To the Editor,
In the majority of coronavirus disease 2019 (COVID-19) patients, respiratory mechanics is different from the “normal” acute respiratory distress syndrome (ARDS) patient. Plateau pressures and driving pressures are often low and respiratory system compliance relatively normal compared to the ARDS patient [1]. Many physicians use high positive end-expiratory pressure (PEEP) for patients with COVID-19 although the potential for recruitment is often low [1, 2]. We fear that the high compliance of the respiratory system in combination with high PEEP will lead to hyperinflation, high dead space, and potentially right ventricular failure.
We have used the following strategy for COVID-19 patients (N = 70): after intubation, immediately prone positioning for at least 3 days, using the lowest possible PEEP to obtain adequate oxygenation with FiO2 of 50%. We assessed the effects of different PEEP levels on respiratory mechanics and ventilation-perfusion mismatching.
Methods
Respiratory mechanics was assessed in COVID-19 patients admitted to the Radboud University Nijmegen Medical Center as part of standard patient care. Brief occlusions were performed to assess end-inspiratory and end-expiratory airway and transpulmonary pressures (absolute and elastance ratio method) and to calculate respiratory and lung compliances as previously described [3, 4]. Dead space ventilation was assessed using two methods:
The Bohr equation using partial pressure of carbon dioxide in alveolar air (PACO2) and mixed expired air (PeCO2): (PACO2 − PeCO2)/PACO2. See our previous work for detailed description [5].
The Enghoff modification of Bohr’s equation using partial pressure of carbon dioxide in arterial blood (PaCO2): (PaCO2 − PeCO2)/PaCO2. Therefore, shunt and diffusion limitations are taken into the equation.
Results
Advanced respiratory mechanics was assessed in 14 patients (8 males and 6 females, age (mean ± SEM) 67 ± 2 years, body mass index 28.0 ± 0.9 kg/m2) between the 19th of March and 2nd of April (Table 1). Compliance of the respiratory system was low (42 ± 3 mL/cmH2O) due to a lower than normal lung compliance (61 ± 5 mL/cmH2O). However, compared to ARDS patients, lung compliance was relatively high, resulting in low end-inspiratory transpulmonary pressures (12 ± 1 cmH2O). Chest wall compliance was slightly lower than normal due to prone positioning in most patients. COVID-19 patients had high dead space ventilation and gas exchange impairment (Bohr 52 ± 3%; Enghoff modification 67 ± 2%).
Table 1.
Respiratory mechanics
| Patient no. | MV days | FiO2 | PaO2/FiO2 (mmHg) | PaCO2 (mmHg) | Pplateau (cmH2O) | Pdrive (cmH2O) | PL,e-i | PL,drive (cmH2O) | Crs (mL/cmH2O) | CL (mL/cmH2O) | Enghoff (%) | Bohr (%) | Position |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 | 0.50 | 156 | 87 | 22 | 8 | 9 | 5 | 55 | 82 | – | – | P |
| 2 | 2 | 0.45 | 208 | 56 | 24 | 7 | 17 | 18 | 54 | 79 | – | – | S |
| 3 | 0.55 | 124 | 57 | 26 | 8 | – | – | 48 | – | 66 | 47 | S | |
| 3 | 0 | 0.50 | 228 | 44 | 23 | 9 | 17 | 16 | 47 | 62 | 66 | 56 | S |
| 4 | 1 | 0.60 | 123 | 44 | – | – | – | – | – | – | 71 | 58 | P |
| 5 | 0 | 0.40 | 214 | 48 | 23 | 13 | 9 | 9 | 40 | 54 | 55 | 42 | P |
| 1 | 0.40 | 278 | 44 | 18 | 10 | 7 | 8 | 50 | 64 | 48 | 38 | P | |
| 6 | 1 | 0.45 | 143 | 49 | – | – | – | – | – | – | 63 | 40 | P |
| 7 | 1 | 0.55 | 183 | 55 | 23 | 14 | 11 | 10 | 36 | 50 | 60 | 42 | P |
| 8 | 1 | 0.40 | 176 | 52 | 16 | 8 | 7 | 5 | 56 | 95 | 64 | 51 | P |
| 9 | 0 | 0.95 | 98 | 61 | 29 | 12 | 14 | 9 | 38 | 50 | – | – | P |
| 5 | 0.60 | 143 | 89 | 27 | 12 | 14 | 9 | 35 | 45 | 72 | 60 | P | |
| 10 | 1 | 0.80 | 125 | 53 | 21 | 10 | 11 | 7 | 36 | 49 | 66 | 52 | P |
| 11 | 2 | 0.55 | 147 | 49 | 21 | 12 | 11 | 10 | 40 | 51 | 69 | 47 | P |
| 12 | 2 | 0.75 | 113 | 59 | 25 | 11 | 11 | 8 | 26 | 37 | 69 | 57 | P |
| 3 | 0.65 | 111 | 47 | 26 | 12 | 11 | 8 | 27 | 40 | 71 | 60 | P | |
| 13 | 1 | 0.50 | 192 | 67 | 24 | 12 | 10 | 7 | 47 | 76 | 82 | 74 | P |
| 14 | 6 | 0.70 | 150 | 62 | 28 | 15 | 15 | 11 | 31 | 43 | 65 | 52 | P |
Crs compliance of respiratory system, CL lung compliance, MV days days of mechanical ventilation at the time of measurement, PL,e-i end-inspiratory transpulmonary pressure, PL,drive transpulmonary driving pressure, P prone position, S supine position
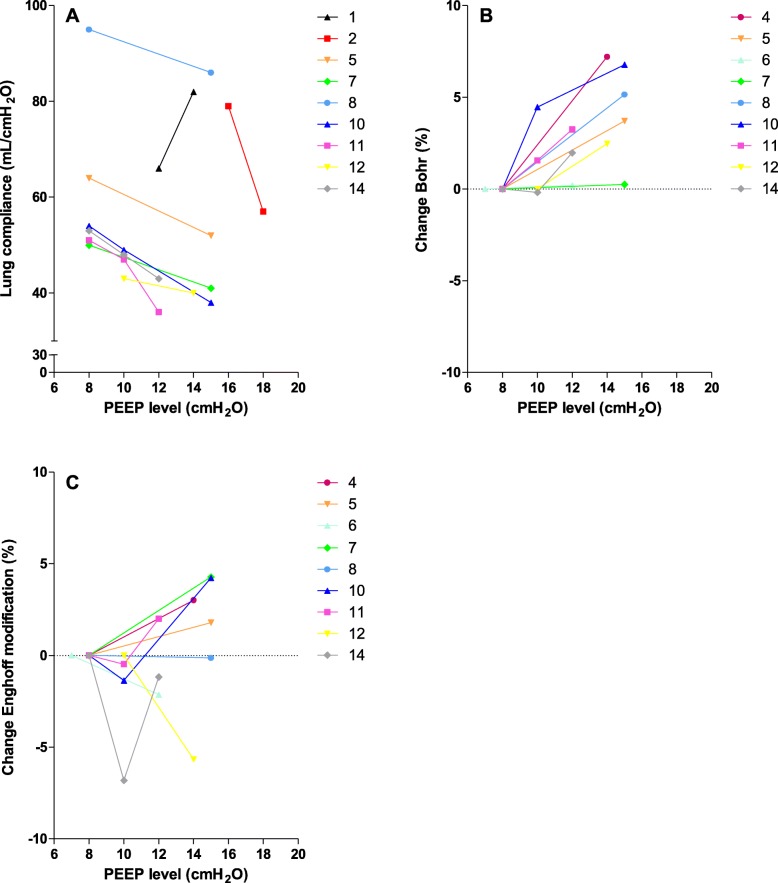
Reducing PEEP resulted in an increase in lung compliance and decrease in dead space ventilation, except for patient 1 (Fig. 1).
Fig. 1.
The effects of increasing positive end-expiratory pressure (PEEP) on lung compliance (N = 9) and dead space ventilation (N = 9). Every patient is depicted with a different symbol and color. a Lung compliance decreased with increasing PEEP levels in 8 patients. b Dead space ventilation according to Bohr increased in all patients with increasing PEEP levels. c In response to higher PEEP levels, dead space ventilation according to Enghoff modification (global gas exchange impairment) increased in 3 patients, first decreased and then increased in 3 patients, decreased in 2 patients, and had no effect in 1 patient
Discussion
We demonstrate that mechanically ventilated patients with COVID-19 have a relatively high lung compliance, high dead space ventilation, and gas exchange impairment. In almost all patients, lung compliance decreased and dead space ventilation increased with increasing PEEP levels.
The decrease in lung compliance and increase in dead space ventilation in response to higher PEEP levels indicate that COVID-19 lesions were not recruited and that higher PEEP levels cause hyperinflation of the more compliant parts of the lung [1]. These results are in accordance with recent findings in COVID-19 patients [2].
When lung compliance increases in response to higher PEEP levels (patient 1), recruitment is likely and PEEP should be set accordingly [1, 2].
All patients responded extremely well to prone positioning, although the exact mechanism is unclear. Redistribution of blood flow seems to be an important mechanism.
In conclusion, we show that higher PEEP levels decrease lung compliance and in most cases increase dead space ventilation, indicating that high PEEP levels probably cause hyperinflation in patients with COVID-19. We suggest using prone position for an extended period of time (e.g., 3–5 days) and apply lower PEEP levels as much as possible.
Acknowledgements
Not applicable.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- COVID-19
Coronary virus disease 2019
- PACO2
Partial pressure of carbon dioxide in alveolar air
- PaCO2
Partial pressure of carbon dioxide in arterial blood
- PeCO2
Partial pressure of carbon dioxide in mixed expired air
- PEEP
Positive end-expiratory pressure
- SEM
Standard error of the mean
Authors’ contributions
Data acquisition: LR. Data analysis: LR. Data interpretation: all authors. Manuscript drafting and revising: all authors. The authors read and approved the final manuscript.
Funding
There was no financial funding.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Due to standard patient care and the urgent need to gain knowledge about this new lung disease, informed consent was deemed unnecessary, but also not feasible in most cases.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 2.Pan C, Chen L, Lu C, Zhang W, Xia JA, Sklar MC, et al. Lung Recruitability in SARS-CoV-2 associated acute respiratory distress syndrome: a single-center, observational study. Am J Respir Crit Care Med. 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 3.Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189(5):520–531. doi: 10.1164/rccm.201312-2193CI. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida T, Amato MBP, Grieco DL, Chen L, Lima CAS, Roldan R, et al. Esophageal manometry and regional transpulmonary pressure in lung injury. Am J Respir Crit Care Med. 2018;197(8):1018–1026. doi: 10.1164/rccm.201709-1806OC. [DOI] [PubMed] [Google Scholar]
- 5.Doorduin J, Nollet JL, Vugts MP, Roesthuis LH, Akankan F, van der Hoeven JG, et al. Assessment of dead-space ventilation in patients with acute respiratory distress syndrome: a prospective observational study. Crit Care. 2016;20(1):121. doi: 10.1186/s13054-016-1311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.