Abstract
Objective
To quantify the implementation of the third Joint British Societies’ Consensus Recommendations for the Prevention of Cardiovascular Disease (JBS3) after coronary event.
Methods
Using a cross-sectional survey design, patients were consecutively identified in 36 specialist and district general hospitals between 6 months and 2 years, after acute coronary syndrome or revascularisation procedure and invited to a research interview. Outcomes included JBS3 lifestyle, risk factor and therapeutic management goals. Data were collected using standardised methods and instruments by trained study nurses. Blood was analysed in a central laboratory and a glucose tolerance test was performed.
Results
3926 eligible patients were invited to participate and 1177 (23.3% women) were interviewed (30% response). 12.5% were from black and minority ethnic groups. 45% were persistent smokers, 36% obese, 52.9% centrally obese, 52% inactive; 30% had a blood pressure >140/90 mm Hg, 54% non-high-density lipoprotein ≥2.5 mmol/L and 44.3% had new dysglycaemia. Prescribing was highest for antiplatelets (94%) and statins (85%). 81% were advised to attend cardiac rehabilitation (86% <60 years vs 79% ≥60 years; 82% men vs 77% women; 93% coronary artery bypass grafting vs 59% unstable angina), 85% attended if advised; 69% attended overall. Attenders were significantly younger (p=0.03) and women were less likely to attend (p=0.03).
Conclusions
Patients with coronary heart disease (CHD) are not being adequately managed after event with preventive measures. They require a structured preventive cardiology programme addressing lifestyle, risk factor management and adherence to cardioprotective medications to achieve the standards set by the British Association for Cardiovascular Prevention and Rehabilitation and JBS3 guidelines.
Keywords: cardiac rehabilitation, hypertension, smoking, epidemiology, delivery of care
Key questions.
What is already known about this subject?
There is strong scientific evidence that lifestyle, risk factor and therapeutic management of patients with CHD will reduce their risk of subsequent events and increase survival. The Joint British Societies’ Consensus Recommendations for the Prevention of Cardiovascular Disease guidelines summarise this scientific evidence for secondary prevention and the British Association for Cardiovascular Prevention and Rehabilitation advocate standards for cardiovascular disease prevention and rehabilitation.
What does this study add?
ASPIRE-3-PREVENT provides a contemporary picture of the implementation of the guidelines and standards in everyday clinical practice across the UK and quantifies the scope for improvement.
How might this impact on clinical practice?
These results will inform commissioners of services about the need to improve delivery of secondary preventive care and how this can be achieved through a modern preventive cardiology programme.
Introduction
ASPIRE-3-PREVENT (A-3-P)1 2 was conducted to evaluate preventive care for patients with coronary heart disease (CHD) in everyday clinical practice. Goals for care were outlined, most recently, in the Joint British Societies’ Consensus Recommendations for the Prevention of Cardiovascular Disease (JBS3)3 and aim to reduce morbidity, improve quality of life and increase life expectancy. A-3-P was conducted as part of the fifth EUROASPIRE survey, which investigated preventive care in patients with CHD from 27 European countries. The European Society of Cardiology (ESC) EUROASPIRE cross-sectional surveys4–8 have repeatedly evaluated implementation of the European prevention guidelines since 1995.
There is substantial evidence that intervening to modify adverse lifestyle behaviours and manage medical risk factors can reduce the risk of future cardiovascular events and improve survival in patients with CHD.3 9–11 Opportunities for delivering quality care for this patient group are offered by comprehensive cardiovascular prevention and rehabilitation programmes. In the third edition of their standards and core components,12 the British Association for Cardiovascular Prevention and Rehabilitation (BACPR) place emphasis on the need to underpin cardiovascular disease (CVD) risk reduction with patient-centred, behavioural and goal-oriented approaches to address risk factor and therapeutic management with timely intervention from a clinically led multidisciplinary team. Audit of outcomes is through the National Audit for Cardiac Rehabilitation’s (NACR)13 latest report 2018.
The specific objectives of this survey were:
To determine adherence to JBS3 guidelines in everyday clinical practice for patients with CHD.
To evaluate diagnostic and therapeutic strategies for dysglycaemia.
To make recommendations to the BACPR, British Cardiovascular Society and Public Health England on ways to improve preventive cardiology care.
This paper describes the principal results of A-3-P in relation to achievement of lifestyle, risk factor and therapeutic targets as defined in the JBS3 guidelines.
Methods
A-3-P was registered as a cross-sectional survey with the National Institute for Health Research Clinical Research Network (CRN) Portfolio on 23 August 2016 and made visible to all CRNs in England wishing to participate. It aimed to secure representation from hospital centres across five English National Health Service regions and in CRNs in Wales, Northern Ireland and Scotland. Thirty-six specialist cardiac centres and district general hospitals were recruited in this convenience sample.
Fieldwork for the survey was led by CRN and hospital Research and Development (R&D) department nurses. Hospitals were asked to create lists of retrospectively identified eligible patients from their clinical coding systems and to recruit consecutively according to admission date for the recruiting event. Eligibility was defined as male and female patients between the ages of 18 and 79 years at the time of diagnosis with new or recurrent ST-elevation myocardial infarction (STEMI), non-STEMI (NSTEMI) or acute ischaemia and/or elective or emergency percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). Patients were entered onto an electronic log and invited to participate, where reason for non-participation was also recorded. Patients were invited to attend an interview and examination with the centrally trained nurses who obtained written, informed consent, collected data using standardised instruments and methods (see box 1), and drew blood, processed and stored it prior to it being sent to the central laboratory in Finland for analysis. Outcome measures can be seen in box 1. Data were entered and managed on the anonymised ESC EurObservational Research Programme electronic online database.
Box 1. Outcome measures and research instruments for the ASPIRE-3-PREVENT (A-3-P) survey.
Current smoking validated with a breath carbon monoxide (CO) test ≥10 ppm (Micro+Smokerlyzer, Bedfont Scientific, Kent, UK).
Persistent smoking: smoking at recruiting event and still smoking at interview.
Overweight26 27: body mass index (BMI) ≥25 kg/m2 (non-Asian); BMI ≥23 kg/m2 (Asian) (height and weight measured in light indoor clothes without shoes with SECA measuring stick model 220 and SECA scale model 701).
Obesity26 27: BMI ≥30 kg/m2 (non-Asian); BMI ≥25 kg/m2 (Asian).
Central overweight26 27: waist circumference (WC) ≥94 cm (non-Asian men); WC ≥90 cm (Asian men); WC ≥80 cm (all women) (WC measured with metal tape measures mid-way between the lower rib margin and the iliac crest).
Central obesity26 27: WC ≥102 cm (non-Asian men); WC ≥90 cm (Asian men); WC ≥88 cm (non-Asian women); WC ≥80 cm (Asian women).
Not performing moderate-intensity physical activity at least 30 min/5 times per week (Godin Leisure-Time Exercise Questionnaire28).
Elevated blood pressure: ≥140 mm Hg and/or ≥90 mm Hg (blood pressure measured twice on the right upper arm in a sitting position after rest for at least 5 min using Omron M6 HEM 7211-E automatic digital sphygmomanometers).
Non-high-density lipoprotein (HDL) cholesterol: ≥2.5 mmol/L (analysed in central laboratory in Finland).
Low-density lipoprotein cholesterol (LDL-C) ≥1.8 mmol/L.
HbA1c ≥7% (≥53 mmol/mol) in patients with type 2 diabetes mellitus.29
New diabetes mellitus (DM); impaired fasting glycaemia (IFG); impaired glucose tolerance (IGT) from oral glucose tolerance test (OGTT) (see box 2 30) in patients without self-reported diabetes and with fasting blood glucose (FBG) <11.1 mmol/L measured with HemoCue near-patient testing monitor.
Cardiovascular drug therapies and medication adherence.31
Hospital Anxiety and Depression Scale (HADS32), HeartQoL,33 EuroQoL (EQ-5D34).
Box 2. Diagnostic criteria for diabetes and dysglycaemia.
Diabetes ☐ No ☐ Yes
Fasting plasma glucose ≥7.0 mmol/L (126 mg/dL)
or
2-hour plasma glucose* ≥11.1 mmol/L (200 mg/dL)
Impaired glucose tolerance (IGT) ☐ No ☐ Yes
Fasting plasma glucose <7.0 mmol/L (<126 mg/dL)
and 2-hour glucose* ≥7.8 and <11.1 mmol/L (140 and <200 mg/dL)
Impaired fasting glucose (IFG) ☐ No ☐ Yes
Fasting plasma glucose ≥6.1 and <6.9 mmol/L (110 and <125 mg/dL)
and 2-hour plasma glucose* <7.8 mmol/L (140 mg/dL)
*Venous plasma glucose 2 hours after the ingestion of 75 g dissolved glucose in 200 mL water.
A sample size of 400 patients attending for interview was sufficient with a precision of at least 5% and a confidence of 95% to estimate prevalence and allow power to stratify by age and gender and to estimate prevalence of single risk factors with 95% CIs of width 10%. All statistical analyses were undertaken using STATA V.11 (StataCorp). The majority of analyses were descriptive aiming to summarise characteristics and outcomes. The exact binomial method was used to calculate CIs for the percentage values. The McNemar test was used to statistically compare the results from hospital discharge and the time of interview for the proportion of patients on the most popular medications.
The cardiac rehabilitation (CR) analyses focused on differences in patients who were advised and not advised and who attended and did not attend a programme. Attendance was defined as one or more of the programme sessions. The groups were compared on key demographic variables and comparisons between the groups were made for lifestyle, risk factors and prescribing. All outcomes were binary and comparisons between groups were made using logistic regression. Adjustments were made if results varied in terms of key demographics.
Results
Figure 1 shows the flow of patients through the study. Non-attendance at interview was driven principally by a failure to respond to the invitation (71% of DNA). As expected in a population of patients with CHD under the age of 80 years, the majority were male (77%). The mean age of the participants was 65.7 years and most were of white ethnicity. Nearly two-thirds of patients identified for the study had either acute STEMI or NSTEMI.
Figure 1.

Flow chart of patients through all phases of data collection in ASPIRE-3-PREVENT. CABG, coronary artery bypass grafting; NSTEMI, non-STEMI; OGTT, oral glucose tolerance test; PTCA, percutaneous transluminal coronary angioplasty; STEMI, ST-elevation myocardial infarction.
Table 1 shows lifestyle habits, medical risk factors, prescribing and psychosocial status in all patients and also according to their attendance at a CR programme. If adhering to national standards (BACPR), CR should have been offered to all patients who have suffered a cardiac event and/or who have been revascularised, that is, all those who were recruited to this survey. In fact, a large proportion of interviewed patients (80.8%) were advised to attend a programme and, of these, 84.8% attended. Out of all 1177 patients, 68.5% attended a programme. Patients most likely to be advised were those who had undergone CABG. Least advised were patients with unstable angina, older patients, women, those not achieving the physical activity target and those with self-reported diabetes. Interestingly, less advised groups were just as likely to attend if so advised (see table 2). There were significant differences between patients who attended and did not attend in relation to mean age (66.6 years in non-attenders compared with 65.4 years in attenders, p=0.03), gender and revascularisation procedure (see table 2) with women and older patients being less likely to attend and those revascularised with CABG and acute myocardial infarction being more likely to attend.
Table 1.
Lifestyle and risk factor outcomes in patients following an acute cardiac event comparing those who attended and those who did not attend cardiac rehabilitation
| Attended cardiac rehabilitation n=800 |
Did not attend cardiac rehabilitation n=368 |
All patients** n=1177 |
|||||||
| M 629 |
F 171 |
All 800 |
M 268 |
F 100 |
All 368 |
M 903 |
F 274 |
All 1177 |
|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Current smoker | 48 (7.6) | 7 (4.1) | 55 (6.9) | 32 (11.9) | 11 (11.0) | 43 (11.7)* | 83 (9.2) | 19 (6.9) | 102 (8.7) |
| Smoker at index event | 107 (17.0) | 32 (18.7) | 139 (17.4) | 53 (19.8) | 20 (20.0) | 73 (19.8) | 163 (18.1) | 53 (19.3) | 216 (18.4) |
| Persistent smoker‡ | 46 (43.0) | 7 (21.9) | 53 (38.1) | 30 (56.6) | 11 (55.0) | 41 (56.2) | 79 (48.5) | 19 (35.9) | 98 (45.4) |
| Not achieving physical activity guidelines | 278 (45.1) | 103 (62.1) | 381 (48.7) | 149 (58.2) | 64 (64.7) | 213 (60.0)** | 428 (48.9) | 169 (63.1)*** | 597 (52.2) |
| Overweight | 488 (77.7) | 126 (74.1) | 614 (76.9) | 213 (79.8) | 83 (83.0) | 296 (80.7) | 705 (78.4) | 212 (77.7) | 917 (78.2) |
| Obese | 221 (35.2) | 62 (36.5) | 283 (35.5) | 95 (35.6) | 43 (43.0) | 138 (37.6) | 316 (35.2) | 106 (38.8) | 422 (36.0) |
| Centrally overweight | 446 (71.4) | 146 (85.9) | 592 (74.5) | 192 (72.5) | 85 (87.6) | 277 (76.5) | 671 (71.7) | 234 (86.7)*** | 875 (75.2) |
| Centrally obese | 296 (47.4) | 105 (61.8) | 401 (50.4) | 135 (50.9) | 76 (78.4) | 211 (58.3) | 432 (48.3) | 184 (68.2)*** | 616 (52.9) |
| BP ≥140/90 mm Hg | 181 (28.8) | 46 (26.9) | 227 (28.4) | 92 (34.3) | 30 (30.0) | 122 (33.2) | 275 (30.5) | 79 (28.8) | 354 (30.1) |
| BP ≥130/80 mm Hg | 363 (57.7) | 89 (52.1) | 452 (56.5) | 158 (59.0) | 58 (58.0) | 216 (58.7) | 525 (58.2) | 150 (54.7) | 675 (57.4) |
| LDL-C ≥1.8 mmol/L | 334 (56.4) | 110 (66.7) | 444 (58.7) | 163 (69.9) | 66 (74.2) | 229 (66.6)† | 500 (58.7) | 177 (68.9)** | 677 (61.0) |
| Non-HDL-C ≥2.5 mmol/L | 292 (48.6) | 103 (62.1) | 395 (51.5) | 143 (55.2) | 65 (69.2) | 208 (58.9)† | 439 (50.7) | 169 *** (64.3) | 609 (53.9) |
| Self-reported diabetes | 127 (20.3) | 34 (20.1) | 161 (20.3) | 79 (29.5) | 23 (23.0) | 102 (27.7) | 209 (23.3) | 58 (21.3) | 267 (22.8) |
| HbA1c ≥7% (≥53 mmol/mol in self-reported diabetes) | 47 (7.9) | 18 (10.8) | 65 (8.5) | 43 (16.7) | 11 (11.7) | 54 (15.4) | 92 (10.7) | 29 (11.0) | 121 0.8) |
| OGTT: IGT | 122 (19.7) | 39 (23.4) | 161 (20.5) | 31 (11.7) | 20 (20.2) | 51 (14.0) | 153 (17.2) | 59 (21.9) | 212 (18.3) |
| IFG | 79 (12.8) | 18 (10.8) | 97 (12.3) | 25 (9.4) | 8 (8.1) | 33 (9.0) | 104 (11.7) | 27 (10.0) | 131 (11.3) |
| New diabetes | 94 (15.2) | 21 (12.6) | 115 (14.6) | 44 16.5) | 11 (11.1) | 55 (15.1) | 138 (15.5) | 32 (11.9) | 170 (14.7) |
| Antiplatelet | 603 (95.9) | 165 (96.5) | 768 (96.0) | 243 (91.0) | 88 (88.0) | 331 (90.2)* | 849 (94.4) | 256 (93.4) | 1105 (94.2) |
| Beta blocker | 503 (80.1) | 129 (75.4) | 632 (79.1) | 199 (75.1) | 68 (68.0) | 267 (73.2) | 704 (78.6) | 198 (72.3) | 902 (77.1) |
| ACE | 402 (63.9) | 87 (50.9) | 489 (61.1) | 160 (59.9) | 53 (53.0) | 213 (58.0) | 565 (62.9) | 142 (51.8) | 707 (60.3) |
| ARB | 98 (15.6) | 41 (24.0) | 139 (17.4) | 36 (13.5) | 18 (18.0) | 54 (14.8) | 134 (14.9) | 60 (21.9) | 194 (16.6) |
| Other BP-lowering drugs | 166 (26.4) | 51 (29.8) | 217 (27.1) | 93 (34.8) | 34 (34.0) | 127 (34.6) | 261 (29.0) | 86 (31.4) | 347 (29.6) |
| Statins | 545 (86.8) | 143 (83.0) | 687 (86.0) | 225 (84.6) | 75 (75.0) | 300 (82.0) | 773 (86.2) | 220 (80.3) | 993 (84.8) |
| Other lipid-lowering drugs | 29 (4.6) | 9 (5.3) | 38 (4.8) | 16 (6.0) | 5 (5.0) | 21 (5.7) | 45 (5.0) | 14 (5.1) | 59 (5.0) |
| Drug adherence§ | |||||||||
| BP lowering | 584/588 (99.3) | 158/159 (99.4) | 742/747 (99.3) | 95/101 (94.1) | 30/31 (96.8) | 125/132 (94.7) | 818/835 (98.0) | 244/246 (99.2) | 1062/1081 (98.2) |
| Lipid lowering | 528/532 (99.3) | 149/150 (99.3) | 677/682 (99.3) | 91/93 (97.9) | 28/29 (96.6) | 119/122 (97.5) | 744/756 (98.4) | 232/234 (99.2) | 990/976 (98.6) |
| Glucose lowering | 169/178 (94.9) | 46/47 (97.9) | 215/225 (95.6) | 34/35 (97.1) | 10/11 (90.9) | 44/46 (95.7) | 261/273 (95.6) | 74/76 (97.4) | 335/349 (96.0) |
| HADS Anxiety ≥8 |
123 (19.8) | 54 (32.3) | 177 (22.4) | 51 (19.5) | 39 (39.8) | 90 (25.1) | 176 (19.8) | 94 (35.1) | 270 (23.4) |
| HADS Depression ≥8 |
75 (12.1) | 17 (10.2) | 92 (11.7) | 38 (14.6) | 24 (24.4) | 62 (17.3) | 113 (12.7) | 41 (15.3) | 154 (13.3) |
| EQ-VAS out of 100¶ | 80 (70, 90) | 80 (60, 90) | 80 (70, 90) | 80 (65, 90) | 75 (50, 90) | 76 (60, 90) | 80 (70, 90) | 75 (60, 90) | 80 (68, 90) |
*P=0.006; **p=0.003; ***p<0.001.
†P=0.02.
‡Persistent smoker: smoking at hospital admission and still smoking at interview.
§Medication adherence denominator included due to variation in drug treatment.
¶Median (IQR).
**No data available on CR attendance in 9 patients
ARB, angiotensin receptor blocker; BP, blood pressure; EQ-VAS, EuroQol Visual Analogue Scale; HADS, Hospital Anxiety and Depression Scale; HDL-C, high-density lipoprotein cholesterol; IFG, impaired fasting glycaemia; IGT, impaired glucose tolerance; LDL-C, low-density lipoprotein cholesterol; OGTT, oral glucose tolerance test.
Table 2.
Characteristics of patients advised to attend, participated if advised and who participated overall in a cardiac rehabilitation programme
| Patient group | Advised to participate | Participated if advised | Participated overall | |||
| n/N (%) | P value | n/N (%) | P value | n/N (%) | P value | |
| All patients | 944/1168 (80.8) | 800/944 (84.8) | 800/1168 (68.5) | |||
| Age <60 | 254/296 (85.8) | 0.01 | 213/254 (83.9) | 0.65 | 213/296 (72.0) | 0.14 |
| Age ≥60 | 690/872 (79.1) | 587/690 (85.1) | 587/872 (67.3) | |||
| Male | 735/897 (81.9) | 0.08 | 629/735 (85.6) | 0.18 | 629/897 (70.1) | 0.03 |
| Female | 209/271 (77.1) | 171/209 (81.8) | 171/271 (63.1) | |||
| AMI (STEMI) | 213/242 (88.0) | <0.001 | 180/213 (84.5) | 0.15 | 180/242 (74.4) | <0.001 |
| AMI (non-STEMI) | 428/514 (83.3) | 356/428 (83.2) | 356/514 (69.3) | |||
| CABG | 99/107 (92.5) | 87/99 (87.9) | 87/107 (81.3) | |||
| PTCA | 153/218 (70.2) | 128/153 (83.7) | 128/218 (58.7) | |||
| Unstable angina | 51/87 (58.6) | 49/51 (96.1) | 49/87 (56.3) | |||
AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; PTCA, percutaneous transluminal coronary angioplasty; STEMI, ST-elevation myocardial infarction.
Tobacco
Around one-fifth reported smoking at the time of their event (table 1) and nearly one-half of these were still smoking at the time of interview. There were no statistical differences between men and women with regard to smoking habit. Among current smokers, two-thirds reported an intention to quit within the next 6 months. There were no gender differences.
While the majority of current smokers or recent quitters reported being advised to quit since the recruiting cardiac event, only a minority were advised to use pharmacological support or referred for specialist help (see table 3). At the time of discharge from hospital, around one-fifth of current smokers and recent quitters were prescribed antismoking treatments (table 4). Around two-thirds of patients made a quit attempt although a relatively small proportion accessed specialist support or pharmacotherapies. Current smokers were significantly less likely to attend a CR programme.
Table 3.
Lifestyle habits and change attempts in patients participating in the A-3-P survey
| Men % | Women % | All % | |
| Tobacco | |||
| Referred to specialist clinic | 32.7 | 40.4 | 34.6 |
| Advised to stop | |||
| Use NRT | 34.4 | 50.0 (p=0.04) | 38.1 |
| Use bupropion | 2.6 | 2.0 | 2.5 |
| Use varenicline | 5.9 | 3.9 | 5.4 |
| Made quit attempt | 60.1 | 71.7 | 63 |
| Tried to reduce | 37.5 | 26.4 | 34.7 |
| Attended specialist clinic | 14.4 | 11.3 | 13.6 |
| Used NRT | 23.8 | 24.5 | 23.9 |
| Used bupropion | 0.0 | 1.9 | 0.5 |
| Used varenicline | 3.3 | 1.9 | 2.9 |
| Diet and weight | |||
| Dietary advice | 75.4 | 71.5 | 74.5 |
| Advised to lose weight | 63.7 | 59.4 | 62.7 |
| Steps taken—improve diet | |||
| Reduce salt | 67.2 | 60.5 (p=0.04) | 65.7 |
| Reduce fat | 69.2 | 69.5 | 69.2 |
| Change fat | 61.6 | 60.3 | 61.3 |
| Increase fruits and vegetables | 72.9 | 65.7 (p=0.02) | 71.2 |
| Eat more fish | 61.3 | 56.4 | 60.1 |
| Reduce sugar | 64.7 | 55.9 (p=0.009) | 62.6 |
| Reduce alcohol | 51.6 | 40.7 (p=0.002) | 49.1 |
| Eat more sterols | 29.4 | 17.1 (p<0.001) | 26.5 |
| Steps taken to lose weight—in overweight patients | 67.6 | 65.6 | 67.1 |
| Physical activity | |||
| Disability/infirmity | 38.1 | 51.1 (p<0.001) | 41.1 |
| Infirmity limits activity | 74.6 | 85 (p=0.01) | 77.6 |
| Professional advice to increase activity | 52.4 | 57.1 | 53.5 |
| Advised to increase everyday activities | 60.6 | 59.9 | 17.2 |
| Advised to join walking group | 16.8 | 18.3 | 68.8 |
| Any steps taken to increase activity | 70.7 | 71.2 | 70.8 |
A-3-P, ASPIRE-3-PREVENT; NRT, nicotine replacement therapy.
Table 4.
Prescription of tobacco cessation drugs at discharge and at interview
| Time point | Treatment | Males n (%) |
Females n (%) |
All n (%) |
| Discharge (from medical notes) | NRT | 27 (17.8) | 14 (28.6) | 41 (20.4) |
| Bupropion | 1 (0.7) | 1 (2) | 2 (1) | |
| Varenicline | 1 (0.7) | 1 (2) | 2 (1) | |
| Interview | NRT | 10 (6.1) | 1 (1.9) | 11 (5.1) |
| Bupropion | 0 (0) | 0 (0) | 0 (0) | |
| Varenicline | 0 (0) | 0 (0) | 0 (0) |
NRT, nicotine replacement therapy.
Overweight and obesity
A large majority of patients were above ideal body mass index (table 1). More than half were centrally obese with a significantly higher prevalence in women. There were no significant differences in prevalence according to attendance at CR. Two-thirds of overweight patients reported being advised to lose weight and attempted to do so.
Physical activity and exercise
Approximately one-half reported not achieving the physical activity recommendation (see table 1). However, 40%, mostly women, reported having a disability of which the majority said limited exercise capacity (see table 3). Around one-half claimed to take regular planned exercise (see figure 2).
Figure 2.
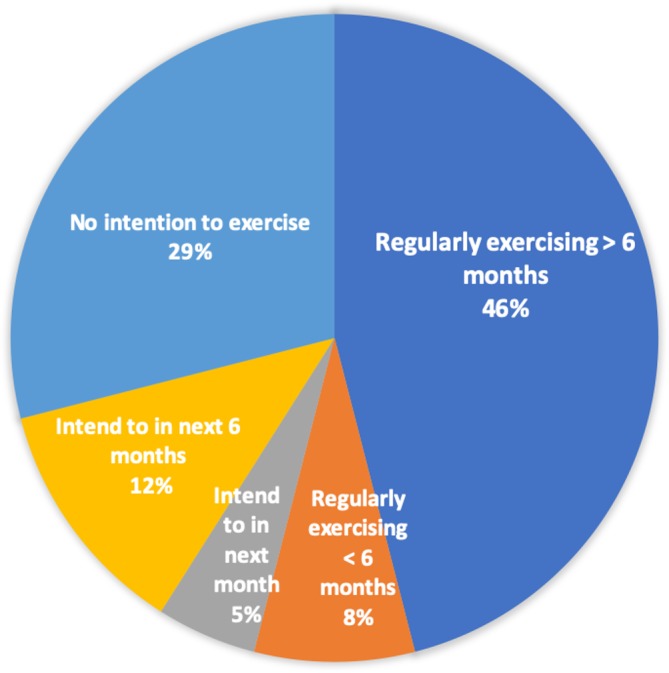
Self-reported regular planned exercise.
Biological risk factors
One-third had raised blood pressure (BP) according to the JBS3 goal (<140 and/or 90 mm Hg) (table 1). However, if more recent guidelines on BP targets from the American Heart Association/American College of Cardiology14 and the ESC/European Society of Hypertension15 are taken into account, this proportion is higher with nearly 60% of patients having a BP of ≥130 mm Hg and/or ≥80 mm Hg. More than one-half of patients are not reaching the JBS3 non-high-density lipoprotein cholesterol (HDL-C) goal of 2.5 mmol/L and these prevalences are significantly higher in women. While one-quarter of patients reported having been previously diagnosed with diabetes, an oral glucose tolerance test (OGTT) picked up new dysglycaemia in a further 44% of patients (see figure 3). 39.7% of patients with self-reported diabetes had a BP <130 mm Hg and/or <80 mm Hg and 50.9% an HbA1c of <7% (<53 mmol/mol). There were no gender differences in relation to BP control; however, low-density lipoprotein cholesterol (LDL-C) and non HDL-C were better managed in men (see table 5).
Figure 3.

Glucose metabolism in 1177 patients based on an oral glucose tolerance test in those without self-reported diabetes.
Table 5.
Therapeutic control of blood pressure, cholesterol and glucose by gender
| Male (n=903) | Female (n=274) | P value | |||
| n | % (95% CI) | n | % (95% CI) | ||
| Blood pressure* | 899 | 258 | |||
| BP at target 1† | 595 | 69.9 (66.7 to 73.0) | 187 | 72.4 (66.6 to 77.8) | 0.43 |
| BP at target 2‡ | 360 | 42.3 (39.0 to 45.7) | 119 | 46.1 (39.9 to 52.4) | 0.28 |
| Cholesterol§ | 759 | 219 | |||
| TC <5.0 mmol/L | 698 | 92.0 (89.8 to 93.8) | 184 | 84.0 (78.5 to 88.6) | <0.001 |
| TC <4.0 mmol/L | 523 | 68.9 (65.4 to 72.2) | 104 | 47.5 (40.7 to 54.3) | <0.001 |
| LDL <1.8 mmol/L¶ | 329 | 43.9 (40.3 to 47.6) | 75 | 34.7 (28.4 to 41.5) | 0.02 |
| LDL <2.0 mmol/L¶ | 441 | 58.9 (55.3 to 62.4) | 93 | 43.1 (36.4 to 49.9) | <0.001 |
| LDL <3.0 mmol/L¶ | 695 | 92.8 (90.7 to 94.5) | 193 | 89.4 (84.5 to 93.1) | 0.10 |
| Non-HDL <2.5 mmol/L | 400 | 52.7 (49.1 to 56.3) | 89 | 40.6 (34.1 to 47.4) | 0.002 |
| Glucose and BP control in diabetics** | 209 | 58 | |||
| Glucose <7 mmol/L†† | 61 | 30.4 (24.1 to 37.2) | 16 | 29.1 (17.6 to 42.9) | 0.86 |
| HbA1c <7% (<53 mmol/mol)‡‡ | 107 | 54.9 (47.6 to 62.0) | 29 | 50.0 (36.6 to 63.4) | 0.51 |
| HbA1c <6% (<42 mmol/mol)‡‡ | 29 | 14.9 (10.2 to 20.7) | 9 | 15.5 (7.3 to 27.4) | 0.90 |
| BP at target‡ | 81 | 38.8 (32.1 to 45.7) | 25 | 43.1 (30.2 to 56.8) | 0.55 |
p values in bold indicate those with statistical significance
*Figures for those on blood pressure-lowering medication.
†Defined as blood pressure <140/90 mm Hg.
‡Defined as blood pressure <130/80 mm Hg.
§Figures for those on lipid-lowering medication and with cholesterol measurements made.
¶Data available for 749 male and 216 female patients.
**Figures for self-reported diabetics only.
††Data available for 201 male patients, 55 female.
‡‡Data available for 195 male patients, 58 female.
BP, blood pressure; HDL, high-density lipoprotein; LDL, high-density lipoprotein; TC, total cholesterol.
Prescribing
Prescribing (table 1) was, on the whole, very good with 85% or more of patients reporting taking antiplatelets, statins and BP-lowering drugs. However, despite more than 90% of patients prescribed BP-lowering drugs, less than three-quarters were at the JBS3 goal. This proportion was much lower (43.2%) if a target of 130/80 mm Hg is used, which is in keeping with recent guidelines.14 15 There is a similar picture for lipid control with less than half at goal despite 83% of patients being prescribed lipid-lowering drugs. In patients with self-reported diabetes at interview, glycaemic control was not at target in one-third of patients. Statins were less frequently prescribed in women (86.2% men, 80.3% women, p=0.02).
Discussion
Our A-3-P survey investigated the implementation of CVD prevention guidelines in the UK and continues to highlight the enormous potential to reduce the risk of recurrent disease and associated disabilities and also to improve survival. In this discussion, we focus on achievement of a healthy lifestyle, treating risk factors—BP, lipids and diabetes—to targets with cardioprotective medications, and the role of prevention and rehabilitation programmes.
Persistent smoking in those who were smoking at the time of their event is high at 45%. There is a lack of support in hospital and after discharge to help patients quit smoking or to maintain abstinence due to underprescribing of drugs and provision of counselling to support smoking cessation. Many of these persistent smokers intend to quit so there is the opportunity for a behavioural intervention supported by pharmacotherapies with proven efficacy,16 17 effectiveness18 and safety19 in this patient population. Some patients were prescribed nicotine replacement therapy at the time of discharge from hospital but what is required is a systematic evidence-based approach which starts on the first day of hospital admission and continues after discharge in the CR programme and in general practice. The recent announcement by the Department of Health to support smokers admitted to hospital to quit will improve smoking cessation rates including for those with coronary disease.20
Encouragingly, more than half of the interviewed patients reported taking regular exercise and one-fifth report that they intended to start doing so within the next 6 months. Taking regular exercise was more common in men; however, the older age of female patients and their associated disabilities should be taken into account. Interestingly, patients not achieving the physical activity target were less likely to be advised to attend CR, and were also less likely to attend a programme, and yet these patients would derive even greater benefits from supervised exercise sessions.
There is a high prevalence of obesity and central obesity in this population, particularly in women. About two-thirds were advised to lose weight, which means that such advice was not given to a significant minority, and a similar proportion reported they were attempting to do so. One caveat is that for those who successfully quit smoking, weight gain is common21 and this may be a confounding factor in this overall picture.
The high prevalence of central obesity is a major factor in the increasing prevalence of diabetes in patients with CHD. Diabetes is commonly associated with coronary disease and, as the results of the OGTT demonstrate there is a considerable overlap with two-thirds of patients with CHD exhibiting some form of dysglycaemia. What is of great concern is that two-thirds of these patients with dysglycaemia had not been identified at the time of their event or subsequently. Diabetes is a major risk factor for coronary disease and patients with both CHD and diabetes are at much greater risk of a further major adverse cardiovascular event. So why are we not screening the population with CHD without self-reported diabetes with an OGTT, the only test which most reliably identifies all patients with diabetes and with impaired glucose tolerance?22 The identification of dysglycaemia will be a stimulus to greater diligence in monitoring for the development of diabetes, and in those with newly diagnosed diabetes, appropriate monitoring for the development of microvascular disease and other complications can be put in place.
Although cardioprotective medications are prescribed in all patients, the extent to which risk factors are controlled to national targets remains disappointing. Just over two-thirds of patients are achieving the conservative BP target of <140 mm Hg and/or 90 mm Hg, but only 42% are achieving the target of <130 mm Hg and/or 80 mm Hg. The non-HDL-C target of <2.5 mmol/L is achieved by just over half of all patients and only 44% are below the more stringent LDL-C target of <1.8 mmol/L. In patients with self-reported diabetes an HbA1c <7.0% is achieved in just over half of all patients. In these patients with diabetes the lower BP target of <130 mm Hg and/or 80 mm Hg is only achieved by just over a third.
After experiencing an acute cardiac event and/or undergoing a revascularisation procedure, patients require an interdisciplinary CR programme which provides expert support to help patients and families change adverse health behaviours, to monitor and manage medical risk factors to targets and promote adherence to prescribed medications over the long term. Such a programme should be an integral part of their care with patients moving seamlessly from acute care to longer term preventive care to further reduce their risk of events, the need for further revascularisation and to improve survival. In this survey, 68% participated in some form of CR programme by comparison with 47% reported by NACR, and therefore our patient sample is skewed towards those undertaking rehabilitation. Participation was higher for younger patients, men and those who had a CABG. Although these patients all had coronary atherosclerosis there was a large differential in participation rates from 81% for CABG to 56% for those presenting with acute myocardial ischaemia. Older patients and women were less likely to be advised to attend a programme and the same was true for patients with acute myocardial ischaemia. There should be no difference in participation rates by diagnostic category or gender given that they all have the same underlying disease.
A recent systematic review and meta-analysis of trials since 2010 of prevention and rehabilitation programmes showed no overall benefit in terms of all-cause mortality, but significant reductions in cardiovascular mortality, including stroke.23 However, comprehensive programmes managing six or more risk factors did reduce all-cause mortality, compared with programmes managing less than six risk factors. Programmes that took responsibility for prescribing, uptitrating and monitoring adherence to cardioprotective medications also reduced all-cause mortality compared with programmes that devolved that responsibility to other healthcare professionals. These results support the view that prevention and rehabilitation programmes should be truly comprehensive, addressing lifestyle, risk factor management and taking responsibility within the service provided to manage all cardioprotective medications.24 25
Exercise-based CR is not truly comprehensive based on the results of this survey. Achievement of physical activity guidelines is better in those attending CR but there is no impact on persistent smoking, the prevalence of obesity and central obesity or control of BP to defined targets. Achievement of the LDL-C goal is significantly better in those attending CR but even so more than half of these patients are not achieving this target. Diabetes control is no better in those attending CR and new diabetes was missed just as frequently in those attending CR compared with those who did not. Cardioprotective medications were prescribed as commonly in those attending and not attending CR apart from antiplatelet medications which were significantly higher in those attending CR. Anxiety and depression outcomes were no different and health-related quality of life was the same. To reduce the risk of a further event and improve life expectancy, it is essential to address all aspects of lifestyle, manage the biological risk factors to targets and ensure adherence with optimally prescribed cardioprotective medications.
The availability and quality of these programmes should be ensured through appropriate funding and support from the Department of Health and auditing their adherence to all BACPR exemplary standards and core components. The ongoing National Audit of Cardiac Rehabilitation does not reflect all these standards and in particular there is no adequate audit of risk factors (BP, lipids and diabetes) or cardioprotective medications. NACR should consider expanding its audit function to embrace CVD as a whole and all important aspects of secondary prevention to become the National Audit for Cardiovascular Disease Prevention and Rehabilitation. The current NACR certification programme is also not comprehensive and BACPR and NACR should work together to develop performance levels which reflect the overall BACPR standards as a basis for certification.
Strengths
The main strength of A-3-P is the real-world national setting of this survey, together with the standardised methodology used to interview and examine patients using the same instruments (measuring stick, scales, tape measure and BP machine), the HemoCue point-of-care device for glucose measurement and a central laboratory for a full lipid profile, glucose, HbA1c and creatinine. All patients without self-reported diabetes were systematically investigated with an OGTT and therefore the metabolic status of all patients with CHD is described. The generic names and total daily doses of all prescribed drugs at hospital discharge and again at follow-up are recorded together with a measure of adherence.
Limitations
This survey of patients with CHD is based on a convenience sample of specialist cardiac centres and district general hospitals across the UK, with most centres located in England, followed by Scotland and fewer in Wales and Northern Ireland, which may not be representative of all hospitals. A systematic retrospective sample of consecutive patients from hospital databases was identified, but the patient response rate to interview and examination was very low, a source of potential bias. The high proportion of patients attending CR, 69% in our survey compared with 47% in the NACR database, indicates a bias towards healthier patients attending rehabilitation and therefore our results overestimate the health status of all patients with CHD and their quality of care in terms of risk factor control and drug prescribing. A truly representative patient sample is likely to include more higher risk patients with less healthier lifestyles and even poorer standards of secondary preventive care. Self-reported lifestyle has limitations but smoking habit was validated by breath carbon monoxide, and validated tools were used to measure physical activity and exercise. Diet could not be assessed using validated methods as these are too complex and time consuming and beyond the capacity of this survey.
Conclusion
The overall standard of preventive care remains suboptimal in the UK for patients with CHD. Although over two-thirds accessed a CR programme, inequalities still exist with less female, older adults and some diagnostic groups not attending. Despite CR attendance, lifestyle and risk factor management for several factors remains inadequate among attenders, reflecting the content and resources for these programmes. Patients require a comprehensive preventive cardiology programme with interdisciplinary expertise to address lifestyle, risk factor management and adherence to cardioprotective medications in order to achieve the standards and the specified core components set out by the BACPR and JBS3 guidelines.
Footnotes
Collaborators: Imperial College London, National Heart and Lung Institute: D Wood*, A Adamska, S Adamska, C Jennings, K Kotseva. England Academic Cardiology, Castle Hill Hospital, Hull: A Hoye* P Atkin, D Fellowes. Barnsley Hospital NHS Foundation Trust: A Negahban*, M Cunningham, A Daniels, L Zeidan. Basildon University Hospital: Dr S Iyer*, A Nicholson, M Ocampo, A Sevillano. Blackpool Teaching Hospitals: G Galasko*, L Benham, S Preston, D Sebastian. Bradford Teaching Hospitals NHS Foundation Trust Bradford: S Lindsay*, C Atkinson, C Kranilla, M Vinod. Hammersmith Hospital: D Wood*, H Abbass, N Rhoualmi. Harrogate District NHS Foundation Trust: Y Beerachee*, C Bennett, M Broome, A Bwalya, Lindsay Caygill, L Dinning, A Gillespie, R Goodfellow, J Guy, T Idress, C Mills, C Morgan, N Oustance, N Singh, M Yare. Hinchingbrooke Hospital: J M Jagoda*, H Bowyer, V Christenssen, A Groves. King’s Mill Hospital Sherwood Forest Hospitals: A Jan PI*, A Riaz*, M Gill, T A Sewell. Lister Hospital, Stevenage: Professor D Gorog*, M Baker, P de Sousa, T Mazenenga. London Northwest University Healthcare NHS Trust, Northwick Park Hospital, London: J Shah*, A Banfield, R Encarnado, A Taylor. Luton and Dunstable University Hospital: C Travill*, S Gent, N Hussain. North West Anglia NHS Foundation Trust: J Porter*, F Haines, T Peachey, J Taaffe, K Wells. Northumbria Healthcare NHS Foundation Trust: D P Ripley*, H Forward, H McKie, S L Pick, H E Thomas. Mid Yorkshire Hospitals NHS Trust: P D Batin*, D Exley, T Rank, J Wright. Milton Keynes University Hospital: A Kardos*, S-B Sutherland, L Wren. Oxford University Hospitals NHS Foundation Trust, John Radcliffe Hospital: P Leeson*, D Barker, B Moreby, J Sawyer. Pennine Acute Hospitals NHS Trust, The Royal Oldham Hospital: J Sobolewska*, D Adams, C Corbett, K Hallett, S Kaye, L Morby, L Winstanley. Royal Berkshire Foundation NHS Trust: J Stirrup*, M Brunton. Royal Devon & Exeter Hospital, Exeter: M M Gandhi*, L Adams, A Hunt. University Hospital Coventry: L Tapp*, V Ansell, S Hyndman. University Hospitals of Morecambe Bay NHS Foundation Trust: A Brodison*, J Craig, S Peters. West Middlesex University Hospital: R Kaprielian*, A Bucaj, K Mahay, M Oblak. Wrightington Wigan and Leigh NHS Foundation Trust: A Sultan*, K Duell, M Gaskell, L Heaton, C Moore, V Parkinson, T Taylor, C Tierney, K Vandesnepscheut-Jones. Yeovil District Hospital: A Broadley*, C Buckley, L Matthews, L Pippard. York Teaching Hospital: C Gale*, M Pye*, Y McGill, H Redfearn, M Fearnley. NIHR Clinical Research Network: East Midlands, Eastern, Greater Manchester, North East and North Cumbria, North Thames, North West Coast, North West London, South West Peninsula, Thames Valley and South Midlands, West Midlands, Yorkshire and Humber. Northern Ireland: Antrim Area Hospital, Northern Ireland: K Lyons*, C Edwards, L Miskelly, S O Mullan. Royal Victoria Hospital, Belfast Health and Social Care Trust: M Spencer*, E McCart. Ulster Hospital Belfast: P Donnelly*, S Kelly, S Regan, D Turnbull. Scotland: Aberdeen Royal Infirmary: Dana Dawson*, B Brikinns, S Joseph. Golden Jubilee National Hospital: Oldroyd K*, Teyhan F, Kelly J, Tewkesbury A. Royal Infirmary of Edinburgh: D Newby*, S Clark, K Combe, L Derr, J Donnelly, L Flint, A Gill, M Glenwright, J Harrison, H Nailon, K Orr, E McDonald, C Mahoney, F Morrow, K Paterson, B Poulose, B Rif, N Spath H Spence, A Sutherland. Raigmore Hospital: J Watt*, C Barr, S Dekker, D McDonald, L O'Keeffe, I Shread. University of Dundee: C C Lang*, A M Choy, L Douglas, S Kalra, M Mohan, R Symon. Wales: Abertawe Bro Morgannwg University Health Board: J Halcox*, K Baldwin, H Goldring, C Thomas. Prince Philip Hospital: L Izzat *, D Evans, Z Omar, E Perkins. (* indicates the principal investigator)
Contributors: All authors contributed to the design, conduct, analysis and interpretation of results and take full responsibility as authors for the manuscript.
Funding: This survey was funded by research grants from Amgen, AstraZeneca, Novartis, Pfizer and Sanofi through the European Society of Cardiology and the Preventive Cardiology Trust (Registered UK Charity).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Ethics approval was obtained from the London Central Research Ethics Committee in Spring 2016 (REC reference number 16/LO/0432).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
Contributor Information
on behalf of the ASPIRE-3-PREVENT Investigators:
D Wood, A Adamska, S Adamska, C Jennings, K Kotseva, A Hoye, P Atkin, D Fellowes, A Negahban, M Cunningham, A Daniels, L Zeidan, S Iyer, A Nicholson, M Ocampo, A Sevillano, G Galasko, L Benham, S Preston, D Sebastian, S Lindsay, C Atkinson, C Kranilla, M Vinod, D Wood, H Abbass, N Rhoualmi, Y Beerachee, C Bennett, M Broome, A Bwalya, Lindsay Caygill, L Dinning, A Gillespie, R Goodfellow, J Guy, T Idress, C Mills, C Morgan, N Oustance, N Singh, M Yare, J M Jagoda, H Bowyer, V Christenssen, A Groves, P I Jan, A Riaz, M Gill, T A Sewell, D Gorog, M Baker, P De Sousa, T Mazenenga, J Shah, A Banfield, R Encarnado, A Taylor, C Travill, S Gent, N Hussain, J Porter, F Haines, T Peachey, J Taaffe, K Wells, D P Ripley, H Forward, H McKie, SL Pick, H E Thomas, P D Batin, D Exley, T Rank, J Wright, A Kardos, S-B Sutherland, L Wren, P Leeson, D Barker, B Moreby, J Sawyer, J Sobolewska, D Adams, C Corbett, K Hallett, S Kaye, L Morby, L Winstanley, J Stirrup, M Brunton, M M Gandhi, L Adams, A Hunt, L Tapp, V Ansell, S Hyndman, A Brodison, J Craig, S Peters, R Kaprielian, A Bucaj, K Mahay, M Oblak, A Sultan, K Duell, M Gaskell, L Heaton, C Moore, V Parkinson, T Taylor, C Tierney, K Vandesnepscheut-Jones, A Broadley, C Buckley, L Matthews, L Pippard, C Gale, M Pye, Y McGill, H Redfearn, M Fearnley, K Lyons, C Edwards, L Miskelly, S O Mullan, M Spencer, E McCart, P Donnelly, S Kelly, S Regan, D Turnbull, Dana Dawson, B Brikinns, S Joseph, K Oldroyd, F Teyhan, J Kelly, A Tewkesbury, D Newby, S Clark, K Combe, L Derr, J Donnelly, L Flint, A Gill, M Glenwright, J Harrison, H Nailon, K Orr, E McDonald, C Mahoney, F Morrow, K Paterson, B Poulose, B Rif, N Spath, H Spence, A Sutherland, J Watt, C Barr, S Dekker, D McDonald, L O'Keeffe, I Shread, C C Lang, AM Choy, L Douglas, S Kalra, M Mohan, R Symon, J Halcox, K Baldwin, H Goldring, C Thomas, L Izzat, D Evans, Z Omar, and E Perkins
References
- 1. Bowker TJ, Clayton TC, Ingham J, et al. A British cardiac Society survey of the potential for the secondary prevention of coronary disease: ASPIRE (action on secondary prevention through intervention to reduce events). Heart 1996;75:334–42. 10.1136/hrt.75.4.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kotseva K, Jennings CS, Turner EL, et al. ASPIRE-2-PREVENT: a survey of lifestyle, risk factor management and cardioprotective medication in patients with coronary heart disease and people at high risk of developing cardiovascular disease in the UK. Heart 2012;98:865–71. 10.1136/heartjnl-2011-301603 [DOI] [PubMed] [Google Scholar]
- 3. Boon N, Boyle R, Bradbury K, et al. Joint British societies' consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart 2014;100 Suppl 2:ii1–67. 10.1136/heartjnl-2014-305693 [DOI] [PubMed] [Google Scholar]
- 4. EUROASPIRE Study Group EUROASPIRE: a European Society of cardiology survey of secondary prevention of coronary heart disease: principal results. Eur Heart J 1997;18:1569–82. 10.1093/oxfordjournals.eurheartj.a015136 [DOI] [PubMed] [Google Scholar]
- 5. EUROASPIRE II Study Group Lifestyle and risk factor management and use of drug therapies in coronary patients from 15 countries; principal results from EUROASPIRE II Euro heart survey programme. Eur Heart J 2001;22:554–72. 10.1053/euhj.2001.2610 [DOI] [PubMed] [Google Scholar]
- 6. Kotseva K, Wood D, De Backer G, et al. EUROASPIRE III: a survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. Eur J Cardiovasc Prev Rehabil 2009;16:121–37. 10.1097/HJR.0b013e3283294b1d [DOI] [PubMed] [Google Scholar]
- 7. Kotseva K, Wood D, De Bacquer D, et al. EUROASPIRE IV: a European Society of cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur J Prev Cardiol 2016;23:636–48. 10.1177/2047487315569401 [DOI] [PubMed] [Google Scholar]
- 8. Kotseva K, De Backer G, De Bacquer D, et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: results from the European Society of cardiology ESC-EORP EUROASPIRE V registry. Eur J Prev Cardiol 2019;26:824–35. 10.1177/2047487318825350 [DOI] [PubMed] [Google Scholar]
- 9. Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease. JAMA 2003;290:86–97. 10.1001/jama.290.1.86 [DOI] [PubMed] [Google Scholar]
- 10. Anderson L, Taylor RS. Cardiac rehabilitation for people with heart disease: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 2014:CD011273 10.1002/14651858.CD011273.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chow CK, Jolly S, Rao-Melacini P, et al. Association of diet, exercise, and smoking modification with risk of early cardiovascular events after acute coronary syndromes. Circulation 2010;121:750–8. 10.1161/CIRCULATIONAHA.109.891523 [DOI] [PubMed] [Google Scholar]
- 12. BACPR The BACPR standards and core components for cardiovascular disease prevention and rehabilitation 2017. 3rd edn, 2017. http://www.bacpr.com/resources/BACPR_Standards_and_Core_Components_2017.pdf [DOI] [PubMed] [Google Scholar]
- 13. The National audit of cardiac rehabilitation annual statistical report, 2018. Available: https://www.bhf.org.uk/informationsupport/publications/statistics/national-audit-of-cardiac-rehabilitation-quality-and-outcomes-report-2018
- 14. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines. Hypertension 2018;71:1269–324. 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 15. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–104. 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 16. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013;5:CD009329 10.1002/14651858.CD009329.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rigotti NA, Munafo MR, Stead LF. Smoking cessation interventions for hospitalized smokers: a systematic review. Arch Intern Med 2008;168:1950–60. 10.1001/archinte.168.18.1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jennings C, Kotseva K, De Bacquer D, et al. Effectiveness of a preventive cardiology programme for high CVD risk persistent smokers: the EUROACTION plus varenicline trial. Eur Heart J 2014;35:1411–20. 10.1093/eurheartj/ehu051 [DOI] [PubMed] [Google Scholar]
- 19. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (eagles): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016;387:2507–20. 10.1016/S0140-6736(16)30272-0 [DOI] [PubMed] [Google Scholar]
- 20. NHS Long term plan, 2019. Available: https://www.longtermplan.nhs.uk/wp-content/uploads/2019/01/nhs-long-term-plan.pdf
- 21. Aubin H-J, Farley A, Lycett D, et al. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ 2012;345:e4439 10.1136/bmj.e4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gyberg V, De Bacquer D, Kotseva K, et al. Screening for dysglycaemia in patients with coronary artery disease as reflected by fasting glucose, oral glucose tolerance test, and HbA1c: a report from EUROASPIRE IV--a survey from the European Society of Cardiology. Eur Heart J 2015;36:1171–7. 10.1093/eurheartj/ehv008 [DOI] [PubMed] [Google Scholar]
- 23. van Halewijn G, Deckers J, Tay HY, et al. Lessons from contemporary trials of cardiovascular prevention and rehabilitation: a systematic review and meta-analysis. Int J Cardiol 2017;232:294–303. 10.1016/j.ijcard.2016.12.125 [DOI] [PubMed] [Google Scholar]
- 24. Connolly SB, Kotseva K, Jennings C, et al. Outcomes of an integrated community-based nurse-led cardiovascular disease prevention programme. Heart 2017;103:840–7. 10.1136/heartjnl-2016-310477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gibson I, Flaherty G, Cormican S, et al. Translating guidelines to practice: findings from a multidisciplinary preventive cardiology programme in the West of Ireland. Eur J Prev Cardiol 2014;21:366–76. 10.1177/2047487313498831 [DOI] [PubMed] [Google Scholar]
- 26. WHO Obesity: preventing and managing the global epidemic. Report of a WHO consultation. Geneva: World Health Organisation, 2004. http://whqlibdoc.who.int/trs/WHO_TRS_894_(part1).pdf [PubMed] [Google Scholar]
- 27. Inoue S, Zimmet P. The Asia-Pacific perspective. redefining obesity and its treatment, 2000. Available: http://www.diabetes.com/pdf/obesity_report.pdf
- 28. Godin G, Shephard RJ. Godin leisure-time exercise questionnaire. Med Sci Sports Exerc 1997;29:S36e8. [Google Scholar]
- 29. NICE Managing diabetes in patients with type 2 diabetes, 2019. Available: http://pathways.nice.org.uk/pathways/type-2-diabetes-in-adults
- 30. WHO Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia [Internet], 2019. Available: https://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/ [Accessed 12 Jun 2019].
- 31. Gehi A, Haas D, Pipkin S, et al. Depression and medication adherence in outpatients with coronary heart disease: findings from the heart and soul study. Arch Intern Med 2005;165:2508–13. 10.1001/archinte.165.21.2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 33. Oldridge N, Höfer S, McGee H, et al. The HeartQoL: Part II. validation of a new core health-related quality of life questionnaire for patients with ischemic heart disease. Eur J Prev Cardiol 2014;21:98–106. 10.1177/2047487312450545 [DOI] [PubMed] [Google Scholar]
- 34. Schweikert B, Hahmann H, Leidl R. Validation of the EuroQol questionnaire in cardiac rehabilitation. Heart 2006;92:62–7. 10.1136/hrt.2004.052787 [DOI] [PMC free article] [PubMed] [Google Scholar]


