Minotti Chiara and colleagues recently published a systematic review that investigated the current knowledge on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cases in children and adults with immunosuppression and concluded that immunosuppressed patients with Coronavirus disease 2019 (COVID-19) seem to be few in relation to the overall figures and to present a favorable outcome as compared to other comorbidities.1 We congratulate and applaud Minotti et al’ important work, but this study drew conclusions only based on the systematic review without a meta-analysis. Therefore, we conducted a systematic review and meta-analysis to quantitatively assess whether immunosuppression and immunodeficiency are associated with increased risk of severe disease and mortality in patients with COVID-19.
We searched the PubMed, EMBASE.com, Web of Science, the Cochrane Central Register of Controlled Trials (CENTRAL), China National Knowledge Infrastructure (CNKI), Chinese Biomedical Literature Database (CBM), and Wanfang Database up to April 25, 2020. The following terms were used for the search: “COVID-19”, “coronavirus disease-19”, “new coronavirus”, “2019-nCoV”, “novel corona virus”, “novel coronavirus”, “nCoV-2019”, “2019 novel coronavirus”, “coronavirus disease 2019”, “SARS-CoV-2”, “severe acute respiratory syndrome coronavirus 2”, “immunosuppression”, “immunosuppressive”, “immunodeficiency”, “HIV”, “clinical characteristic”, “clinical feature”, “risk factor”, and “comorbidities”. Reference lists of eligible studies and relevant systematic reviews were manually searched for potentially eligible studies.
We included studies that met the following criteria: (1) patients have a laboratory-confirmed diagnosis of COVID-19; (2) provided data of immunosuppression, immunodeficiency, or human immunodeficiency virus (HIV) between patients with severe or non-severe disease or between non-survivors and survivors; (3) studies published in Chinese and English. We excluded following studies: (1) studies with a sample size of no more than 10 patients; (2) studies did not provide the prevalence of immunosuppression, immunodeficiency, or HIV; (3) studies without comparisons (e.g. severe versus non-severe patients); (4) review articles, abstracts, letters, and editorials. In the current analysis, we considered a severe disease as patients experiencing acute respiratory distress syndrome (ARDS), requiring vital life support, requiring mechanical ventilation, or requiring intensive care unit admission (ICU) support.2
The primary outcome was the association between immunosuppression or immunodeficiency and risk of severe disease in patients with COVID-19. The secondary outcome was the association between immunosuppression or immunodeficiency and risk of mortality in COVID-19 patients.
Two reviewers conducted study selection and data extraction. Disagreements were resolved by consensus or by a discussion with a third reviewer. The abstracted data included: first author, year of publication, country of the corresponding author, publication language, recruitment time frame, age and sex of patients, sample size, and outcomes of interest.
Stata 13.0 (Stata Corporation, College Station, Texas, USA Stata) was used to estimate pooled odds risk (OR) and its 95% confidence interval (CI) for dichotomous outcomes using the Mantel-Haenszel statistical method with the random-effects model. Statistical heterogeneity was evaluated using I2 statistic, and values of <25%, 26-50%, and >50% considered as low, moderate, and high degrees of heterogeneity, respectively. Subgroup analysis was conducted for the primary outcome between different countries. We also performed sensitivity analyses by excluding studies published in Chinese to assess the stability of results.
2176 records were obtained through systematic electronic searches. After screening titles, abstracts, and full texts, 8 studies3., 4., 5., 6., 7., 8., 9., 10. were included for analysis. All the included studies were published in 2020, incorporated a total of 4007 patients (2256 males) between December 11, 2019 and April 15, 2020. Two studies3 , 9 published in Chinese, 6 studies4., 5., 6., 7., 8. , 10 published in English, 7 studies3., 4., 5., 6., 7., 8., 9. from China, and 110 study from the USA. The sample size per study ranged from 79 to 1,590 (Table 1 ).
Table 1.
Characteristics of included studies.
| Study | Country | Language | Recruitment time frame | Sample | Age, yearsa | Sex |
Immunosuppression | Immunodeficiency/HIV | |
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | ||||||||
| Fang XW3 | China | Chinese | 2020.1.22-2020.2.18 | 79 | 45.1(16.6) | 45 | 34 | 1(1.27%) | |
| Feng Y4 | China | English | 2020.1.1-2020.2.15 | 476 | 53(40-64) | 271 | 205 | 7(1.47%) | |
| Zhang GQ5 | China | English | 2020.1.2-2020.2.10 | 221 | 55(39-66.5) | 108 | 113 | 3(1.36%) | |
| Guan WJ6 | China | English | 2019.12.11-2020.1.31 | 1590 | 48.9(16.3) | 904 | 686 | 3(0.19%) | |
| Wang DW7 | China | English | 2020.1.1-2020.1.28 | 138 | 56 (42-68) | 75 | 63 | 2(1.45%) | |
| Wu J8 | China | English | 2020.1.20-2020.2.19 | 280 | 43.1(19.0) | 151 | 129 | 1(0.36%) | |
| Yuan J9 | China | Chinese | 2020.1.24-2020.2.23 | 223 | 46.5(16.1) | 106 | 117 | 1(0.45%) | |
| Argenziano MG10 | USA | English | 2020.3.1-2020.4.15 | 1000 | 61.7(17.5) | 596 | 404 | 21(2.10%) | |
Age data presented as median (IQR) or mean (SD). HIV: human immunodeficiency virus.
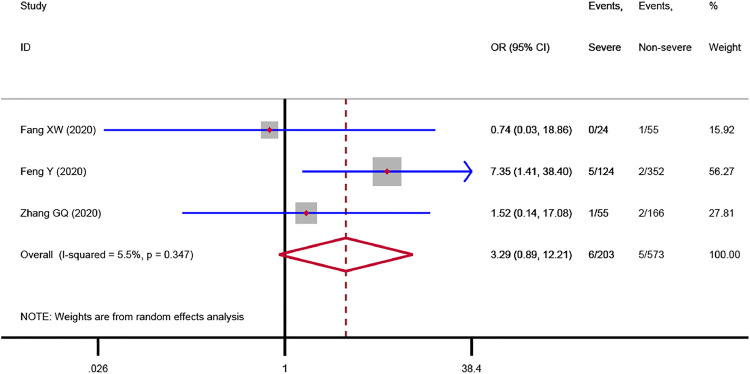
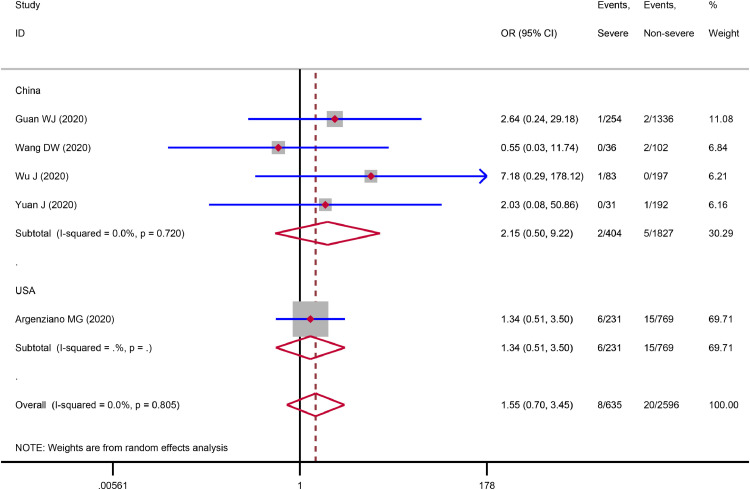
The meta-analysis showed that immunosuppression was associated with a 3.29-fold increased risk of severe COVID-19 disease (3 studies,3., 4., 5. 776 patients; OR = 3.29, 95%CI: 0.89 to 12.21, P = 0.075; I2 = 5.5%), although the statistical difference was not significant (Fig. 1 ). Sensitivity analysis indicated the result (OR = 4.32, 95%CI: 1.00 to 18.64) did not change substantially after excluding the Chinese study.3 We found that immunodeficiency was associated with a 1.55-fold increased risk of severe COVID-19 disease (5 studies,6., 7., 8., 9., 10. 3,231 patients; OR = 1.55, 95%CI: 0.70 to 3.45, P = 0.285; I2 = 0.0%), but the statistical difference was not significant (Fig. 2 ). Sensitivity analysis by excluding the Chinese study9 showed a similar result (OR = 1.52, 95%CI: 0.67 to 3.47). The subgroup analysis based on countries indicated the association between immunodeficiency and severe COVID-19 disease in China (OR = 2.15, 95%CI: 0.50 to 9.22) was stronger than that in the USA (OR = 1.34, 95%CI: 0.51 to 3.50), Fig. 2. One study6 involving 1,590 patients reported the data on immunodeficiency between dead and surviving COVID-19 patients. The result revealed that there was no correlation (P = 1.000) between immunodeficiency and the risk of death in patients with COVID-19.
Fig. 1.
Association between immunosuppression and severe COVID-19 disease
Fig. 2.
Association between immunodeficiency and severe COVID-19 disease
Our study showed that immunosuppression and immunodeficiency were associated with the increased risk of severe COVID-19 disease, although the statistical differences were not significant. These findings suggest that healthcare professionals need to be alert to the increased risk of serious diseases associated with COVID-19 infection in patients with immunosuppression and immunodeficiency/HIV. In response to the COVID-19 pandemic, special preventive and protective measures should be provided for patients with immunosuppression and immunodeficiency/HIV. Future researchers should focus on the treatment and management strategies for patients with immunosuppression and immunodeficiency/HIV during the COVID-19 pandemic, as well as the care and treatment strategies for COVID-19 patients with immunosuppression and immunodeficiency/HIV. However, our study was limited by small sample size, the results should be interpreted with caution. As more data becomes available, these findings should be re-analyzed to provide more reliable evidence.
In conclusion, immunosuppression and immunodeficiency were associated with increased risk of severe COVID-19 disease, although the statistical differences were not significant. Further high-quality studies are needed to provide robust evidence of the association between immunosuppression and immunodeficiency and COVID-19.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Declarations
Funding: This work was supported by the Emergency Research Project of Key Laboratory of Evidence-based Medicine and Knowledge Translation of Gansu Province (Grant No. GSEBMKT-2020YJ01).
Role of the Funding Source: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Minotti C., Tirelli F., Barbieri E., Giaquinto C., Donà D. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? A systematic review. The Journal of infection. 2020 doi: 10.1016/j.jinf.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020 doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 3.Fang X.W., Mei Q., Yang T.J., Zhang L., Yang Y., Wang Y.Z. [Clinical characteristics and treatment strategies of 79 patients with COVID-19] Chinese Pharmacological Bulletin. 2020;36(4):453–459. [Google Scholar]
- 4.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J. COVID-19 with Different Severity: A Multi-center Study of Clinical Features. American journal of respiratory and critical care medicine. 2020 doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2020;127 doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M. Comorbidity and its impact on 1590 patients with Covid-19 in China: A Nationwide Analysis. The European respiratory journal. 2020;55(5) doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J., Li W., Shi X., Chen Z., Jiang B., Liu J. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) Journal of internal medicine. 2020 doi: 10.1111/joim.13063. [DOI] [PubMed] [Google Scholar]
- 9.Yuan J., Sun Y.Y., Zuo Y.J., Chen T.Y., Cao Q., Yuan G.D. [A Retrospective Analysis of the Clinical Characteristics of 223 NCP Patients in Chongqing] Journal of Southwest University (Natural Science Edition) 2020;42:17–24. [Google Scholar]
- 10.Argenziano M.G., Bruce S.L., Slater C.L., Tiao J.R., Baldwin M.R., Barr R.G. Characterization and Clinical Course of 1000 Patients with COVID-19 in New York: retrospective case series. medRxiv. 2020 doi: 10.1136/bmj.m1996. 2020.04.20.20072116. [DOI] [PMC free article] [PubMed] [Google Scholar]