Abstract
Background
Patients with BRCA1-associated protein 1 (BAP1)-mutant clear cell renal cell carcinoma (ccRCC) have worse prognosis. C-C chemokine receptor 5 (CCR5) plays an important role in ccRCC development and its expression is elevated in BAP1-mutant tumors.
Methods
533 patients with ccRCC from The Cancer Genome Atlas cohort and 797 patients with ccRCC from the Shanghai cohort were enrolled. In vitro and in vivo studies were conducted with human ccRCC tumors and murine tumor models. The association between BAP1 and CCR5 or its ligands was assessed by immunohistochemistry, flow cytometry, real-time PCR and ELISA. Survival was compared between different subpopulations of patients using Kaplan-Meier curve. Therapeutic effect of CCR5 blockade was validated using human ccRCC tumors and murine models.
Results
Expression of CCR5 and its ligands were elevated in BAP1-mutant patients with ccRCC. High CCR5 expression was indicative of poor prognosis in BAP1-low group of patients. CCR5 blockade prolonged the survival of tumor-bearing mice, resulting in enhanced cytotoxicity of T cells and antigen presentation of dendritic cells but repressed immune checkpoint expression. CCR5 ligands could recruit CCR5+ regulatory T cells to the tumor microenvironment. Additionally, BAP1-mutant ccRCC tumor cells secreted CCR5 ligands, which increased programmed cell death ligand 1 expression. However, both processes could be inhibited by CCR5 blockade. Study limitations include the unclear impact of CCR5 expressed by other cell populations.
Conclusions
CCR5 in BAP1-mutant ccRCC results in an immune-suppressive microenvironment. Targeting CCR5 could provide a potential therapeutic benefit for patients.
Trial registration number
NCT01358721, CA209-009.
Keywords: urology, immunology, oncology
Background
Clear cell renal cell carcinoma (ccRCC) represents 75%–85% of kidney cancer cases, and its incidence has been increasing in recent years.1 Genomics studies have revealed that multiple genes with significant mutations, including the von Hippel-Lindau (VHL) gene, polybromo-1 (PBRM1) gene and BRCA1-associated protein 1 (BAP1) gene, are present in ccRCC and are capable of driving tumor development and progression.2 Existing studies indicate that the mechanism of ccRCC development first involves VHL gene mutation on chromosome 3 associated with short-arm deletion of homologous chromosomes; then, the tumor progresses in one of two directions, PBRM1 gene mutation or BAP1 gene mutation. Patients with ccRCC with BAP1 mutation are often with higher grade, metastasis development and worse prognosis.3
Notably, ccRCC ranks first in common tumors for both the immune infiltration score and T-cell infiltration score.4 Therefore, immune checkpoint inhibitors (ICIs) have drawn much attention in the treatment of renal cancer. Several clinical trials have investigated the therapeutic effect of programmed cell death 1 (PD-1) inhibitors in advanced kidney cancer, some of which obtained positive feedback.5 6 Previous study has demonstrated relatively high immune activity in BAP1-mutant ccRCC (online supplementary figure 1a).7 However, whether BAP1-mutant ccRCC is sensitive to ICIs has rarely been documented. We analyzed data from two clinical trials8 of anti-PD-1 therapy in metastatic ccRCC and found that BAP1-mutant patients with ccRCC responded poorly to inhibitors targeting PD-1 (online supplementary figure 1b-c). Therefore, it is particularly essential to find new therapeutic targets for BAP1-mutant patients with ccRCC.
jitc-2019-000228supp001.pdf (908.9KB, pdf)
C-C chemokine receptor 5 (CCR5) is found to associate with renal cell carcinoma (RCC) development. It was reported that CCR5 and its ligands was significantly upregulated in high-stage or high-grade tumors.9 Thus, CCR5 might be a potential therapeutic target. Maraviroc, a Food and Drug Administration (FDA)-approved CCR5 antagonist, was found able to induce cytotoxic and apoptotic effects in both primary and metastatic colon cancer cells.10 Additionally, it inhibited breast cancer and liver cancer progression in mouse models as well,11–13 but whether it has a therapeutic effect in ccRCC remains unknown.
In the present study, we investigated whether blockade of CCR5 receptor affected the progression of this type of tumor. We found that CCR5 blockade might serve as a novel cancer-immunotherapy strategy to induce a robust immune response and improve clinical outcomes.
Materials and methods
Study population
The Shanghai cohort consisted of 809 patients with ccRCC from Zhongshan Hospital Fudan University (Shanghai, China). All these patients received partial or radical nephrectomy between January 7, 2008 and December 23, 2010, and corresponding archived formalin-fixed paraffin-embedded specimen were preserved for immunological staining. None of these 809 patients received neoadjuvant radiotherapy or chemotherapy. Twelve patients with distant metastatic diseases were excluded. Baseline demographic, clinical and laboratory data were collected simultaneously, MRI and CT scans were reassessed in radiology units and all archived diagnostic H&E slides were pathologically central reviewed independently. Patient characteristics are presented in online supplementary table 1.
Sixteen ccRCC fresh tumor specimens (8 BAP1-mutant ccRCC and 8 BAP1-wildtype ccRCC) and five paired peripheral blood samples were collected from Zhongshan Hospital Fudan University. Patients enrolled underwent nephrectomy and were treatment-naïve.
The Cancer Genome Atlas (TCGA) data were downloaded from Genomic Data Commons Data Portal (https://portal.gdc.cancer.gov/) in August 2016.14 Five hundred thirty-three patients with ccRCC who underwent renal mass surgery between 1998 and 2013 were enrolled. During survival analysis, 518 patients with intact follow-up data were enrolled. Likewise, the number of patients with recurrence data was 516. RNA expression level was normalized as Z-score or log2 (expression +1) before analysis.
The primary outcomes of survival analyzes were overall survival (OS) and recurrence-free survival (RFS), representing the time from the date of surgery to the date of death (OS) and recurrence (RFS), or to the date of last follow-up. Further details of the materials and methods used in this study were presented in the online supplementary materials and methods.
Results
Expression of CCR5 and its ligands are elevated in BAP1-mutant ccRCC
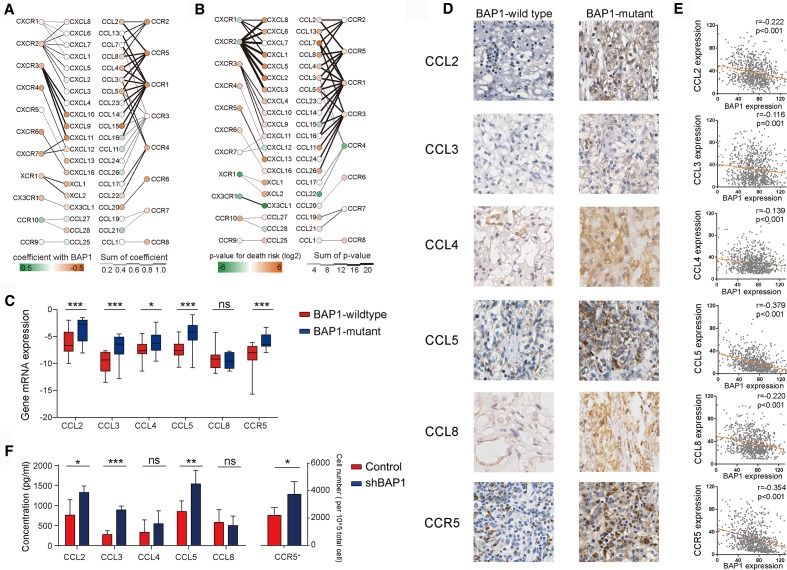
The mutation rate of BAP1 in ccRCC was 14%–15%. Genome sequencing in the TCGA cohort identified 40 BAP1-mutant patients with 42 mutations, about 24% (10/42) of which was frameshift mutation, 26% (11/42) of which was nonsense mutation, 12% (5/42) of which splice mutation, 7% (3/42) of which was translation start site mutation. In-frame deletion and missense mutation accounted for 2.4% (1/42) and 28.5% (12/42) cases, respectively. Missense mutations did not affect protein levels. Frameshift mutations led to the degradation of proteins due to translation errors. Nonsense mutations and translation start site mutation led to the failure of protein transcription. Splice site mutation resulted in post-transcriptional splicing errors. Above all, about 70% of these mutations induced a decrease in BAP1 protein expression. Another cohort found that nearly 90% (22/25) BAP1-negative patients with ccRCC measured by immunohistochemistry (IHC) had BAP1 mutation.9 Thus, we use BAP1 mRNA/protein level as proxy of mutation in patients with ccRCC in this study (online supplementary figure 2d). Consistent with previous reports, we found that BAP1 mutation was typically associated with loss of the protein and indicated poor prognosis for patients with renal cell carcinoma9 15 (online supplementary figure 2a-c and online supplementary table 2). To understand the possible mechanism underlying this phenomenon, we analyzed the correlation between BAP1 mRNA expression and various chemokines plus their receptors. We found that the expression of CCR5 and its ligands were anticorrelated with BAP1 (figure 1A). Additionally, patients with higher expression of CCR5 and its ligands were at greater risk of death (figure 1B). Real-time PCR analysis confirmed this in BAP1-mutant patients with ccRCC (figure 1C). IHC analysis further revealed that BAP1 protein was negatively associated with CCR5 and its ligands (figure 1D-E). Next, we stably integrated an RNA interference vector into murine RENCA cells and established a BAP1 knockdown cell line, which was then used to construct a subcutaneous tumor-bearing mouse model. Flow cytometry and ELISA results showed that the proportion of CCR5+ cells and the expression levels of CCL2, CCL3 and CCL5 in ccRCC tumors were higher than those in the control group (figure 1F). These results indicated the association existing between BAP1 gene mutation and high expression of CCR5 and its ligands.
Figure 1.
The expression of C-C chemokine receptor 5 (CCR5) and its ligands increases in BRCA1-associated protein 1 (BAP1)-mutant clear cell renal cell carcinoma (ccRCC). (A) Association between BAP1 RNA expression vs various chemokines and their receptors RNA expression in patients with ccRCC from The Cancer Genome Atlas (TCGA) cohort. ‘Coefficient with BAP1’ means the Spearman's rank correlation coefficients between chemokines or their receptors and BAP1, or ‘Spearman's Rho’. Death risk is the HRs of the overall survival (OS) calculated with Cox model by inputting continuous variables. (B) Association between chemokines and their receptors RNA expression vs risk of death in patients with ccRCC from the TCGA cohort. (C) CCL2-5, CCL8 and CCR5 mRNA expression levels in fresh BAP1-mutant and BAP1-wildtype ccRCC tumor specimens measured by real-time PCR (n=8 per group). Box-and-whisker diagrams were used (median, lower and upper quartiles; horizontal lines define min and max). (D) Representative images showing CCL2-5, CCL8 and CCR5 expression in BAP1-wildtype and BAP1-mutant ccRCC tumor specimens via immunohistochemistry. (E) Correlation between chemokines RNA expression and BAP1 RNA expression from the Shanghai cohort. (F) Left: CCL2-5, CCL8 expression levels in BAP1 knockdown tumor-bearing mice measured by ELISA; right: proportion of CCR5+ cells determined by flow cytometry (n=10 per group).
CCR5 blockade retards the progression of BAP1-mutant ccRCC
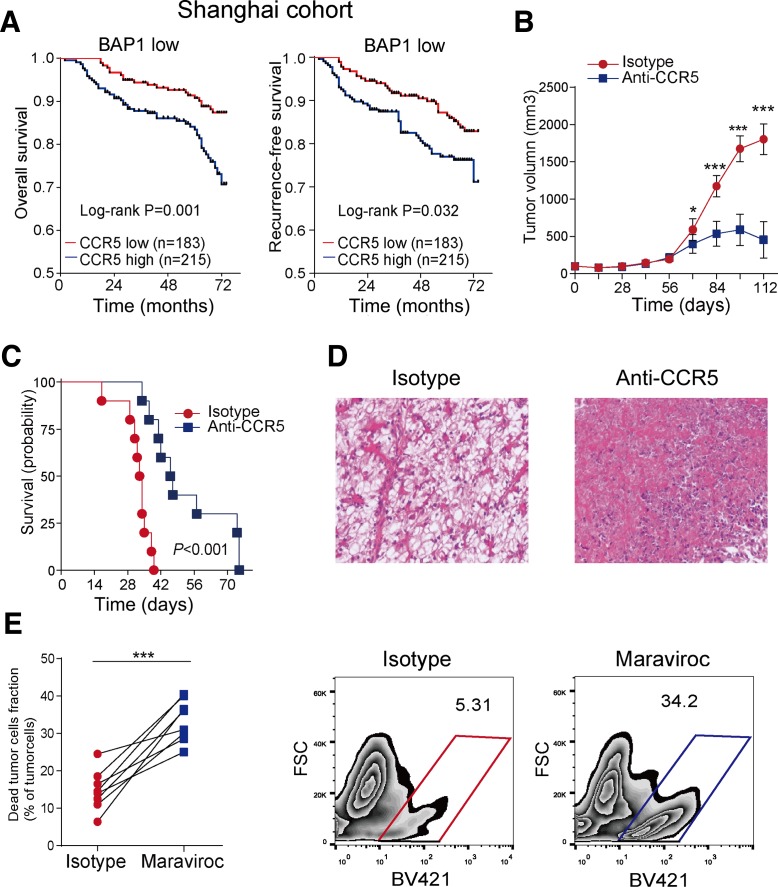
The above-mentioned results suggested that CCR5 might be a potential therapeutic target for BAP1-mutant ccRCC. Indeed, we found that in BAP1-low group of patients, increased CCR5 expression was indicative of poor OS and RFS (figure 2A), while no difference was observed in BAP1-high group of patients (online supplementary figure 3a). In BAP1-knockdown subcutaneous tumor-bearing mouse model, tumor volume was significantly reduced after treating with an anti-CCR5 antibody (figure 2B). A survival benefit was observed in the orthotopic mouse model as well (figure 2C). Moreover, we found that the use of anti-CCR5 antibody caused extensive necrosis of tumor cells (figure 2D) and a similar result was observed by flow cytometry analysis. Our previous study16 established a novel in vitro culture system incorporating bulk single cell suspensions from resected ccRCC tumors to simulate the in vivo tumor immune system of patients. And similar models were used to test the therapeutic effect of histone deacetylation inhibitor in lung cancer.17 In our study, resected ccRCC specimens were disaggregated and cultured in vitro, and maraviroc (an FDA-approved anti-CCR5 antagonist) was added for activity testing. The fraction of dead renal tumor cells in BAP1-mutant patients was significantly higher than that in the isotype control (figure 2E). However, anti-CCR5 antibody had no effect in BAP1-wildtype ccRCC (online supplementary figure 3b-c). These findings suggested that blockade of CCR5 could effectively inhibit the progression of BAP1-mutant ccRCC.
Figure 2.
C-C chemokine receptor 5 (CCR5) antagonist prolongs survival and causes tumor cell death in BRCA1-associated protein 1 (BAP1)-mutant clear cell renal cell carcinoma (ccRCC) mice. (A) Overall survival (OS) and recurrence-free survival (RFS) curves for BAP1-low patients with ccRCC according to immunohistochemical CCR5 staining from a shanghai cohort. (B) Tumor volume from BALB/c mice subcutaneously injected with BAP1-knockdown RENCA cells and treated with anti-CCR5 or isotype (n=10 per group). (C) OS curve for BALB/c mice orthotopically injected with BAP1-knockdown RENCA cells and treated with anti-CCR5 or isotype antibodies (n=10 per group). (D) Typical images of mouse tumorous tissue sections with H&E staining after treatment with anti-CCR5 or isotype antibodies. (E) Left: dead tumor cell fraction in cultured human tumors treated with maraviroc or isotype antibodies (n=8 per group). Dead cells were identified as populations that stained positive for the viability dye (Zombie Violet for 405 nm excitation); right: typical images gated by Epcam+CD45− cells.
CCR5 blockade activates the antitumor immunity in BAP1-mutant ccRCC
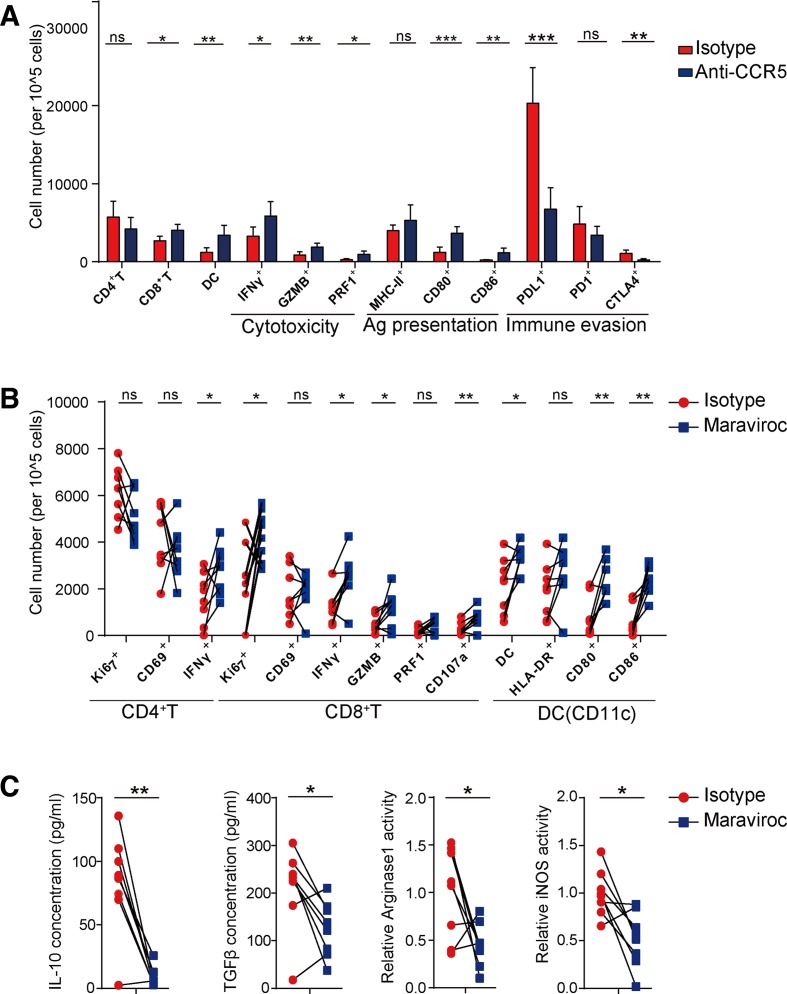
After observing a large number of immune cells around the necrotic foci of renal tumor cells treated with the anti-CCR5 antibody, we postulated that blockade of CCR5 could enhance the antitumor immune response. Thus, we investigated the infiltrating level of local immune cells in tumor-bearing mice. We found that the proportions of tumor-infiltrating CD8+ T cells and dendritic cells (DCs) were increased in mice treated with anti-CCR5 antibody and the corresponding proportions of interferon (IFN)-γ+, GZMB+, PRF1+, CD80+ and CD86+ cells were also increased, indicating enhanced cell cytotoxicity and antigen presentation (figure 3A). Meanwhile, the proportions of programmed cell death ligand 1 (PD-L1)+ and CTLA-4+ cells were significantly decreased (figure 3A). We further digested fresh tissue specimens from BAP1-mutant patients with ccRCC into single cell suspensions and cultured the cells in vitro with maraviroc as the negative allosteric modulator for 4 days. Subsequently, flow cytometry revealed no significant differences in the activation marker CD69 of CD4+ T and CD8+ T cells; however, the proportion of CD4+ T cells secreting the functional effector molecule IFN-γ and CD8+ T cells secreting IFN-γ and GZMB or expressing CD107a were significantly increased. The number of DCs increased together with elevated expression of CD80 and CD86; however, the change in HLA-DR was not significant. The proliferation ability of CD8+ T cells and DCs were enhanced as well (figure 3B). In addition, interleukin-10 (IL-10), transforming growth factor-β (TGF-β), arginase and inducible nitric oxide synthase expression levels in tumor supernatants were measured. We found that the expression levels of all these inhibitory molecules decreased following maraviroc treatment (figure 3C). However, the effect of CCR5-blocking antibody on the antitumor immune response was not significant in BAP1-wildtype ccRCC mice (online supplementary figure 3d).
Figure 3.
Blockade of C-C chemokine receptor 5 (CCR5) reactivates the antitumor immune response. (A) Number of intratumoral CD4+T, CD8+T, dendritic cell (DC), interferon (IFN)-γ+, GZMB+, PRF1+, CD80+, CD86+, MHC-II+, programmed cell death ligand 1 (PD-L1)+, programmed cell death 1 (PD-1)+, CTLA-4+ cells from BALB/c mice orthotopically injected with BRCA1-associated protein 1 (BAP1)-knockdown RENCA cells and treated with anti-CCR5 or isotype antibodies (n=10 per group). (B) Expression of cytotoxic cytokines, Ki67 and CD69 in CD4+ or CD8+ T cells and molecules related to antigen presentation in DC from cultured human tumors treated with maraviroc or isotype antibodies (n=8 per group). Measured by flow cytometry. (C) Interleukin-10 (IL-10), transforming growth factor-β (TGF-β), arginase 1 and inducible nitric oxide synthase (iNOS) levels measured by ELISA and enzyme activity assays in supernatants of tumor tissue cultures treated with maraviroc and the control. ns. not significant. *P<0.05; **p<0.01; ***p<0.001.
CCR5 blockade suppresses the recruitment of regulatory T cells
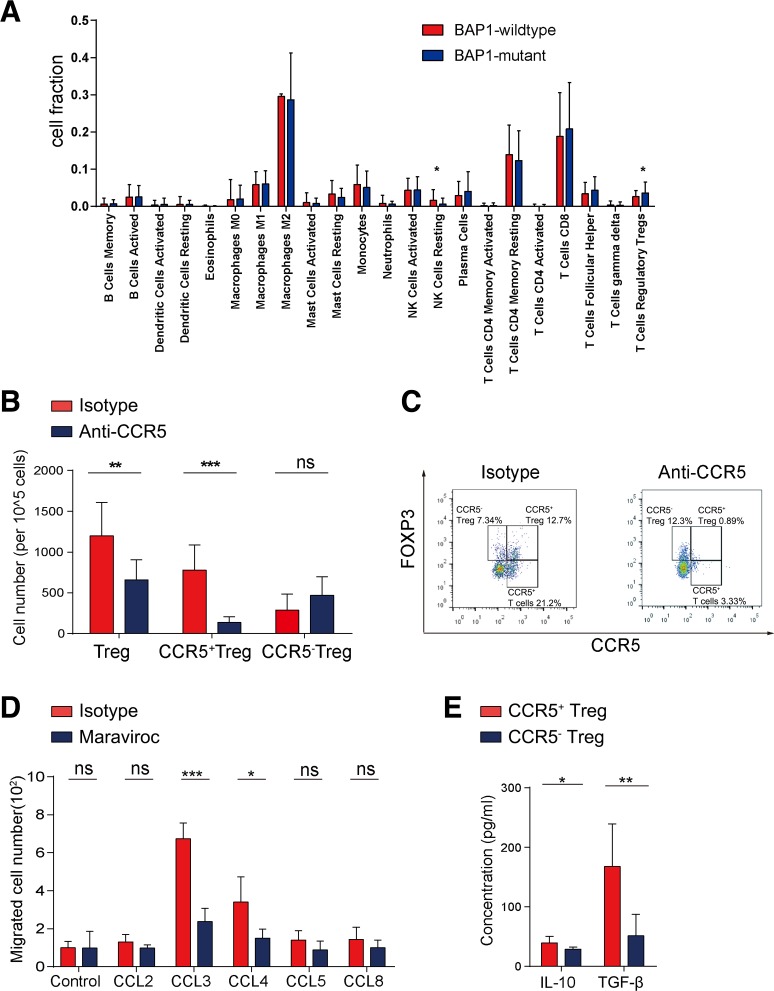
We next explored the specific mechanism by which blockade of CCR5 activated the antitumor immunity. We analyzed the fraction of infiltrating immune cells in ccRCC using the CIBERSORT method18 and found a higher fraction of Tregs in BAP1-mutant patients with ccRCC (figure 4A). A previous study indicated that CCR5 was partially expressed on the surface of Tregs and blockade of CCR5 affected the recruitment of Tregs.19 Likewise, we found that the total number of Tregs, especially CCR5+ Tregs, was decreased after treatment with the CCR5-blocking antibody compared with the control group, but no obvious change was found in CCR5− Tregs (figure 4B, C). In addition, we found that total CD8+ T cells increased and CD4+ T cells and macrophages decreased slightly after CCR5 blockade, but there was no significantly difference in total number of CD8+ T cells, CD4+ T cells and macrophages or corresponding CCR5+ or CCR5− immune cell subtypes between the two groups (online supplementary figure 4a). It suggested that CCR5+ Tregs might play an important role in this process. We then isolated human peripheral blood-derived CCR5+ Tregs and found that CCR5+ Tregs were recruited by CCL3 and CCL4, but this chemotaxis was significantly attenuated after maraviroc treatment (figure 4D). Next, CCR5+ Tregs and CCR5− Tregs were isolated to investigate their functional differences. Compared with CCR5− Tregs, CCR5+ Tregs secreted higher levels of IL-10 and TGF-β (figure 4E), which indicated their stronger immunosuppressive function.
Figure 4.
Blockade of CCR5 prevents the recruitment of CCR5+ Tregs. (A) The CIBERSORT method was performed to analyze the proportion of 22 immune cells among BAP1-mutant or BAP1-wildtype patients with ccRCC from The Cancer Genome Atlas cohort. (B) Number of Tregs, CCR5+ Tregs and CCR5− Tregs in BALB/c mice orthotopically injected with BAP1-knockdown RENCA cells and treated with anti-CCR5 or isotype antibodies (n=10 per group). (C) Representative images of CCR5+ Tregs and CCR5− Tregs, gated by CD3+CD4+CD25+ cells. (D) Migration of PBMC-derived CCR5+ Tregs in response to CCR5 ligands in the presence of maraviroc or isotype control was analyzed with a migration assay (n=5 per group). (E) PBMC-derived CCR5+ Tregs and CCR5− Tregs were isolated by FACS from BAP1-mutant patients with ccRCC and cultured for 3 days before measurement of interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) concentrations in supernatants via ELISA (n=5 per group). ns. not significant. *P<0.05; **p<0.01; ***p<0.001.
CCR5 blockade represses PD-L1 expression by tumor cells in BAP1-mutant ccRCC
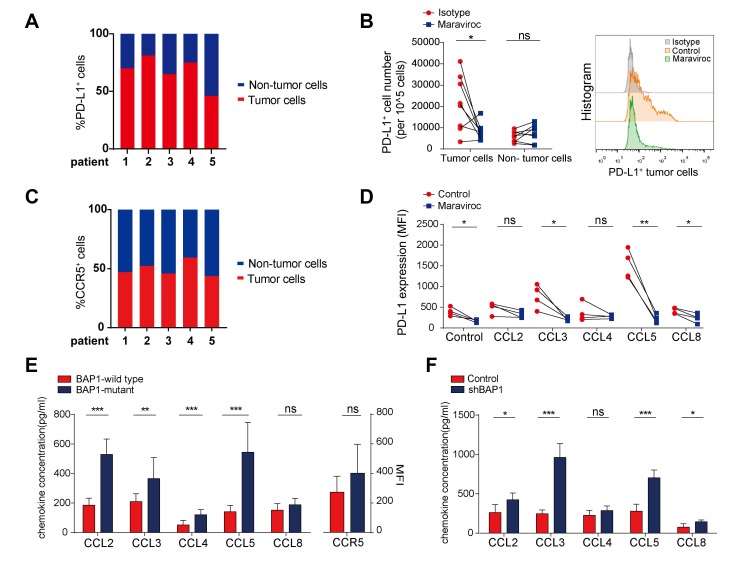
Given that research has shown positive PD-L1 expression in tumor cells20 and that our study revealed a decreased number of tumor-infiltrating PD-L1+ cells after treatment with the CCR5-blocking antibody (figure 3A), we analyzed the distribution of PD-L1 in BAP1-mutant tumor tissues and found that tumor cells had a significantly higher proportion of PD-L1+ cells than non-tumor cells (figure 5A). The proportion of PD-L1+ tumor cells decreased, while the proportion of PD-L1+ non-tumor cells did not change substantially after maraviroc treatment (figure 5B).
Figure 5.
Blockade of C-C chemokine receptor 5 (CCR5) represses the expression of programmed cell death ligand 1 (PD-L1) by tumor cells. (A) Proportion of PD-L1+ tumor and non-tumor cells in fresh BRCA1-associated protein 1 (BAP1)-mutant tumor specimens (n=5). (B) Left: number of PD-L1+ tumor and non-tumor cells from cultured human tumors treated with maraviroc or isotype antibodies (n=8 per group); right: typical image of flow cytometry. (C) Proportion of CCR5+ tumor and non-tumor cells in fresh BAP1-mutant tumor specimens (n=5). (D) Renal tumor cells were isolated from BAP1-mutant patients with clear cell renal cell carcinoma (ccRCC). One group was treated with maraviroc and the other group with isotype antibody. The PD-L1 expression level on the tumor cell surface was measured after 4 days of stimulation with CCR5 ligands in vitro. (A–D) All measured by flow cytometry. (E) Left: renal tumor cells were isolated from BAP1-wildtype and BAP1-mutant patients with ccRCC and cultured in vitro for 3 days and CCL2-5 and CCL8 secretion in supernatants was measured via ELISA; right: CCR5 expression level on the surface of renal tumor cells was measured via flow cytometry. (F) CCL2-5 and CCL8 secretion in culture supernatants of BAP1-knockdown RENCA cell line measured via ELISA. ns. not significant. *P<0.05; **p<0.01; ***p<0.001.
Interestingly, tumor cells also included a relatively high proportion of CCR5+ cells (figure 5C). We stimulated the isolated BAP1-mutant renal tumor cells with CCR5 ligands in vitro with addition of maraviroc. Subsequent analysis revealed CCL5 could significantly enhanced PD-L1 expression by tumor cells, followed by CCL3 and CCL8. However, this effect was reversed after blocking CCR5 (figure 5D). Moreover, blockade of CCR5 caused PD-L1 expression to decrease without addition of exogenous chemokines. We isolated tumor cells from BAP1-wildtype and BAP1-mutant patients with ccRCC and cultured them for 3 days in vitro. The concentration of CCL2-5 was significantly higher in BAP1-mutant ccRCC (figure 5E). A similar result was obtained from the BAP1-knockdown RENCA cell line constructed with BAP1 short hairpin RNA (figure 5F).
Discussion
BAP1 is known to interact with BRCA1 and possess deubiquitinating enzyme activity and thus BAP1 can prevent further degradation of the protein.21 Therefore, mutations in the BAP1 gene are often associated with decreases in protein expression or changes in gene expression. In the present study, we found BAP1 gene mutation in approximately 10% of patients from TCGA database. This patient population had a short survival time and was prone to tumor recurrence. The poor clinical outcome of BAP1-protein-low patients was also confirmed in the Shanghai cohort. Therefore, BAP1 mutation induces a decrease in target protein expression, which is likely to affect tumor development and progression through downstream pathways.22
We used multilevel data to confirm that BAP1 expression was negatively correlated with CCR5 and its ligands. Moreover, we confirmed that high CCR5 expression was only suggestive of poor prognosis for BAP1-low patients, which was possibly due to the immunosuppressive environment. Previous studies have reported the high expression of CCR5 and its ligand CCL5 in basal and HER-2 breast cancer, revealing that CCR5 plays a crucial role in cancer progression.11 23 Taken together, these results suggested that targeting CCR5 might serve as a novel strategy in cancer treatment.
In the murine model, we found that CCR5 antibody treatment prolonged survival and delayed tumor progression. A study by Song et al24 demonstrated that CCR5 promoted tumor progression and further found that CCR5 deficiency led to apoptotic melanoma cell death. In the present study, we noted that immunosuppressive microenvironment was interrupted after CCR5 blockade. Specifically, the number of cells related to cytotoxicity and antigen presentation was increased, while the number of cells mediating immunosuppression was decreased. In vitro studies revealed an elevated expression of functional effector molecules by lymphocytes and antigen-presenting molecules by DCs after maraviroc treatment. Meanwhile, a decrease in the protein expression levels of inhibitory molecules in culture supernatants was observed. Taken together, these results indicated that CCR5 blockade could enhance the ability of the host immune system to eliminate tumor cells.
We further explored the specific mechanisms by which CCR5 blockade enhanced the antitumor immunity. First, we found a large number of locally infiltrating Tregs in BAP1-mutant ccRCC. Oldham et al25 reported that CCR5 was significantly overexpressed on tumor infiltrating lymphocytes, of which were mostly FOXP3+ cells in RCC. Additionally, CCL5-CCR5 axis was found to play critical roles in immune cell migration and chemotaxis axis and in inducing Tregs polarization.26 27 Based on these findings, we presumed that recruitment of Tregs was affected following the use of CCR5 inhibitors. By transwell assays, we found that CCL3 and CCL4 could recruit CCR5+ Tregs and that CCL3 has the strongest chemotactic effect. Similarly, Zhang et al found that colon cancer cells recruited CCR5+ Tregs to local tumors by secreting CCL5, which in turn suppressed the cytotoxicity of CD8+ T cells and mediated immune evasion.19 28 Intriguingly, we observed stronger immunosuppressive capacity in CCR5+ Tregs than in CCR5− Tregs. A similar result was also confirmed by Ward et al29 in human colorectal cancer. In addition, enhanced infiltration and more IL-10 secreted by CCR5+ Tregs than CCR5− Tregs in lymphoid and central nervous system tissues retarded encephalitis progression in mice model.30 However, the detailed phenotype and function of CCR5+ or CCR5− Tregs in ccRCCs are still needed to be explored.
Besides recruiting CCR5+ Tregs, we also found that BAP1-mutant tumor cells generated CCL2, CCL3 and CCL5, which bound to CCR5 on the cell surface and induced PD-L1 expression. This process could be attenuated by CCR5 inhibitors. ICIs have developed rapidly for clinical treatment of kidney cancer. However, only a relatively small proportion of patients with ccRCC respond to ICI treatment.31 This implies that other immune evasion mechanisms might exist, and thus new therapeutic targets are needed to improve the therapeutic effect of ICIs. Yang et al32 found that maraviroc reduced myeloid-derived suppressor cell infiltration in tumor parenchyma and retarded tumor progression in gastric cancer, and its combined use with an anti-PD-1 antibody enhanced the therapeutic effect. These results suggested that combining use of PD-1 inhibitor and maraviroc in BAP1-mutant patients with ccRCC is a direction worth exploring in the future.
The present work has some limitations. The specific mechanism that the CCL5-CCR5 axis induces tumor cells to express PD-L1 at high levels needs to be further elucidated. Additionally, the mechanism by which BAP1 mutation leads to increased expression of CCR5 and its ligands remains to be investigated.
Conclusion
We demonstrated that BAP1 mutation led to increased expression of CCR5 on Tregs and tumor cells. Application of anti-CCR5 antibody induced the antitumor immune response and effectively inhibited tumor growth. The present study revealed that tumor cells could secrete CCR5 ligands, which could bind with the tumor cell surface receptor and induce increased PD-L1 expression, and recruit CCR5+ Tregs to the local tumor microenvironment and promote immune evasion. CCR5 blockade could prohibited both of these processes and might serve as a potential new therapeutic approach.
Acknowledgments
The authors would like to thank Dr Lingli Chen (Department of Pathology, Zhongshan Hospital Fudan University, Shanghai, China) and Dr Peipei Zhang (Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China) for their excellent pathological technology help.
Footnotes
QZ, YQ, ZW and HZ contributed equally.
Contributors: QZ, YQ, ZW and HZ for acquisition of data, analysis and interpretation of data, statistical analysis and drafting of the manuscript; HZ, ZL, QH, YX, JW, YC, QB, YX, YW, LL, LX, BD and JG for technical and material support; YZ, WZ and JX for study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding and study supervision. All authors read and approved the final manuscript.
Funding: This study was funded by grants from National Natural Science Foundation of China (81671628, 31770851, 81702496, 81702497, 81702805, 81772696, 81871306, 81872082, 81902556, 81902563, 81902898, 81974393), National Key R&D Program of China (2017YFC0114303), Shanghai Municipal Natural Science Foundation (16ZR1406500, 17ZR1405100, 19ZR1431800), Guide Project of Science and Technology Commission of Shanghai Municipality (17411963100), Shanghai Sailing Program (18YF1404500, 19YF1407900, 19YF1427200), Shanghai Municipal Commission of Health and Family Planning Program (20174Y0042, 201840168, 20184Y0151), Fudan University Shanghai Cancer Center for Outstanding Youth Scholars Foundation (YJYQ201802) and Shanghai Cancer Research Charity Center.
Disclaimer: We analyzed the cytokines expression and immune components in BAP1-mutant ccRCC and found that CCR5 blockade could reverse the immune-suppressive microenvironment and might become a promising therapeutic approach. All the study sponsors have no roles in the study design, in the collection, analysis and interpretation of data.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Written informed consent was obtained from each patient included and the protocol of all study cohorts were approved by the institutional review board and ethics committee of Zhongshan Hospital, Fudan University.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. All data generated that are relevant to the results presented in this article are included in this article. Other data that were not relevant for the results presented here are available from the corresponding author Dr Xu on reasonable request.
References
- 1.Siegel R, Miller K, Jemal A. Cancer statistics, 2017 Ca cancer. J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Sato Y, Yoshizato T, Shiraishi Y, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet 2013;45:860–7. 10.1038/ng.2699 [DOI] [PubMed] [Google Scholar]
- 3.Brugarolas J. Molecular genetics of clear-cell renal cell carcinoma. J Clin Oncol 2014;32:1968–76. 10.1200/JCO.2012.45.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshihara K, Shahmoradgoli M, Martínez E, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun 2013;4:2612. 10.1038/ncomms3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson RH, Dong H, Lohse CM, et al. Pd-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res 2007;13:1757–61. 10.1158/1078-0432.CCR-06-2599 [DOI] [PubMed] [Google Scholar]
- 6.Şenbabaoğlu Y, Gejman RS, Winer AG, et al. Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger RNA signatures. Genome Biol 2016;17:231. 10.1186/s13059-016-1092-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorsson V, Gibbs DL, Brown SD, et al. The immune landscape of cancer. Immunity 2018;48:812–30. 10.1016/j.immuni.2018.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miao D, Margolis CA, Gao W, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 2018;359:801–6. 10.1126/science.aan5951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, et al. Bap1 loss defines a new class of renal cell carcinoma. Nat Genet 2012;44:751–9. 10.1038/ng.2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pervaiz A, Ansari S, Berger MR, et al. Ccr5 blockage by maraviroc induces cytotoxic and apoptotic effects in colorectal cancer cells. Med Oncol 2015;32:158. 10.1007/s12032-015-0607-x [DOI] [PubMed] [Google Scholar]
- 11.Velasco-Velázquez M, Jiao X, De La Fuente M, et al. Ccr5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res 2012;72:3839–50. 10.1158/0008-5472.CAN-11-3917 [DOI] [PubMed] [Google Scholar]
- 12.Velasco-Velázquez M, Pestell RG. The CCL5/CCR5 axis promotes metastasis in basal breast cancer. Oncoimmunology 2013;2:e23660. 10.4161/onci.23660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochoa-Callejero L, Pérez-Martínez L, Rubio-Mediavilla S, et al. Maraviroc, a CCR5 antagonist, prevents development of hepatocellular carcinoma in a mouse model. PLoS One 2013;8:e53992. 10.1371/journal.pone.0053992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas Research Network Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013;499:43–9. 10.1038/nature12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapur P, Peña-Llopis S, Christie A, et al. Effects on survival of BAP1 and PBRM1 mutations in sporadic clear-cell renal-cell carcinoma: a retrospective analysis with independent validation. Lancet Oncol 2013;14:159–67. 10.1016/S1470-2045(12)70584-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu Q, Xu L, Wang Y, et al. Tumor-Associated macrophage-derived interleukin-23 interlinks kidney cancer glutamine addiction with immune evasion. Eur Urol 2019;75:752–63. 10.1016/j.eururo.2018.09.030 [DOI] [PubMed] [Google Scholar]
- 17.Adeegbe DO, Liu Y, Lizotte PH, et al. Synergistic immunostimulatory effects and therapeutic benefit of combined histone deacetylase and bromodomain inhibition in non-small cell lung cancer. Cancer Discov 2017;7:852–67. 10.1158/2159-8290.CD-16-1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 2015;12:453–7. 10.1038/nmeth.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang N, Schröppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity 2009;30:458–69. 10.1016/j.immuni.2008.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Q, Cai M-Y, Weng D-S, et al. PD-L1 expression patterns in tumour cells and their association with CD8+ tumour infiltrating lymphocytes in clear cell renal cell carcinoma. J Cancer 2019;10:1154–61. 10.7150/jca.29052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheuermann JC, de Ayala Alonso AG, Oktaba K, et al. Histone H2A deubiquitinase activity of the polycomb repressive complex PR-DUB. Nature 2010;465:243–7. 10.1038/nature08966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bott M, Brevet M, Taylor BS, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet 2011;43:668–72. 10.1038/ng.855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiao X, Velasco-Velázquez MA, Wang M, et al. Ccr5 governs DNA damage repair and breast cancer stem cell expansion. Cancer Res 2018;78:1657–71. 10.1158/0008-5472.CAN-17-0915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song JK, Park MH, Choi D-Y, et al. Deficiency of C-C chemokine receptor 5 suppresses tumor development via inactivation of NF-κB and upregulation of IL-1ra in melanoma model. PLoS One 2012;7:e33747. 10.1371/journal.pone.0033747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oldham KA, Parsonage G, Bhatt RI, et al. T lymphocyte recruitment into renal cell carcinoma tissue: a role for chemokine receptors CXCR3, CXCR6, CCR5, and CCR6. Eur Urol 2012;61:385–94. 10.1016/j.eururo.2011.10.035 [DOI] [PubMed] [Google Scholar]
- 26.Liston A, Gray DHD. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol 2014;14:154–65. 10.1038/nri3605 [DOI] [PubMed] [Google Scholar]
- 27.Sakaguchi S, Vignali DAA, Rudensky AY, et al. The plasticity and stability of regulatory T cells. Nat Rev Immunol 2013;13:461–7. 10.1038/nri3464 [DOI] [PubMed] [Google Scholar]
- 28.Chang L-Y, Lin Y-C, Mahalingam J, et al. Tumor-derived chemokine CCL5 enhances TGF-β-mediated killing of CD8(+) T cells in colon cancer by T-regulatory cells. Cancer Res 2012;72:1092–102. 10.1158/0008-5472.CAN-11-2493 [DOI] [PubMed] [Google Scholar]
- 29.Ward ST, Li KK, Hepburn E, et al. The effects of CCR5 inhibition on regulatory T-cell recruitment to colorectal cancer. Br J Cancer 2015;112:319–28. 10.1038/bjc.2014.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JH, Patil AM, Choi JY, et al. CCR5 ameliorates Japanese encephalitis via dictating the equilibrium of regulatory CD4(+)Foxp3(+) T and IL-17(+)CD4(+) Th17 cells. J Neuroinflammation 2016;13:223. 10.1186/s12974-016-0656-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flynn M, Pickering L, Larkin J, et al. Immune-checkpoint inhibitors in melanoma and kidney cancer: from sequencing to rational selection. Ther Adv Med Oncol 2018;10:1758835918777427. 10.1177/1758835918777427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, Wang B, Qin J, et al. Blockade of CCR5-mediated myeloid derived suppressor cell accumulation enhances anti-PD1 efficacy in gastric cancer. Immunopharmacol Immunotoxicol 2018;40:91–7. 10.1080/08923973.2017.1417997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2019-000228supp001.pdf (908.9KB, pdf)