Abstract
Background
Preemptive kidney transplants result in better outcomes and patient experiences than transplantation after dialysis onset. It is unknown how often a person initiates maintenance dialysis before living kidney donor transplantation when their donor candidate evaluation is well underway.
Methods
Using healthcare databases, we retrospectively studied 478 living donor kidney transplants from 2004 to 2014 across 5 transplant centers in Ontario, Canada, where the recipients were not receiving dialysis when their donor’s evaluation was well underway. We also explored some factors associated with a higher likelihood of dialysis initiation before transplant.
Results
A total of 167 (35%) of 478 persons with kidney failure initiated dialysis in a median of 9.7 months (25th-75th percentile, 5.4-18.7 months) after their donor candidate began their evaluation and received dialysis for a median of 8.8 months (3.6-16.9 months) before kidney transplantation. The total cohort’s dialysis cost was CAD $8.1 million, and 44 (26%) of 167 recipients initiated their dialysis urgently in hospital. The median total donor evaluation time (time from evaluation start to donation) was 10.6 months (6.4-21.6 months) for preemptive transplants and 22.4 months (13.1-38.7 months) for donors whose recipients started dialysis before transplant. Recipients were more likely to start dialysis if their donor was female, nonwhite, lived in a lower-income neighborhood, and if the transplant center received the recipient referral later.
Conclusion
One third of persons initiated dialysis before receiving their living kidney donor transplant, despite their donor’s evaluation being well underway. Future studies should consider whether some of these events can be prevented by addressing inappropriate delays to improve patient outcomes and reduce healthcare costs.
A preemptive kidney transplant avoids the risks of initiating dialysis and results in better outcomes and patient experiences compared with other treatment options available to patients with kidney failure.1,2 Deceased donor preemptive kidney transplants are rare, as most patients wait on a list for several years before an offer for a deceased donor kidney becomes available.3 For this reason, preemptive kidney transplants are typically achieved from a living donor.
There are many challenges in receiving a preemptive living donor kidney transplant. First, the intended recipient needs to be referred to a transplant program, thoroughly evaluated, and approved to receive a kidney transplant. Second, the transplant should be timed such that the intended recipient’s native kidneys have not failed to the extent of initiating dialysis urgently, but not too early so that the recipient can make use of any remaining native kidney function.4 Third, a living donor has to be identified.5 Finally, the living kidney donor candidate needs to be thoroughly evaluated and approved for kidney donation. For this last consideration, there is a growing appreciation that the living donor evaluation process for many motivated donor candidates is lengthy, difficult to navigate, and challenging.6–8 The 2017 Kidney Disease: Improving Global Outcomes “Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors” recommends that transplant programs should conduct as efficient a donor evaluation as possible, meeting the needs of donor candidates, intended recipients, and transplant programs.9 Using data from a multicenter study, the median estimated donor evaluation time (time from first contact to nephrectomy) was 10.3 months.10 In some cases, a prolonged donor evaluation process may prevent a preemptive transplant.
In this study, we focused on a cohort of patients with kidney failure, all who received a living donor kidney transplant. We studied persons not receiving dialysis when their donor candidate’s evaluation was well underway and determined how often maintenance dialysis was initiated before receipt of the living kidney donor transplant. We assessed the cost of dialysis treatments, and whether dialysis was started urgently in a hospital setting. Finally, we explored whether some unmodifiable and modifiable factors were associated with dialysis initiation before transplant.
MATERIALS AND METHODS
Design and Setting
We conducted a retrospective analysis of living donor kidney transplants using linked databases for the entire province of Ontario, Canada. Ontario has a current population of 13.7 million people and residents receive access to publicly insured hospital and physician services. In 2016, there were approximately 10 000 patients receiving dialysis, and 20 000 patients were followed up in clinics for advanced chronic kidney disease; living kidney donor transplants took place in 5 transplant centers. This study was approved by the research ethics board at Sunnybrook Health Sciences Centre, Toronto, Canada. Data sets were linked using unique encoded identifiers and analyzed at the Institute for Clinical Evaluative Sciences (ICES). The study was conducted according to a prespecified protocol and reporting of the study followed standardized guidelines (Appendix 1, SDC, http://links.lww.com/TP/B548).
Variables and Data Sources
We ascertained demographic characteristics, clinical factors, and outcomes using several linked databases. Information on all living kidney donors and recipients in Ontario were obtained from Trillium Gift of Life Network, chart abstraction, and the Canadian Organ Replacement Register databases, and included race, blood type, and donor-recipient relationship. Additional donor information included the donor’s estimated glomerular filtration rate (eGFR) before donation. Additional recipient information included primary cause of kidney failure, prior transplant history, and serum creatinine, hemoglobin, and albumin at the time of dialysis initiation. Recipient referral dates were available for recipients transplanted after 2010. Demographic variables were obtained from the Registered Persons Database (age, sex, postal codes to calculate the Euclidean distance to the transplant center and to obtain neighborhood income quintiles from the 2006 Canada Census). The Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD) and the Ontario Health Insurance Plan (OHIP) data sets were used to determine if and when dialysis was initiated (and whether it was started in the hospital or outpatient setting), as well as to identify various nonrenal comorbidities among recipients (Appendix 2, SDC, http://links.lww.com/TP/B548).11 The ICES Physician Database and OHIP were used to determine the start date of the living donors’ evaluation (Appendix 3-5, SDC, http://links.lww.com/TP/B548). Linked laboratory databases were used to obtain the most recent recipient serum creatinine at the time their donor initiated their evaluation (±3 months) and at the time of referral (±3 months) in a subset of patients. This database, the Ontario Laboratory Information System, includes inpatient and outpatient test values from hospital and commercial laboratories, together accounting for 91% of Ontario’s laboratory results by 2016. Estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (in mL/min per 1.73 m2).12 Dialysis costs were estimated for recipients who started dialysis after March 2006 (which was the first available date in our data sources when dialysis costs could be reliably ascertained). Costs were tabulated from the public payers’ perspective using OHIP billing codes (Appendix 2, SDC, http://links.lww.com/TP/B548) plus resource intensity weights multiplied by the cost per weighted case to calculate the cost per case (ie, consumable materials, nursing staff, machine costs).13
Selection
The selection of living donor kidney transplants for this study is presented in Figure 1. This study was restricted to patients who received a living kidney donor transplant, where the transplants occurred between April 1, 2004, and March 31, 2014. In this study, we focused on the subset of living donor transplants where the recipient was a first-time kidney transplant recipient and was not on dialysis when the evaluation process of the candidate who ultimately donated to them was well underway. Living donors were required to be Ontario residents for at least 2 years before donation to ensure that information on the donor evaluation process was complete and available in our data. We excluded donors who were missing a donation date, a nephrology consult, or a surgery consult (Figure 1), because these donors were likely from outside of Ontario or may have participated in a national kidney paired donation program. We also excluded donors with unreasonable patterns of procedures (ie, nephrectomy codes before donation date) and those with a late-stage procedure captured as the first procedure (ie, a living donor evaluation would not begin with a nephrology consultation).
FIGURE 1.
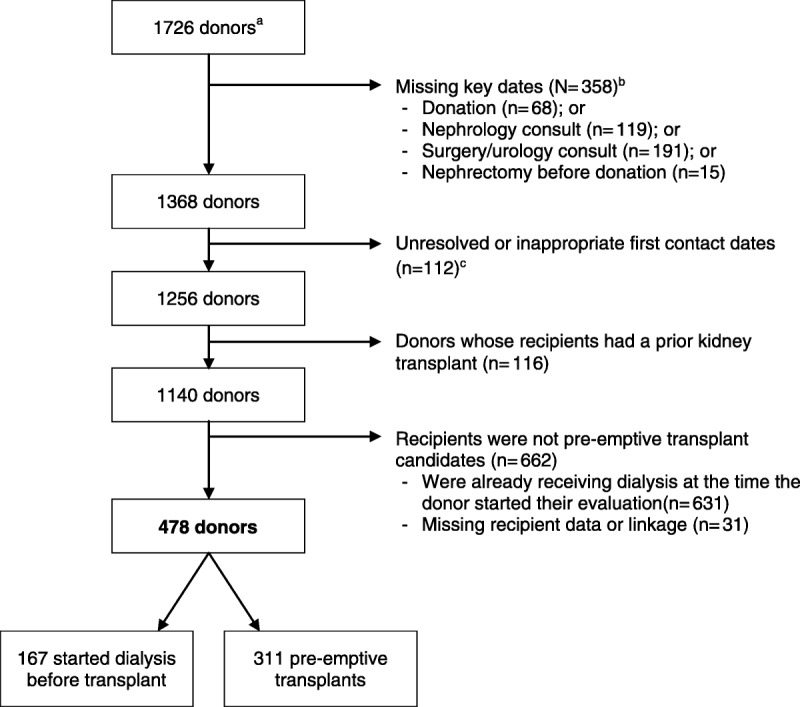
Overview of inclusion/exclusion criteria for living kidney donors in this study. a Living kidney donors were identified through Trillium Gift of Life Network. All living donors have a unique identification number that allows linkage across data sets. b These exclusions are not mutually exclusive so do not sum to 358; nephrology consults within 2 weeks of donation and surgical consults within 2 days of donation were not considered true consults (part of the preadmission process).c Healthcare procedures deemed appropriate start points for the living donor evaluation.
Measurements
In this study, a preemptive transplant was defined as the absence of dialysis billing codes for the recipient before their transplant. We considered a preemptive transplant potentially possible if the recipient did not receive dialysis within 92 days after the donors’ evaluation start date. For these recipients, if dialysis was initiated before transplant, it was considered a “potential unrealized preemptive transplant.” Our opinion is that 92 days (3 months) is a reasonable buffer time to complete the evaluation (which would be the case if the donor was motivated and eligible to donate). In sensitivity analysis, we extended this period to 4 and 6 months. The United Kingdom 2020 strategy suggests that all potential donors should be offered to complete the donor assessment within 4.5 months of referral (where appropriate).14 With the data available to us, we could not reliably assess how many unrealized preemptive kidney transplants were preventable (ie, there were modifiable reasons [inappropriate waiting] that could be addressed to realize the preemptive kidney transplant). For this reason, we deliberately use the wording “potential” unrealized preemptive kidney transplant in this article.
We defined the total evaluation time as the time when the donor started the evaluation (the earliest documented evaluation testing) until the nephrectomy. We defined the total approval time as the time from the donor evaluation start until the last specialist consult preceding nephrectomy. The procedures that defined the start of the evaluation and the consults that defined the approval date are presented in Appendix 5, SDC (http://links.lww.com/TP/B548). We defined the time for consults as the time from the first to the last nephrology, psychosocial, or surgical evaluation; this was restricted to donors who had all 3 consults and was limited to the most recent of the 3 consults. These 3 consults are a standard part of the donor candidate evaluation in all Ontario transplant programs. All times were expressed in months.
Statistical Methods
Descriptive statistics included the mean (standard deviation [SD]), median (25th, 75th percentile), and proportion (95% confidence intervals), where appropriate.
We used a recommended approach to report risk ratios for the association between characteristics and dialysis initiation (ie, a potential unrealized preemptive kidney transplant; yes/no) (estimates derived from modified Poisson regression models [proc genmod using a log link], a Poisson distribution, and a repeated statement [for individuals] for robust standard error estimation).15 To assess whether the results differed across the 5 Ontario transplant programs that performed living donor nephrectomies during the study period, we calculated the intraclass correlation coefficient using mixed models treating the transplant program as the clustering variable (as a measure of the proportion of the variance of the outcome accounted for by differences in transplant program).
To comply with privacy regulations for minimizing the chance of patient identification, 5 or fewer participants are reported as less than 6. For similar reasons, the names of the transplant programs and the number of transplants per program were also suppressed. We used Statistical Analysis Software Enterprise Guide version 6.1 (2013 by SAS Institute Inc., Cary, NC) for all analyses.
RESULTS
Patient Population
A total of 478 living kidney donor transplants were included in the primary analysis (Figure 1). Donors were a mean 46 (SD, 11) years of age at the time of donation, most were white (79%), female (63%), lived in an urban area (87%), had higher neighborhood income (24% were in the highest income quintile versus 13% in the lowest), and lived a median of 33 (16-74) km from the transplant center where they donated (Table 1). The predonation eGFR was greater than 80 mL/min per 1.73 m2 in 79% of donors. Recipients were similar to donors with respect to age at transplant (mean, 44; SD, 14 years), percent living in urban areas (87%), and neighborhood income (24% in the highest income quintile), but were more likely to be male (63%).
TABLE 1.
Donor, recipient, and transplant characteristics
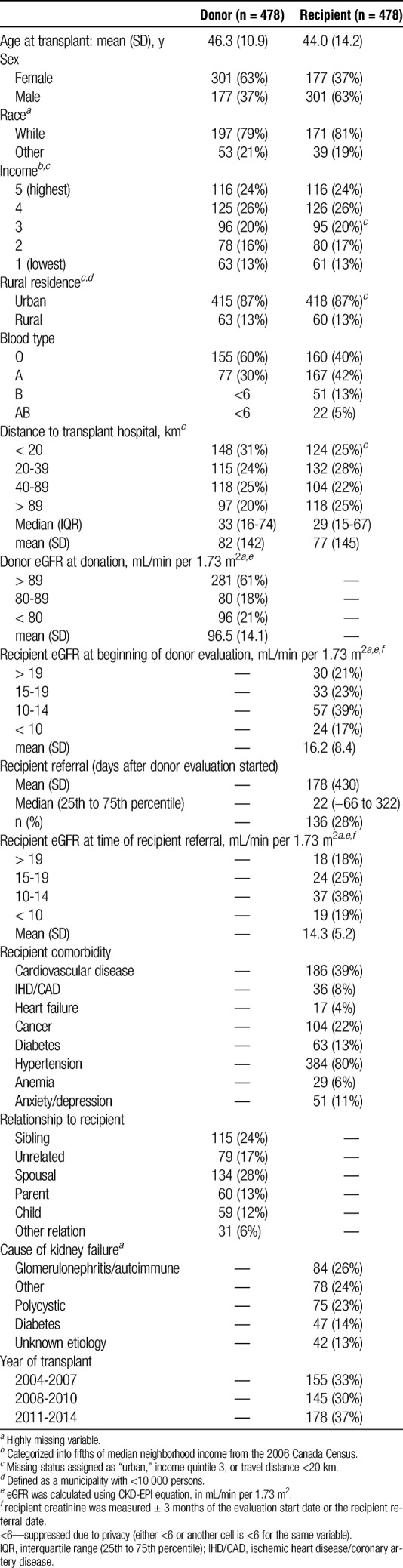
Most transplants occurred between spouses (28%), siblings (24%), or unrelated donor-recipient pairs (17%) (Table 1). The proportion of living donor transplants performed in Ontario ranged from 6% to 31% across the 5 transplant programs.
Potential Unrealized Preemptive Kidney Transplant
A total of 478 persons (all who ultimately received a living kidney donor transplant) were not on dialysis when the donor candidate (who ultimately donated to them) was being evaluated for at least 3 months. Recipient eGFR at the start of their donors’ evaluation was a mean (SD) of 16.2 (8.4) mL/min per 1.73 m2, and in those with available data the recipient eGFR at recipient referral was 14.3 (5.3) mL/min per 1.73 m2. For pairs with available data, the recipient referral predated the date the donor candidate first contacted the transplant program in 55 (40%) of 136 patients (mean [SD] of −5.2 [4.8] months). Donor candidate first contact predated the recipient referral in 80 (59%) of 136 patients (mean [SD], 13.5 [13.9] months). The transplant programs in Ontario typically put the donor candidate evaluation on hold until the intended recipient is referred for transplant evaluation.
A total of 167 (35%) of 478 recipients initiated dialysis before receipt of their transplant, which we consider potential unrealized preemptive kidney transplants. In sensitivity analyses, requiring the donor candidate to be evaluated for at least 4 or 6 months when their recipient (who was not on dialysis) entered the cohort meant 144 (32%) of 451 recipients and 111 (27%) of 412 recipients, respectively, initiated dialysis before transplant.
The mean (SD) eGFR at the time of dialysis initiation was 8.5 (7.2) mL/min per 1.73 m2, serum albumin was 35.2 (7.0) g/L, and serum hemoglobin was 105 (42) g/L. A total of 44 (26%) of the 167 recipients started dialysis as an inpatient in the hospital setting. Recipients, who started dialysis during their donors’ evaluation did so in a median of 9.7 (5.4-18.7) months after their donor started the evaluation, were transplanted a median 8.8 (3.6-16.9) months after starting dialysis, and accrued a mean of CAD $48 717 (SD, CAD $55 249) in dialysis costs, totalling CAD $8.1 million for the cohort of 167 recipients (2017 Canadian dollars). For recipients with available data, the transplant program received the referral for recipient evaluation a mean of 68 (SD, 913) days (median, 363 [198-448] days) before dialysis started.
Characteristics Associated With a Potential Unrealized Preemptive Kidney Transplant
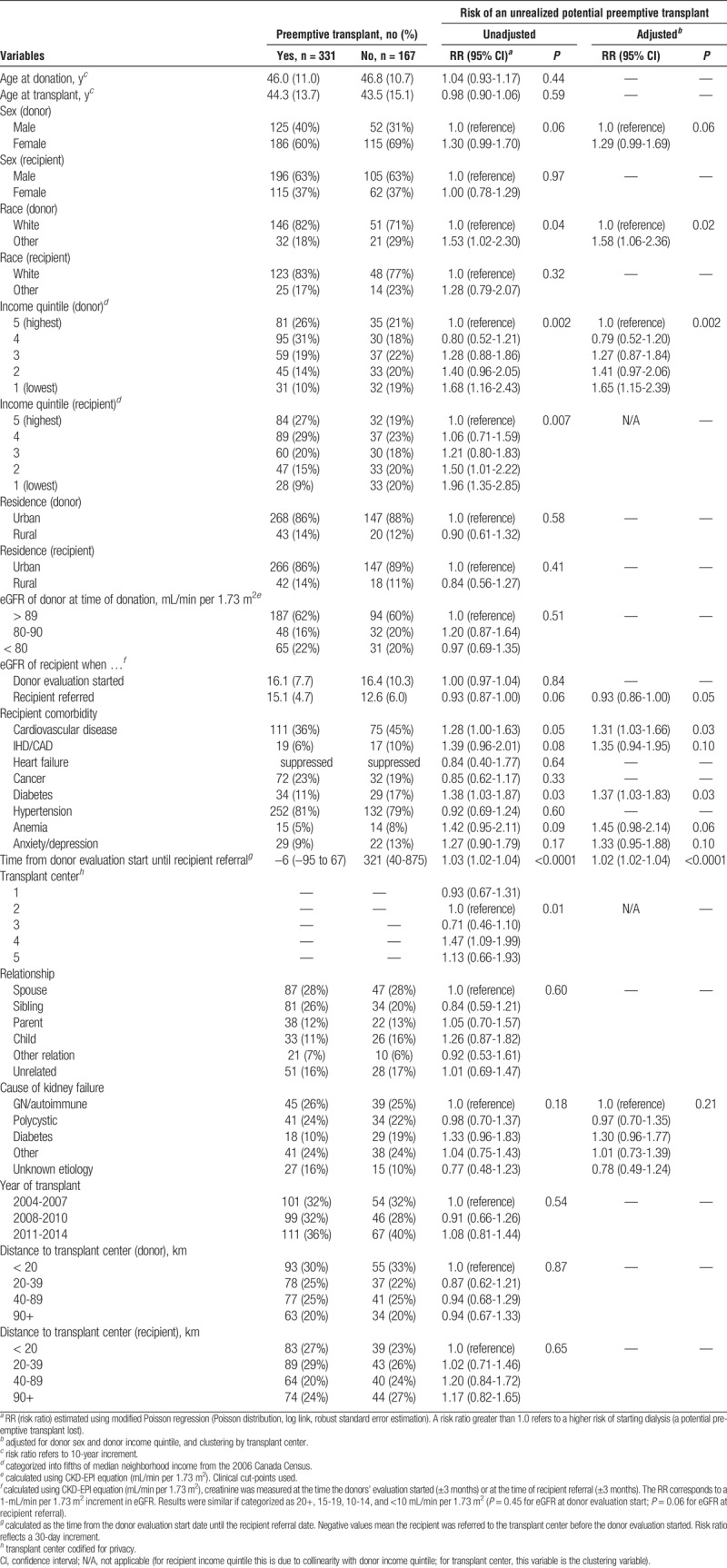
Associations between various characteristics and a potential unrealized preemptive transplant in an exploratory analysis are presented in Table 2. The recipient was more likely to start dialysis if their donor was female (risk ratio [RR], 1.30; 0.99-1.70), if either the donor or recipient was from a lower-income neighborhood (RR, 1.68 [1.16-2.43 and RR, 1.96 [1.35-2.85] for the lowest quintile vs the highest), and if the donor was nonwhite (RR, 1.53 [1.02-2.30]). Recipient nonrenal comorbidity was also a significant predictor of starting dialysis, particularly the presence of cardiovascular disease (RR, 1.31 [1.03-1.66]) and diabetes (RR, 1.37 [1.03-1.83]). Nonsignificant associations were observed for anemia [RR 1.45 (0.98-2.14)], ischemic heart disease or coronary artery disease (RR, 1.35 [0.94-1.95]), and anxiety or depression (RR, 1.33 [0.95-1.88]). For recipients with available data, dialysis before transplant was more likely if there was a longer delay between the donor’s evaluation start date and the date the transplant program subsequently received the referral to begin the intended recipient’s evaluation [RR, 1.03 [1.02-1.04]) per 30-day delay. Furthermore, a lower recipient eGFR at referral was associated with an increased likelihood of starting dialysis (RR, 0.93 [0.86-1.00]), whereas no such association was observed for recipient eGFR at the donor’s evaluation start date. There were significant differences across transplant programs (P = 0.01), where 1 program was 29% less likely to have a potential unrealized preemptive transplant, whereas another program was 47% more likely to do so when compared with a reference. However, between-center variability only accounted for 2.8% of the total variability in potential unrealized preemptive transplant rates (P = 0.16). After adjusting for donor sex, donor income, and clustering by transplant program, the strength of these associations changed very little (Table 2).
TABLE 2.
Characteristics associated with an unrealized potential preemptive transplant
Time to Complete the Living Donor Evaluation
The median total donor evaluation time among donors whose recipients were transplanted preemptively was 10.6 (6.4-21.6) months (mean, 15.3 [12.0] months). For those who started dialysis during the evaluation, the median was twice as long: 22.4 (13.1-38.7) months (mean, 25.4 [14.0] months) (P < 0.0001) (Figure 2). Similar results were observed for the time until approval: median, 9.13 (5.9-20.2) months (mean, 14.3 [12.0] months) versus median 20.9 (11.7-37.8) months (mean, 24.2 [14.0] months) (P < 0.0001), respectively. In contrast, we did not observe a relationship with a prolonged time to complete the major consultations with a higher likelihood of potential unrealized preemptive transplant: median, 6.01 (1.77-17.7) months (mean, 11.0 [11.9] months) for preemptive transplants, median, 6.47 (2.50-15.8) months (mean, 11.2 [11.4] months) for an unrealized potential preemptive transplant (P = 0.87).
FIGURE 2.
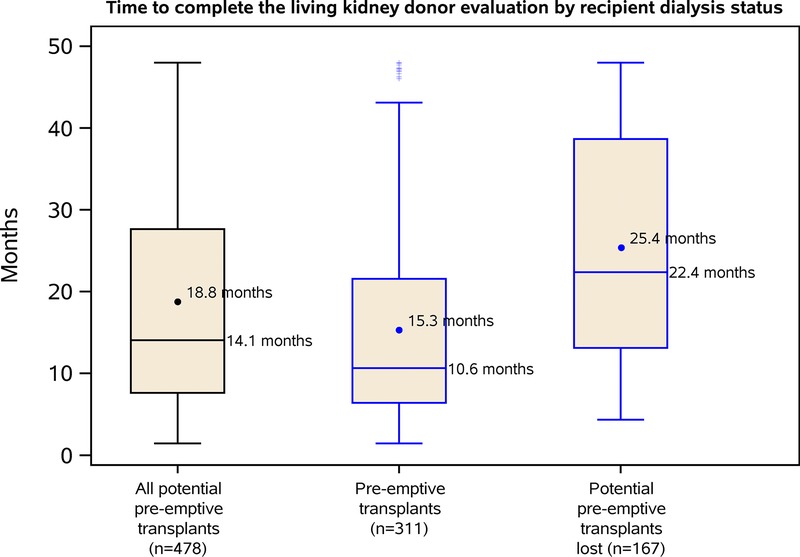
Boxplots showing the distribution of donor evaluation times. The time to complete the evaluation was defined as the period from evaluation start until nephrectomy. Boxes represent interquartile ranges (25th to 75th percentile). Horizontal lines indicate median (50th percentile). Circles represent means. Horizontal lines represent the upper fence (75th percentile plus 1.5× interquartile range) and lower fence (25th percentile minus 1.5× interquartile range). Plus symbols indicate points that fall outside the fence.
DISCUSSION
To our knowledge, no prior study has described recipient outcomes in the context of the time to evaluate a living kidney donor candidate. To address this, we studied a group of people across 5 transplant programs in Ontario, Canada, for the period 2004 to 2014, all who received living donor kidney transplants. We found that a third of persons not receiving dialysis when their donor’s evaluation was well underway initiated dialysis before receiving their living donor kidney transplant. This dialysis cost was CAD $8.1 million, and 44 of (26%) 167 recipients initiated their dialysis urgently in hospital.
A recently published guideline in the United Kingdom has recommended that 50% of all eligible recipients are transplanted preemptively, and that all donors are able to complete their workup in 18 weeks should they choose to do so.14 We agree with this and believe that, for a healthy, motivated donor whose intended recipient has been cleared for transplant, 4 months is sufficient to complete a thorough evaluation while providing sufficient time for donor reflection. The time to complete the necessary nephrology, surgery, and psychosocial consultations, therefore, should not be measured on the order of months and presents an opportunity for improvement. We are aware that some centers (including ours) have transitioned toward scheduling these consults on the same day or within 2 consecutive days of each other, particularly for donor candidates who live far from the transplant center. There is some evidence to suggest that centers that conduct same-day consults may have a faster time until approval.10 There appeared to be a fair amount of consistency on how Ontario transplant programs evaluate living kidney donor candidates, which was evident when setting standards for the Canadian national kidney paired donation program.16 However, operational decisions are made by individual living donor programs, and there is currently no recommendation on the timeliness of the evaluation.9 Thus, we do expect variability in preemptive transplantation rates across transplant programs, much like variability in recipient referral rates observed across dialysis centers.17 Some of this variability may be due to donor evaluation protocols at each program and determining how protocols affect the timeliness of the evaluation should be a focus of future work.
We believe these novel observations should be the focus of quality improvement efforts.18 In the current study, we did not address the degree to which these dialysis starts could have been prevented, nor did we have information on reasons for the length of the evaluation for the donor or the intended recipient. Some of the delay in the donor candidate evaluation process may be because of the unpredictable nature of kidney failure. For example, it is possible that the recipient’s health suddenly deteriorated, placing the living donors’ evaluation on hold until the recipient was well enough after receiving dialysis to receive a kidney transplant. This may avoid unnecessary donor workup in case the recipient is no longer eligible for transplantation or avoid expiration of some donor’s test results until the recipient is eligible again. Conversely, deterioration of the recipient’s health may result in an expedited living donor evaluation to transplant the intended recipient before their health deteriorates further (ie, before dialysis initiation, before potential transplant ineligibility). Although the donor and recipient evaluations are mostly independent, there is some communication that attempts to optimize coordination, outcomes, resource utilization, and donor burden. Other reasons for delay may result when more time is needed to complete a thorough evaluation, including initial test results that required further investigation, clearance of the donor related to any preexisting comorbidity, or the requirement that some donor candidates change their lifestyle (eg, lose weight or reduce their smoking).19,20 Delays because of these reasons are appropriate and may be necessary to uphold the quality of the evaluation and the safety of donor candidate approval. However, in this study, the living donor evaluation was underway for almost 10 months before 50% of the recipients in this group started dialysis, a sufficient amount of time to complete an evaluation even in the presence of some delay. Moreover, delays may stem from the donor or the intended recipient as they come to terms with living donor kidney transplantation.21,22 Determining what factors are modifiable will be critical to be able to modify them and reduce the proportion of recipients starting dialysis.
This study also has other important limitations that should be addressed in future studies. First, the date of first contact and date of approval were obtained by proxy. Although our estimates of the total evaluation time (which includes all the time until nephrectomy) aligns with our clinical experience and is consistent with prior reports,23 the validity of this estimate needs to be substantiated by using more accurate (and agreed-upon) start dates. The date the living donor first contacted the transplant program was unavailable, but is now being actively collected by Ontario transplant programs. The date of approval is important because many factors can influence the time until donation even after the donor has been approved to donate. Also, because evaluation practices in Ontario may differ from those used in other regions, the time until approval may allow additional comparisons to be made, and multiple metrics may be more informative than single metrics in isolation. Second, only patients who received a living kidney donor transplant were included in this study. It remains to be established whether improvements in the time to evaluate donor candidates can prevent lost opportunity for living donor transplants (eg, due to competing events like intended recipient illness, death, or deceased donor kidney transplantation)24,25 or influences candidates who drop out during the evaluation process.18 Donor candidates who did not donate are not currently identifiable from administrative data sets alone. Further, many data on recipient referral dates were missing, and we did not have information on when the intended recipient was approved for transplantation. Finally, among recipients who had no relation to their donors, we were unable to disentangle the effects of nondirected anonymous donation versus kidney paired donation.26,27
In our exploratory analysis, several characteristics were associated with a greater likelihood of not realizing a potential preemptive living donor kidney transplant. Donors who were female, nonwhite, and lived in a low-income neighborhood were all less likely to donate preemptively. These characteristics are all difficult or impossible to modify, but understanding the mechanism may suggest areas where potential modifications may be possible. We did find dialysis before transplant was more likely if the recipient was referred with a lower eGFR, and if there was a longer delay between the donor’s evaluation start date and the date the transplant program subsequently received the referral to begin the intended recipient’s evaluation. These suggest earlier recipient referrals may prevent some recipients from starting dialysis. In Ontario, there is a guideline for intended recipients to undergo several tests, including cardiac assessment organized by their nephrologist before submitting a referral package to a transplant program for evaluation.28 Often, donor candidates contact transplant programs while pretransplant-referral testing for their recipient is underway, but the transplant programs usually do not advance the donor candidate evaluation until they receive a referral package for the intended recipient (as is the general approach in Ontario). From 1 perspective, it may not be worthwhile spending resources evaluating donors before their intended recipient is referred because many of these recipients may not be eligible for transplant or may never be referred, thereby wasting time and resources that could be spent on other donor evaluations. On the other hand, the potential implications of a late referral could at least partly be offset by a donor evaluation that is either quicker or starts before the recipient is referred. If the recipient is never referred or is not a transplant candidate, then this may result in some donor candidates pursuing nondirected donation instead. There is clearly a tradeoff here that should be studied, because this is a potentially modifiable area for quality improvement. In this study, we only reported data from 5 transplant programs in Ontario; our impression is these programs are similar to others throughout Canada, but we do not have data to corroborate this. We believe that this metric (the proportion of potential preemptive transplants that were unrealized) should be measured and reported by all programs nationally and internationally to facilitate comparisons and quality improvement efforts.
In conclusion, by linking donor evaluation times with recipient outcomes, this study raises the possibility of some modifiable adverse impact of a prolonged living donor evaluation process. These effects might not only be restricted to recipient health outcomes but also may extend to the living donor’s experience and to healthcare costs attributable to starting and/or maintaining dialysis until transplantation.29,30 These findings inform future research and quality improvement activities that aim to help patients with kidney failure improve their chances of realizing a preemptive kidney transplant from a living donor.
ACKNOWLEDGMENTS
The authors thank Nancy Moore, Patti Lake, and Kathleen Murphy, patient partners whose experiences informed this research.
Footnotes
Funding for this analysis was provided by Ontario’s Trillium Gift of Life Network. Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease (CAN-SOLVE CKD) is a patient-orientated research network to transform the care of people affected by kidney disease. It is led by Drs. Adeera Levin and Braden Manns. Patient partnerships in this project were supported by CAN-SOLVE. S.H. is supported by the Canadian Institutes of Health Research Frederick Banting and Charles Best Canada Doctoral Scholarship (reference number: GSD 140313). Dr. Ngan N. Lam was supported by a Kidney Research Scientist Core Education and National Training Program (KRESCENT) New Investigator Award. A.G. is supported by the Dr. Adam Linton Chair in Kidney Health Analytics, and a Canadian Institutes of Health Research Clinician Investigator Award.
A.G. received partnership funding from Astellas for a research grant funded by the Canadian Institutes of Health Research. The other authors declare no conflicts of interest.
The Institute for Clinical Evaluative Sciences (ICES) is a nonprofit research corporation funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The MOHLTC provided large administrative data sets to which the Trillium Gift of Life Network data were linked. Parts of this material are based on data and information compiled and provided by CIHI. The study was conducted at the ICES Western facility, which receives financial support from the Academic Medical Organization of Southwestern Ontario, the Schulich School of Medicine and Dentistry at Western University, the Lawson Health Research Institute and multiple clinical Departments. The study was conducted through the ICES Kidney, Dialysis and Transplantation (KDT) Research Program, which receives programmatic support from the Canadian Institutes of Health Research.
The study design and conduct, opinions, results and conclusions reported in this paper are those of the authors and are independent of the funding sources. No endorsement by ICES, CIHI, or the MOHLTC is intended or should be inferred. Amit Garg was supported by the Dr. Adam Linton Chair in Kidney Health Analytics and a Canadian Institutes of Health Research Clinician Investigator Salary Award. Graduate students who worked on this project were provided space in the Lilibeth Caberto Kidney Clinical Research Unit.
S.H., E.M., S.N.D., S.M., C.G.-O., N.N.L., K.L.L., C.D., K.L., M.A.B., S.S., A.X.G. participated in research design. S.H., E.M., S.N.D., S.M., C.G.-O., N.N.L., K.L.L., C.D., K.L., M.A.B., S.S., A.X.G. participated in the writing of the article. S.H., E.M., S.N.D., S.M., C.G.-O., S.S., A.X.G. participated in the performance of the research. S.H., E.M., S.N.D., C.G.-O., S.S., A.X.G. participated in data analysis.
Correspondence: Amit X. Garg, MD, PhD, Institute for Clinical Evaluative Sciences Western facility (ICES Western) Victoria Hospital, Room ELL-215, 800 Commissioners Rd, Victoria Hospital, London, Ontario, Canada N6A 5W9. (amit.garg@lhsc.on.ca).
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
This analysis using healthcare databases from 5 transplant centers in Canada confirms the difficulties in performing preemptive kidney transplants even with expedited living donor evaluation. Yet, not all the factors accounting for this observation can be promptly optimized. Supplemental digital content is available in the text.
REFERENCES
- 1.Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis Transplantation 2002. 741377–1381 [DOI] [PubMed] [Google Scholar]
- 2.Meier-Kriesche HU, Port FK, Ojo AO. Effect of waiting time on renal transplant outcome Kidney Int 2000. 581311–1317 [DOI] [PubMed] [Google Scholar]
- 3.Grams ME, Chen BP, Coresh J. Preemptive deceased donor kidney transplantation: considerations of equity and utility Clin J Am Soc Nephrol 2013. 8575–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grams ME, Massie AB, Coresh J. Trends in the timing of pre-emptive kidney transplantation J Am Soc Nephrol 2011. 221615–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garonzik-Wang JM, Berger JC, Ros RL. Live donor champion: finding live kidney donors by separating the advocate from the patient Transplantation 2012. 931147–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore DR, Serur D, Rudow DL. Living donor kidney transplantation: improving efficiencies in live kidney donor evaluation—recommendations from a consensus conference Clin J Am Soc Nephrol 2015. 101678–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaPointe Rudow D, Hays R, Baliga P. Consensus conference on best practices in live kidney donation: recommendations to optimize education, access, and care Am J Transplant 2015. 15914–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferriman A. Becoming a live kidney donor BMJ 2008. 3361374–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lentine KL, Kasiske BL, Levey AS. KDIGO clinical practice guideline on the evaluation and care of living kidney donors Transplantation 2017. 101S7–S105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habbous S, Arnold J, Begen MA, et al. Duration of Living Kidney Transplant Donor Evaluations: Findings From 2 Multi-center Cohort Studies. Am J Kidney Dis. 2018; DOI: 10.1053/j.ajkd.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 11.Fleet JL, Dixon SN, Shariff SZ. Detecting chronic kidney disease in population-based administrative databases using an algorithm of hospital encounter and physician claim codes. BMC Nephrol. 2013;14:81. doi: 10.1186/1471-2369-14-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lujan PR, Chiurchiu C, Douthat W. CKD-EPI Instead of MDRD for Candidates to Kidney Donation Transplantation 2012. 94637–641 [DOI] [PubMed] [Google Scholar]
- 13.Wodchis WP, Bushmeneva K, Nikinovic M, et al. Guidelines on Person-Level Costing Using Administrative Databases in Ontario. Vol 1 2013. [Google Scholar]
- 14.Living Donor Kidney Transplantation 2020: A UK Strategy. NHS. odt.nhs.uk/pdf/ldkt_2020_strategy.pdf. Accessed November 30, 2017. [Google Scholar]
- 15.Zou G. A modified poisson regression approach to prospective studies with binary data Am J Epidemiol 2004. 159702–706 [DOI] [PubMed] [Google Scholar]
- 16.Richardson R, Connelly M, Dipchand C. Kidney paired donation protocol for participating donors 2014 Transplantation 2015. 99S1–S88 [DOI] [PubMed] [Google Scholar]
- 17.Patzer RE, Plantinga LC, Paul S. Variation in dialysis facility referral for kidney transplantation among patients with end-stage renal disease in Georgia JAMA 2015. 314582–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham JM, Courtney AE. The adoption of a one-day donor assessment model in a living kidney donor transplant program: a quality improvement project Am J Kidney Dis 2018. 71209–215 [DOI] [PubMed] [Google Scholar]
- 19.Underwood PW, Sheetz KH, Cron DC. Cigarette smoking in living kidney donors: donor and recipient outcomes Clin Transplant 2014. 28419–422 [DOI] [PubMed] [Google Scholar]
- 20.Sachdeva M, Sunday S, Israel E. Obesity as a barrier to living kidney donation: a center-based analysis Clin Transplant 2013. 27882–887 [DOI] [PubMed] [Google Scholar]
- 21.Boulware LE, Hill-Briggs F, Kraus ES. Identifying and addressing barriers to African American and non-African American families’ discussions about preemptive living related kidney transplantation Prog Transplant 2011. 2197–104 [DOI] [PubMed] [Google Scholar]
- 22.Boulware LE, Hill-Briggs F, Kraus ES. Effectiveness of educational and social worker interventions to activate patients’ discussion and pursuit of preemptive living donor kidney transplantation: a randomized controlled trial Am J Kidney Dis 2013. 61476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanner MA, Lagging E, Tibell A. The kidney recipient’s path to transplantation: a comparison between living and deceased kidney donor recipients in Stockholm, Sweden Nephrol Dial Transplant 2011. 261053–1057 [DOI] [PubMed] [Google Scholar]
- 24.Connaughton DM, Harmon G, Cooney A. The Irish living kidney donor program—why potential donors do not proceed to live kidney donation? Clin Transplant 2016. 3017–25 [DOI] [PubMed] [Google Scholar]
- 25.Norman SP, Song PX, Hu Y. Transition from donor candidates to live kidney donors: the impact of race and undiagnosed medical disease states Clin Transplant 2011. 25136–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dew MA, Jacobs CL, Jowsey SG. Guidelines for the psychosocial evaluation of living unrelated kidney donors in the United States Am J Transplant 2007. 71047–1054 [DOI] [PubMed] [Google Scholar]
- 27.Cantwell L, Woodroffe C, Holdsworth R. Four years of experience with the Australian kidney paired donation programme Nephrology (Carlton 2015. 20124–131 [DOI] [PubMed] [Google Scholar]
- 28.Trillium Gift of Life Network. Kidney Transplant Referral Form. https//www.giftoflife.on.ca/resources/pdf/transplant/Ontario_Kidney_Referral_Form_Final_2017-04_EN.pdf; Accessed Sep 19, 2017. [Google Scholar]
- 29.Agerskov H, Ludvigsen MS, Bistrup C. Living kidney donors’ experiences while undergoing evaluation for donation: a qualitative study J Clin Nurs 2015. 242258–2267 [DOI] [PubMed] [Google Scholar]
- 30.Barnieh L, Yilmaz S, McLaughlin K. The cost of kidney transplant over time Prog Transplant 2014. 24257–262 [DOI] [PubMed] [Google Scholar]


