data (Table 1) report all registrations and deceased organ transplants for the years 2013 to 2016 (inclusive of TFT, TFO, and NRA). The 2016 Country of Origin report in text and Table 2 excluded registrations of patients removed from the waitlist after living donor transplantation; thus, reports fewer kidney and liver registrations and transplants than Table 1.
TABLE 1.
Deceased donor transplants and waitlist additions for NC/NR 2013 to 2016—TFT, TFO, and NRA listed before March 2012
TABLE 2.
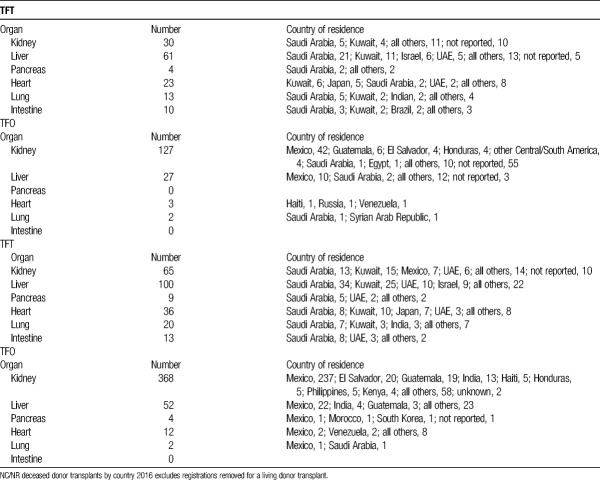
NC/NR deceased donor transplants and registrations by country
The data calculating the median time to transplant was based on registrations added to the waitlist from January 2010 to June 2015. The time estimates were derived from SRTR Program-Specific Reports released in June 2016.
The waitlist mortality data were based upon registrations of patients ever waiting on the list between January 2014 and December 2015. Mortality estimates were also derived from SRTR Program-Specific Reports released in June 2016. The transplant centers with the most NC/NR liver and kidney registrations and transplants between January 2013 and December 2015 were evaluated by the proportion of NC/NR patients that were listed and underwent transplantation and also by evaluating the percentage of registrations removed from the waiting list because of death.
High-volume centers for analyzing NC/NR transplants and waitlist registrations were defined as either performing more than 5% of kidney or liver transplants to NRA and NC/NR recipients between 2014 and 2016 or if the percentage of candidates listed for kidney or liver transplantation exceeded 5% NC/NR. Centers performing pediatric transplants and transplant centers that performed less than 10 NC/NR deceased donor kidney transplants in the 3-year cohort were excluded from analysis.
RESULTS
Deceased Donor Transplants for NC/NR
All Organ Transplants
During the period of 2013 to 2016, 1176 deceased donor transplants (of all organs) were performed n NC/NR candidates (Table 1). 532 of these 1176 transplants (45%) were performed in NC/NR candidates who had traveled to the United States for the purpose of organ transplantation (TFT) (Table 1). The remaining 460 NC/NR candidates underwent transplantation categorized by the transplant center as residing temporarily in the United States for a purpose other than transplantation (TFO) (Figure 1 and Table 1). Between 2013 and 2016, there were 99 285 organ transplants performed from deceased donors in United States. Of this total of nearly 100 000 transplants, the 1176 performed in NC/NR candidates represent 1.2% of the total number of transplants.
FIGURE 1.
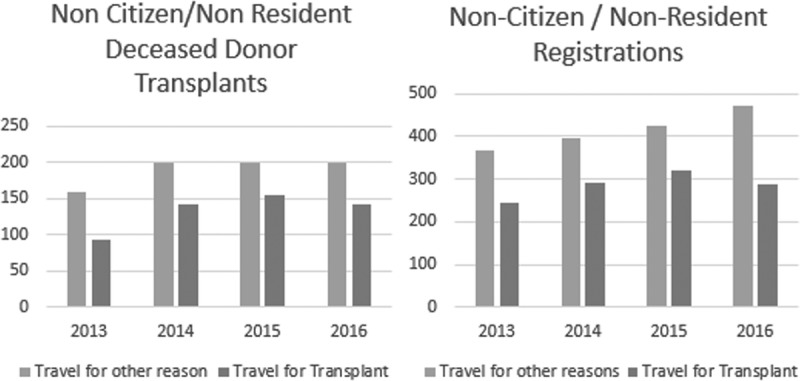
Deceased donor transplants and waitlist additions for all NC/NR that either traveled to the United States for the purpose of organ transplantation (TFT) or traveled to the United States for other reasons (TFO) and yet underwent deceased donor organ transplantation.
We focused on kidney and liver transplants because most of the NC/NR registrations and transplants were with liver and kidney allografts transplanted from deceased donors (Table 1).
Kidney Transplants
There were 5 high-volume NC/NR transplant centers that performed more than 5% of the deceased donor kidney transplants in NC/NR during the years 2014 to 2016 (range, 6-53%). During this period, these 5 centers performed a total of 147 deceased donor kidney transplants in NC/NR (TFT + TFO). NC/NR transplants at these 5 centers constituted 41% of all NCNR during that period (see also Table 1).
Of the 157 kidney NC/NR transplants in 2016, the majority (127) were TFO with patients from Mexico representing the largest number of candidates (42 of 127) Table 2. Of the 30 TFT patients, Saudi Arabia3 and Kuwait4 represented the most frequently reported countries of residence (Table 2). However, there were 65 (of 157) NC/NR transplants where country of origin was not reported.
The Kidney Donor Profile Index (KDPI) of the deceased donor kidneys transplanted into any NRA and NC/NR, during the period of 2014 to 2016, was comparable to the KDPI of kidneys transplanted into non NC/NR during this same period at these high-volume centers. With the KDPI analyzed by categories of 0 to 20, 21 to 34, 35 to 85, and 85 to 100, greater than 40% of KDPI kidneys transplanted were categorized between 0 and 34 for NC/NR patients and for all others. Thus, NC/NR candidates are not receiving lower-quality deceased donor kidneys.
Liver Transplants
There were 7 high-volume NC/NR transplant centers that performed more than 5% of the deceased donor liver transplants in NC/NR during the years of 2014 to 2016 (range, 5-16%). During this period, these 7 transplant centers performed a total of 120 deceased donor liver transplants in NC/NR (TFT + TFO) with 1797 liver total liver transplants performed at these centers (6.6% NC/NR). NC/NR transplants at these 7 centers constituted 42% (120/288) of all NC/NR during that 3-year period (see also Table 1). The Model for End-Stage Liver Disease (MELD) score at the time of deceased donor liver transplantation for NC/NR patients was less than the MELD score for all other patients at these transplant centers. Evident in the data review were 2 high-volume centers performing NC/NR transplants on patients with relatively low MELD Scores (Tables 3A, 3B and 4A, 4B).
TABLE 3A.
Liver high-volume center 11 562 laboratory MELD/PELD at transplant NCNR vs all others
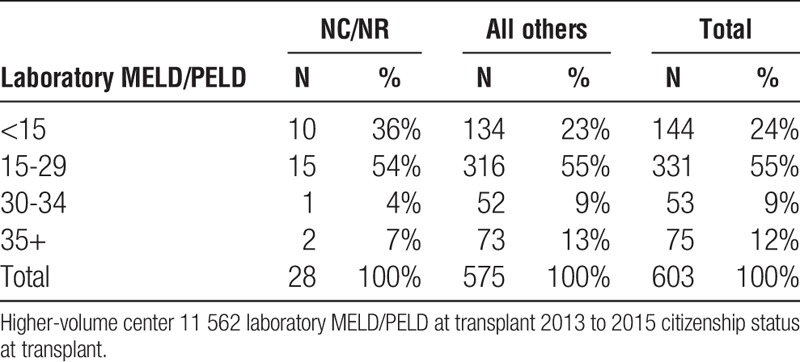
TABLE 3B.
Liver high-volume center 11 562 allocation MELD/PELD at transplant NCNR vs all others
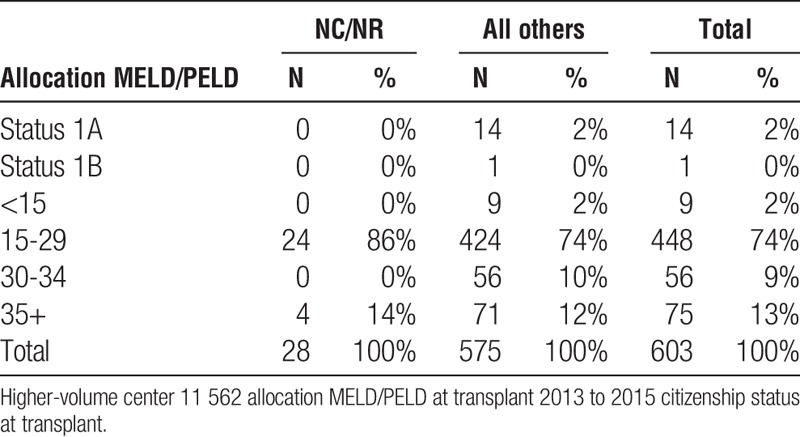
TABLE 4A.
Liver high-volume center 37 553 laboratory MELD/PELD at transplant NCNR vs all others
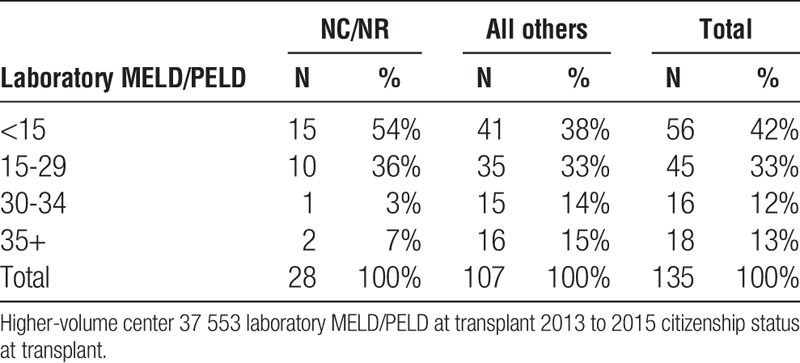
TABLE 4B.
Liver high-volume center 37 553 allocation MELD/PELD at transplant NCNR vs all others
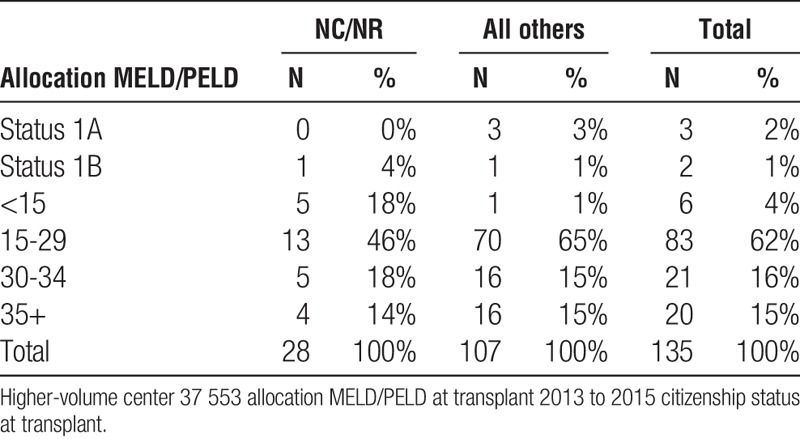
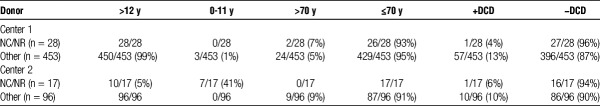
At the 2 transplant centers performing the largest (most) number of NC/NR deceased donor liver transplants, the quality of these liver allografts (donor age, >70 years and DCD) transplanted to NC/NR patients was not inferior to donor livers transplanted into all other recipients (Table 5).
TABLE 5.
Donor quality in NC/NR vs. all other liver transplants at the 2 centers performing the most NC/NR liver transplants versus all other liver recipients
NC/NR Waitlist Registrations
During the period of 2013 to 2016, 2806 NC/NR candidates were registered with 1149 declared as patients traveling to the United States specifically for the purpose of transplantation (TFT). Regions 5 and 9 (mainly California and New York) have the highest percentage of NC/NR candidates and transplants, representing approximately 2.5% of all candidate listings and deceased donor transplants in their respective regions. UNOS region 3 (Southeast United States inclusive of Louisiana and Florida) had the most registrations and deceased donor transplants for NC/NR-TFT candidates in 2016 (kidney, 20 registrations, 10 transplants; liver, 23 registrations; 19 transplants).
In 2016, all NC/NR patients comprised 1.3% of all waitlist additions, with the number (TFT and TFO) increasing steadily between 2013 and 2016 (Figure 1).
During the period of 2014 to 2016, there were 5 high-volume NC/NR centers that listed more than 5% of their kidney candidates as NC/NR. Three of these 5 centers overlapped with listing greater than 5% of their candidates as NC/NR and performing greater than 5% of the deceased donor kidney transplants to NC/NR patients.
During the period of 2014 to 2016, there were 7 high-volume NC/NR centers that listed more than 5% of their liver candidates as NC/NR, 6 of these 7 centers with listing more than 5% of their candidates as NC/NR and performing greater than 5% of the deceased donor liver transplants to NC/NR patients.
The majority of NC/NR patients undergoing deceased donor liver transplantation and being listed for liver transplantation are residing in a Middle East Country (Table 2).
Time to Transplantation and Waitlist Mortality for Kidney and Liver Registrants
To evaluate access for transplantation at the centers performing the most NC/NR transplants the percent of candidates who underwent organ transplantation within 1 and 3 years was compared with other centers in their respective UNOS Regions. Data were available between 2013 and 2015. Four of the 7 kidney transplant centers performing the most NC/NR transplants had a noticeably lower percentage of registrations undergoing kidney transplantation within 1 year, and 5 of 7 had a lower percentage at 3 years, when compared with their respective Region (Figure 2). There were no noticeable differences for liver registrants waiting transplantation.
FIGURE 2.
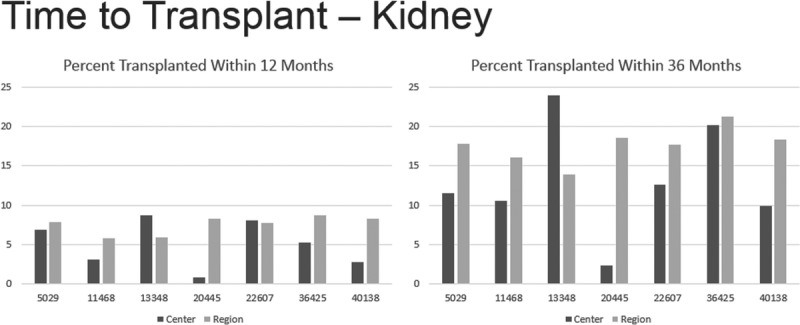
Time to transplant—kidney.
The waitlist mortality was evaluated for the transplant centers performing the most NC/NR transplants between 2013 and 2015. Two kidney centers had a noticeably higher waitlist mortality within 3 years of listing for kidney transplantation, when compared with their respective UNOS Region (Figure 3). There were no observable differences for liver waitlist mortality in this period of 2013 to 2015.
FIGURE 3.
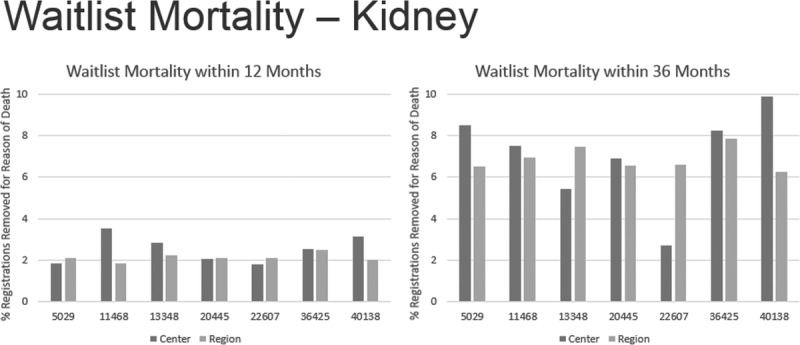
Waitlist mortality—kidney.
NC/NR Countries of Origin
Candidates from the Gulf Countries of the Middle East, particularly Saudi Arabia and Kuwait comprised the largest number of NC/NR-TFT registrations and transplants of organs (Table 2).
In 2016, there were 36 heart, 20 lung, 100 liver and 65 kidney registrations of NC/NR added to the waitlist. The most NC/NR TFT registrations for kidney and liver combined were from Saudi Arabia (47 total; 13 kidney and 34 liver) and Kuwait (40 total; 15 kidney and 25 liver). During 2013, 2014, and 2015, 31 of 971 heart, 33 of 1468 lung, 90 of 1642 liver, and 21 of 1701 kidney transplants were performed from deceased donors to NC/NR candidates. Forty-nine percent of the NC/NR patients undergoing liver and kidney transplantation were from Saudi Arabia and Kuwait.
The largest proportion of NC/NR TFT candidates coming to the United States for deceased donor transplantation were for liver and kidney allografts; in 2016, totaling 165 registrations (100 liver and 65 kidney).
In 2016, 47 residents of Saudi Arabia (34 liver, 13 kidney) were listed for liver or kidney and 26 (21 liver, 5 kidney) received deceased donor transplant. The second highest volumes were from Kuwait with 40 listings (25 liver, 15 kidney) and 15 transplants (11 liver, 4 kidney).
The largest number of TFO was from Central America (notably Mexico). These data are presented in Table 2.
DISCUSSION
A policy of transparency has emerged from the OPTN/UNOS that fulfills a Guiding Principle by the World Health Organization (WHO) for member states to make evident a registry of organ transplants that is transparent and available to the public—as the accountable source of donor organs (both living and deceased). Such a registry should identify the relationship of the donor and recipient, the country of origin from which transplants are being performed and the survival outcome of recipients and living donors.5
The WHO has encouraged countries to achieve self-sufficiency in organ donation and transplantation providing organs for patients within their governmental jurisdiction,3,4 consistent with the Declaration of Istanbul.6 The WHO has also emphasized that the practice of organ transplantation requires this policy of transparency, maintaining public trust and to provide regularly updated data on the allocation of organs that assures their equitable distribution and an assessment of self-sufficiency performance.7,8
We performed an analysis of the data available from OPTN/UNOS for public review that has substantive limitations but accomplishes (as comprehensively possible at this time), the purpose of the OPTN/UNOS to collect such data—fulfilling the WHO transparency principle. These data have been the subject of review by the OPTN/UNOS Ad Hoc International Relations Committee who has been charged with this responsibility.
The limitations of the data available currently from UNOS are evident. The data have not been validated after the submission by transplant centers (self-reported), and the data have a categorical distinction of NC/NR undergoing transplantation by intention or purpose of travel (TFT vs TFO) that also is not validated. Our review involved the retrieval of data with inconsistent time frames of analysis of NC/NR transplants and registrations; these are the data that were accessible. Finally, we recognize that the review of waitlist mortality and time to transplantation are not precise metrics of access to transplantation, but it is a reflection of NC/NR experience at certain centers that should elicit their review of accepting NC/NR patients.
Despite these limitations, this detailed report is useful not only in deriving summary observations of the NC/NR experience and a conclusion to suggest transplant center monitoring, but also in shaping recommendations for the improvement of such analyses in the future.
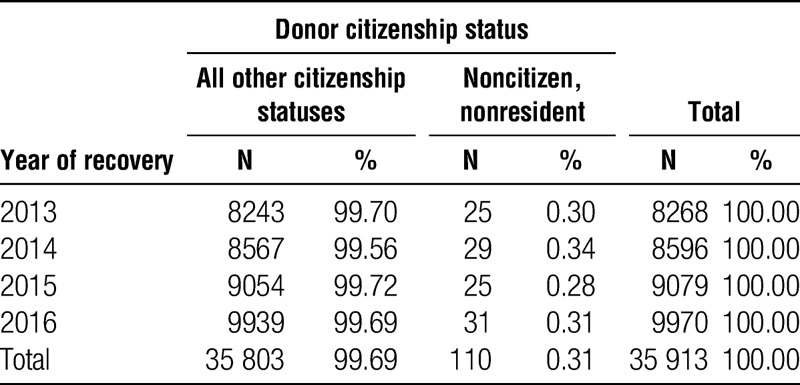
There are a substantial number of NC/NR being listed and undergoing organ transplantation in the United States, irrespective of whether they travel to the US for the purpose of transplantation (TFT) or not (TFO) as evident in Tables 1 and 2. The categorical distinction of TFT versus TFO was understandably developed in its intent to distinguish those NC/NR who were residing in the United States at the time of unexpectedly developing organ failure that required transplantation. However, that categorical separation elicits skepticism with the large number of TFO undergoing transplantation and no data regarding how long they had resided in the United States before transplantation. The increasing trend of TFO as illustrated in Figure 1 also underscores our concern regarding NC/NR transplants, with a skepticism that these patients are correctly categorized. The availability of organs for NC/NR transplants in the United States is not matched by the availability of organs for US patients to undergo transplantation in NC/NR countries, especially those who have not satisfactorily or responsibly addressed their self-sufficiency for organ donation. Finally, there was no (speculated) equivalency of NC/NR transplants with the number of NC/NR deceased organ donors in the United States (Table 6).
TABLE 6.
Deceased donors recovered from 2013 to 2016 by citizenship status
The Declaration of Istanbul defines travel for transplantation to be transplant tourism if it involves organ trafficking and/or transplant commercialism or if the resources (organs, professionals and transplant centers) devoted to providing transplants to patients from outside a country undermine the country’s ability to provide transplant services for its own population.1 Because few countries are currently able to meet the needs of all the patients awaiting transplantation, virtually any transplants performed in individuals from other countries could fall under the definition of tourism unless there are reciprocal arrangements of providing deceased donor organs between countries, as in Eastern Europe. There are no deterrents in countries that are systematically sending their patients to the United States for deceased organ donor transplants because of inadequate programs of deceased donation in their own countries. That reality is the basis of providing the transparency evident in this report.
The percentage of organs transplanted to NC/NR annually is certainly small (<1% of the total transplants performed) for the United States to be perceived as a site of transplant tourism. Nevertheless, the waitlist registrations of NC/NR have been increasing, especially for liver transplantation (Figure 1 and Table 2). These registrations are concentrated at a few transplant centers that overlap but are not identical to the specific centers that are performing the most NC/NR organ transplants.
Noteworthy also are the data of Figure 2, which reveal kidney transplant centers whose percentage of candidates undergoing transplantation within 1 and 3 years after listing were less than other centers in their UNOS respective regions. If they are performing noticeably less transplants than other centers in the region, and have a higher rate of registrants removed from the list because of death (Figure 3), these centers should reckon with such data when contemplating the acceptance of NC/NR candidates—especially listing NC/NR from countries that have not fulfilled a WHO direction of addressing self-sufficiency and otherwise maybe denying the opportunity of transplantation for US residents.
The disproportionate percentage of waitlist registrations from countries in the Middle East that have done little to establish successful programs of deceased donation (Table 2) should be a concern for the United States and international community. There should be no cultural basis for the inadequacy of these countries to establish programs of deceased donation, especially because the governments of these countries do not object to such NC/NR patients undergoing deceased donor transplantation in the United States.
The data also make clear that the organs provided for NC/NR patients are of comparable quality to those organs transplanted to US recipients. NC/NR patients are not being disadvantaged or only assigned organs of marginal quality (Table 5). Moreover, the data also surprisingly reveal lower MELD scores for NC/NR patients at the time of transplant than for US recipients undergoing transplantation at the same center (Tables 3A, 3B and 4A, 4B).
Our analysis excludes NC/NR pediatric transplantation recognizing the inability of some countries to provide organ transplants for this patient population and the history of US transplant centers providing compassionate care for patients, irrespective of their country of origin. Between 2013 and 2015, there were 32 pediatric NC/NR heart transplants, 9 pediatric lung transplants, 62 pediatric liver transplants, and 31 pediatric kidney transplants.
The relatively large number of NC/NR patients undergoing kidney transplantation designated from Mexico (Table 2) may reflect compassionate care of undocumented individuals laboring in the United States. This report also does not address living donor NC/NR transplants in the United States, and it also has the self-reporting inadequacy of either not reporting the country of origin or the NC/NR patient listed from “other” countries of origin.
Finally, a relatively small number high-volume centers for liver7 and kidney3 transplantation would seem to require proper monitoring to assess whether these centers are soliciting NC/NR patients or have developed a systematic referral pattern for NC/NR candidates when the percentage of transplants performed exceeds 5%. We used the 5% metric to define a high-volume NC/NR center recalling the previous threshold based on the prevailing UNOS policy at the time that would have triggered a center review of such transplant activity. Such a review should include heart and lung registrations and transplants as well.
There are no sanctions that are contemplated for centers with high rates of NC/NR registrations or transplants; nevertheless, those centers will be exposed transparently to attention regarding the practices that we anticipate a responsibility of centers to address the concerns of their patient population as to the time waiting for transplantation and mortality on the list.
CONCLUSIONS
We commend OPTN/UNOS for collecting and providing the data to present the analysis of this report. However, the transparency of this report, when viewed with a public awareness of deceased donor organ shortages, suggests the need for a more comprehensive understanding of current NC/NR activity in the United States. Patterns of organ-specific NC/NR registrations and transplantations at high-volume centers should prompt a review of transplant center practices to determine whether the deceased donor and center resources may be compromised for their US patients.
ACKNOWLEDGMENTS
The authors thank Eric Beeson and Sarah Taranto of UNOS Research Department for their contribution in the provision of data for this report.
Footnotes
The authors declare no funding or conflicts of interest.
Please be advised that all authors contributed.
The initial writing was done by F.D. S.G. and N.A. The other authors reviewed the drafts for their revisions. Similarly for the resubmission, F.D., S.G. and N.A. developed draft that each author commented and contributed.
Correspondence: Francis L. Delmonico, MD, 617 413 5311, Massachusetts General Hospital, 55 Fruit Street/White Bldg 505, Boston, MA 02114-2696. (francis_delmonico@neds.org).
The authors analyze the UNOS data regarding citizenship and residency status of all organ recipients within the USA. On top of getting current and accurate data, the authors discuss the potential deleterious consequences for US residents and the applicability to all other countries facing the same issue.
REFERENCES
- 1.Glazier AK, Danovitch GM, Delmonico FL. Organ transplantation for nonresidents of the United States: a policy for transparency Am J Transplant 2014. 141740–1743 [DOI] [PubMed] [Google Scholar]
- 2. http://www.pewresearch.org/fact-tank/2017/04/27/5-facts-about-illegal-immigration-in-the-u-s/accessed November 17, 2017. [Google Scholar]
- 3.White SL, Hirth R, Mahíllo B. The global diffusion of organ transplantation: trends, drivers and policy implications Bull World Health Organ 2014. 92826–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO; Transplantation Society (TTS); Organizatión Nacional de Transplantes (ONT). Striving to achieve self-sufficiency, March 23–25, 2010, Third WHO Global Consultation on Organ Donation and Transplantation. Madrid, Spain. Transplantation. 2011;91(Suppl 11):S27–S28. [DOI] [PubMed] [Google Scholar]
- 5. http://www.wpro.who.int/health_technology/documents/docs/HumanOrganTransplantationMeetingReport.pdf. [Google Scholar]
- 6.Steering Committee of the Istanbul Summit. Organ trafficking and transplant tourism and commercialism: the Declaration of Istanbul. Lancet. 2008;372:5–6. [DOI] [PubMed] [Google Scholar]
- 7.Danovitch GM, Chapman J, Capron AM. Organ trafficking and transplant tourism: the role of global professional ethical standards—the 2008 Declaration of Istanbul Transplantation 2013. 951306–1312 [DOI] [PubMed] [Google Scholar]
- 8.Delmonico FL, Domínguez-Gil B, Matesanz R. A call for government accountability to achieve national self-sufficiency in organ donation and transplantation Lancet 2011. 3781414–1418 [DOI] [PubMed] [Google Scholar]