Dear Editor,
We read with great interest in a recent article by Liu, et al.1 on the clinical and CT findings of pregnant patients and children with COVID-19. It is clinically oriented, and of great value to the medical workers on the frontline. It revealed that the clinical symptoms of pregnant women were atypical, despite unavailable data about pregnancy outcome in the study. We mainly focused on the pregnancy outcome in patients with COVID-19. It seems that SARS-CoV-2 would be more friendly than its members of the coronavirus family,2 such as SARS-CoV-1 and MERS-CoV, which caused severe maternal and neonatal complications.3 Currently, it is too early yet to explicitly determine the effects of SARS-CoV-2 on pregnant women and their fetuses.4 Here we explored the impact on pregnancy in patients with COVID-19 from multiple medical centers outside Wuhan, China.
We retrospectively analyzed data from 8 pregnant patients who were laboratory-confirmed from January 24 to February 19, 2020. A detailed analysis of clinical features was shown in Table 1 . The age range was 27–33 years. Two (20%) patients had uterine scarring and one patient was twin pregnancy. Five patients (62.5%) developed mild symptoms; three patients (37.5%) showed severe or critical illness requiring ICU admission, one of which undergone ECMO support; four patients (50%) were performed emergency deliveries because of fetal distress or premature rupture of the membrane (PROM). Specially, patient 6 with twin pregnancy had preeclampsia with high blood pressure of 180/100 mmHg and later developed into eclampsia; patient 7 presented with mild symptoms at first and her condition deteriorated rapidly within 6 h after admission, with severe complications including septic shock, septic cardiomyopathy, ARDS, MODS, requiring intubation and mechanical ventilation. Six livebirths and one stillbirth were analyzed. Half of the livebirths were premature and admitted to NICU; one twin died at the 18 days of birth with severe pneumonia, referred to “white lung”; another twin showed suspected viral pneumonia on chest CT (Fig. 1 ) and survived after treatment. RT-PCR tests of throat swab specimens for all livebirths were negative.
Table 1.
Characteristics, maternal and neonatal outcomes from patients of COVID-19.
| Patient | Patient | Patient | Patient | Patient | Patient | Patient | Patient | |
|---|---|---|---|---|---|---|---|---|
| Characteristics | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Age (years) | 29 | 27 | 28 | 33 | 29 | 29 | 32 | 32 |
| Gravida, Parity | G3P1 | G1P0 | G2P0 | G4P1 | G2P1 | G2P0 | G5P2 | G3P1 |
| Gestational weeks | 30 | 34 | 39+3 | 38 | 37+4 | 31+2 | 35+2 | 28+1 |
| Contact history | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Medical history | None | None | None | Uterine scarring | None | Twin pregnancy | Uterine scarring | None |
| Complications | Mild anemia | None | None | None | Mild anemia | HF, RF, Mild anemia Eclampsia, PROM | Septic shock, SICM ARDS, MODS | Moderate anemia |
| Delivery mode | Ongoing pregnancy | Ongoing pregnancy | Cesarian section | Cesarian section | Emergency Caesarean section | Emergency Vaginal delivery | Emergency Caesarean section | Emergency Caesarean section |
| Signs and symptoms | ||||||||
| Fever ( °C) * | 36.3 | 36.6 | 37.1 | 37.7 | 38.6 | 39.1 | 39.3 | 38.9 |
| Cough | + | + | + | + | − | + | + | + |
| Rhinorrhea | − | − | − | + | + | − | + | − |
| Sputum production | − | + | + | − | − | − | − | − |
| Pharyngalgia | + | − | − | − | − | − | + | − |
| Myalgia or fatigue | − | + | − | − | − | − | + | + |
| Dyspnea | − | − | − | − | − | + | + | + |
| SpO2 | 99% | 98% | 98% | 95% | 98% | 82% | 94% | 93% |
| Blood Pressure | 103/68 | 134/100 | 118/84 | 110/77 | 114/76 | 180/110 | 100/50 | 113/74 |
| Pulse | 78 | 75 | 100 | 95 | 101 | 136 | 140 | 102 |
| Respiratory rate | 18 | 19 | 16 | 21 | 19 | 24 | 35 | 25 |
| Fetal distress | No | No | No | No | Yes | Yes, Yes | Yes | Yes |
| Laboratory Results† | ||||||||
| WBC (× 109/L) | 7.83 | 6.98 | 7.6 | 6.98 | 6.48 | 13.16 | 6.8 | 14.48 |
| NEUT (× 109/L) | 6.25 | 5.07 | 5.5 | 5.07 | 5.18 | 11.02 | 2.28 | 12.75 |
| LY (× 109/L) | 1.11 | 1.53 | 1.19 | 1.53 | 0.01 | 1.31 | 0.884 | 1.09 |
| Eosinophils (× 109/L) | 0.06 | 0.02 | 0.00 | 0.02 | 0.01 | 0.00 | 0.00 | 0.03 |
| HGB (g/L) | 102 | 134 | 133 | 125 | 102 | 108 | 110 | 99 |
| D-dimer (μg/mL) | 0.62 | 1.09 | 0.40 | 0.52 | 1.41 | 3.76 | 2.89 | 0.95 |
| ALT (U/L) | 18 | 26 | 29 | 13 | 11 | 19.1 | 137 | 17 |
| AST (U/L) | 10.9 | 29 | 22 | 24 | 18 | 47.9 | 190 | 29 |
| LDH (U/L) | 155 | None | None | None | None | 450 | 529 | 276 |
| CK (U/L) | 20 | 66 | 55 | 46 | None | 258 | 190 | 23 |
| Creatinine (μmol/L) | 47 | 72.3 | 47 | 63.2 | None | 27.9 | 85 | 38 |
| BUN (mmol/L) | 4.0 | 3.6 | 5.4 | 1.27 | None | 3.75 | 2.3 | 5.1 |
| CRP (mg/L) | 1.3 | 18.4 | 2.4 | 55.8 | 8.43 | 73.6 | >200.0 | 62.37 |
| Procalcitonin (ng/ml) | 0.050 | 0.270 | 0.136 | 0.072 | None | 3.580 | 26.800 | 0.31 |
| Delivery outcomes | ||||||||
| Umbilical cord | None | None | A nuchal cord | Normal | Normal | Normal | Normal | Normal |
| Placenta | None | None | Normal | Normal | Normal | Normal | Normal | Normal |
| Amniotic fluid | None | None | Normal | Normal | Normal | Normal | Normal | Opacity, Hypamnion |
| Maternal outcomes | Survived | Survived | Survived | Survived | Survived | Survived | Survived | Survived |
| Neonatal outcomes | ||||||||
| Gestational age | None | None | 39+3 | 38+4 | 37+5 | 31+2 | 35+2 | 28+1 |
| Birthweight (g) | None | None | 4200 | 2367 | 2585 | 1520,1720 | 2700 | 1530 |
| Apgar score (1,5/min) | None | None | 9, 10 | 10, 10 | 8, 9 | 8,8 and 8,8 | 1,1 | 8,9 |
| Severe neonatal asphyxia | None | None | No | No | No | Yes, Yes | Yes | Yes |
| Neonatal death | None | None | No | No | No | Yes, No | Yes | No |
| Fetal death or stillbirth | None | None | No | No | No | No, No | Yes | No |
| Admitted to NICU | None | None | No | No | No | Yes, Yes | No | Yes |
| RT-PCR test | None | None | Negative | Negative | Negative | Negative, Negative | None | Negative |
Abbreviations.
PROM, Premature rupture of membrane. MODS, Multiple organ dysfunction syndrome. ARDS, Acute Respiratory Distress Syndrome. SCIM, Septic induced ischemic cardiomyopathy. HF, Heart failure. RF, Respiratory failure. BUN, Blood urea nitrogen.
RT-PCR, Reverse transcription polymerase chain reaction.
Shown are the highest intrafebrile temperature.
Reference ranges are as follows: WBC, 3.5 × 109 to 9.5 × 109/L; NEUT,1.8 × 109 to 6.3 × 109/L; LY, 1.1 × 109 to 3.2 × 109/L; Eosinophils, 0.02 × 109 to 0.032 × 109/L; HGB, 115 to 150 g/L; d-dimer, 0 to 0.5 μg/mL; ALT, 7 to 40 U/L; AST, 13 to 40 U/L; LDH 120 to 250 U/L; CK, 40 to 200 U/L; Creatinine, 41 to 73 μmol/L; BUN, 2.6 to 7.5 mmol/L; CRP, 0.068 to 8.2 mg/L; Procalcitonin, 0 to 0.046 ng/ml.
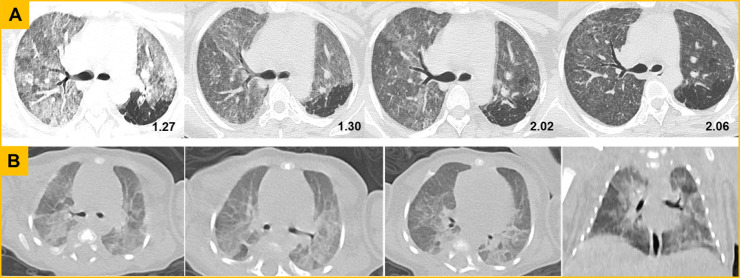
Fig. 1.
Chest CT screening from the mother (patient 6) and her twin neonate. (A) CT findings from the mother. The first axial image showed extensive ground-glass opacities (GGO) and nodules. On the following days, the intensity decreased, indicating the lesions were gradually absorbed after effective treatment. (B) CT findings from the twin neonate. The axial and coronal images at the nineteenth day of birth presented with extensive GGO along the bronchovascular bundle or in the peripheral area, and the localized consolidation in dorsal segment of right lower lobe.
The cases here highlight three issues that worth stressing. Firstly, unlike previous reports about the favorable outcomes, SARS-CoV-2 might have similar behavior to SARS and MERS, resulting in severe maternal and neonatal outcomes including preterm delivery, PROM, fetal distress, stillborn or neonatal death, admission to ICU or NICU, undergoing endotracheal intubation, septic shock, eclampsia, and MODS. Secondly, those who are older or those who have medical histories such as hypertension and cardiovascular disease tend to fare worse,5 other than that, immunocompromised status and physiological adaptive changes during pregnancy might contribute to the rapid deterioration into severe or critical illness. Considering the unfavorable outcomes in pregnant patients, any suspected cases during pregnancy should be systematic screening; closely follow-up for mothers and their fetuses after diagnosis should be emphasized. Finally, despite all livebirths were tested negative for SARS-CoV-2, the stillbirth and one neonatal death at 18-days of birth indicated the potential risk of intrauterine infection.6 There should be more evidence to deep investigation in the future study.
The limitation of our analysis is the absence of data on the amniotic fluid, cord blood, vaginal secretion, and breastmilk samples, as all resources were stretched in a pandemic. Moreover, no data about patients at the first or second trimester was reported, since SARS-CoV-2 infection in different trimester might be associated with different outcomes.
In conclusion, SARS-CoV-2 infection during late pregnancy would have severe maternal and neonatal complications, even the neonatal death. Efforts to limit exposure of pregnant women should be strengthened during the outbreak of COVID-19.
References
- 1.Liu H., Liu F., Li J. Clinical and CT imaging features of the COVID-19 pneumonia: Focus on pregnant women and children. J Infect. 2020;80:e7–e13. doi: 10.1016/j.inf.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H., Guo J., Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. The Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assiri A., Abedi G.R., Al Masri M. Middle east respiratory syndrome coronavirus infection during pregnancy: a report of 5 cases from Saudi Arabia. Clin Infect Dis. 2016;63:951–953. doi: 10.1093/cid/ciw412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo Y., Yin K. Management of pregnant women infected with COVID-19. Lancet Infect Dis. 2020;20:513–514. doi: 10.1016/S1473-3099(20)30191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong L., Tian J., He S. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020 doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]