Abstract
Global health care is experiencing an unprecedented surge in the number of critically ill patients who require mechanical ventilation due to the COVID-19 pandemic. The requirement for relatively long periods of ventilation in those who survive means that many are considered for tracheostomy to free patients from ventilatory support and maximise scarce resources. COVID-19 provides unique challenges for tracheostomy care: health-care workers need to safely undertake tracheostomy procedures and manage patients afterwards, minimising risks of nosocomial transmission and compromises in the quality of care. Conflicting recommendations exist about case selection, the timing and performance of tracheostomy, and the subsequent management of patients. In response, we convened an international working group of individuals with relevant expertise in tracheostomy. We did a literature and internet search for reports of research pertaining to tracheostomy during the COVID-19 pandemic, supplemented by sources comprising statements and guidance on tracheostomy care. By synthesising early experiences from countries that have managed a surge in patient numbers, emerging virological data, and international, multidisciplinary expert opinion, we aim to provide consensus guidelines and recommendations on the conduct and management of tracheostomy during the COVID-19 pandemic.
Introduction
The COVID-19 pandemic has led to an unprecedented increase in the number of patients who are critically ill and require mechanical ventilation. Although severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is associated with lower mortality than the related viruses that cause severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome, it has higher infectivity and rates of transmission.1 SARS-CoV-2 has spread far more widely and rapidly than these other viruses, resulting in catastrophic loss of life globally. Hospitals are overwhelmed, and medical professionals must make difficult decisions regarding the care of patients who are critically ill.
Tracheostomy is a common procedure in critically ill patients who require an extended period of time on mechanical ventilation. Use of tracheostomy can facilitate weaning from ventilation and potentially increase the availability of intensive care unit (ICU) beds. When the COVID-19 pandemic spread to Italy and Spain, ICUs had a massive influx of patients who were critically ill, many of whom became candidates for tracheostomy. However, tracheostomy is an aerosol-generating procedure, so health-care workers are at risk of infection during insertion and subsequent care, even when appropriate personal protective equipment (PPE) is used. Aerosol-generating procedures were identified as a leading cause of viral transmission during the SARS outbreak in 2003, with super-spreading events occurring throughout hospitals in Hong Kong, China, and Canada.2 Reports of infections related to aerosol-generating procedures have also emerged in the current pandemic.3
Although global community-based strategies to manage the impact of COVID-19 are a priority for the general population, tracheostomy is one of a number of important clinical considerations for the optimal management of patients who are critically ill during the pandemic.4 We aim to provide authoritative guidance for health-care providers and health-care systems, highlighting the range of considerations for tracheostomy during the current COVID-19 pandemic, by synthesising experience, currently available evidence, and lessons from history. Owing to the urgent need for guidance and the lack of robust ICU outcome data, we make pragmatic recommendations and suggestions primarily on the basis of international, multidisciplinary expert opinion.
COVID-19 in the context of previous epidemics
In response to the threat of Avian influenza, caused by the influenza A H5N1 virus, in 2005, a great deal of pandemic preparedness planning began, with a view not only to treating patients, but also to mitigating the spread of a lethal and easily transmitted respiratory infection. Some of these methods would only be required in worst-case scenarios, such as that seen during the influenza pandemic of 1918–19, because they are socially and economically disruptive. The hypothesis behind community mitigation has now become popularly known as “flattening the curve”.5 By delaying the peak of the epidemic, less strain is put on health-care capacity, and the burden on hospitals and infrastructure is reduced. In turn, the overall case numbers and health impacts might be reduced, allowing more time for the development of better medical therapies and preventive vaccines. An evaluation of these strategies in the context of the 1918–19 influenza pandemic indicated that cities that acted early had much lower morbidity and mortality than those that did not.6
As we apply these community mitigation strategies on a global level that has never been seen before in human history, future evaluation will determine their effect on the impact of the COVID-19 pandemic. Tracheostomy was an essential clinical strategy for managing epidemics associated with respiratory failure during the 20th century, including those of poliomyelitis and diptheria,4 and we hope that the careful conduct and management of tracheostomy during the current pandemic will help to reduce the impact of COVID-19.
Methods
We (BAM, MJB, SJW, VP, AA, NT, and DJF-K) recruited and convened an international tracheostomy consensus working group by identifying individuals with relevant expertise in tracheostomy and previous experience in the development of guidelines or consensus documents. The group has international, multidisciplinary representation, including critical care, anaesthesiology, pulmonology, otolaryngology, nursing, respiratory (physio) therapy, speech and language pathology, virology and immunology, medical ethics, medical history, and patient and family stakeholders.
The project scope was determined through a review of available consensus statements on tracheostomy care (appendix pp 4–7). We did a literature and internet search to identify and extract data from primary sources, and gathered first-hand accounts of tracheostomy during the current pandemic from discussion among working group members. We gathered additional information by dissemination of targeted questions and data collection forms, and a Qualtrics-XM survey (SAP Walldorf, Germany) that allowed for ranking, scoring, and free-text response completed by members of the consensus working group (the survey is registered with the Institutional Review Board in compliance with University of Michigan institutional policy [HUM00182021]).
The consensus working group recommendations in this Health-care Development paper are expert opinions (denoted by “we suggest” throughout the paper) informed by the best available evidence, or published supporting statements (denoted by “we recommend”). We used an iterative approach, adapted from the Delphi method, to prioritise topics for inclusion and to reach consensus on recommendations. The first round consisted of a core writing group (BAM, MJB, SJW, VP, AA, NT, and DJF-K) that drafted distinct statements as part of the paper, making recommendations on case selection, timing, performance of tracheostomy, and management after tracheostomy, on the basis of the primary data sources retrieved. These recommendations were circulated among all consensus working group members, inviting both opinion and any additional recommendations. The second round involved circulating all new recommendations received, along with those recommendations from the first round that had received favourable comment from the majority (over half) of the respondents (appendix pp 2–3). A final review of the recommendations was made during a videoconference, which included 29 of the group members, followed by electronic correspondence and telephone discussions, facilitating iterative review and refinement over three rounds to achieve consensus agreement for the content of this paper.
Patient selection for tracheostomy
First we considered the role of tracheostomy in critical illness and respiratory failure. Approximately 8–13% of patients admitted to modern ICUs who require mechanical ventilation have a tracheostomy.7 The major indication for tracheostomy remains the facilitation of mechanical ventilation for a long period, while minimising complications from a translaryngeal endotracheal tube and weaning from ventilation. Tracheostomy might also be required for actual or threatened airway obstruction, laryngeal oedema (which might be an emerging feature of COVID-19)8 or unsuccessful extubation due to weakness, poor cough, tenacious secretions, or a combination of these factors. Decision making around access to critical care and tracheostomy during the COVID-19 pandemic is based mainly on existing standards of practice, although the evidence base for tracheostomy timing in those who are critically ill is not substantial.9 Tracheostomy in the context of critical illness is not always in the patient's best interests. Among patients without COVID-19 who require tracheostomy after an extended period of mechanical ventilation, at least half do not survive for more than 1 year, and at 1 year fewer than 12% are at home and functionally independent.10 Similarly, tracheostomy for patients with COVID-19 might not always be beneficial, and the procedure and subsequent care puts health-care workers at increased risk of SARS-CoV-2 infection. Patients and surrogates need information and discussion in the context of a multidisciplinary team about trade-offs, challenges, and the outcomes of tracheostomy; that tracheostomy in this context will often be followed by long periods of functional dependency and rehabilitation should be explained. These decisions might become more important in an overwhelmed health-care system with few resources to care for patients who are critically ill, recovering, or highly dependent. Although rationing in health care is not unprecedented, in the modern age we have never before been faced with the prospect of having to ration medical goods and services on such a large scale. Decision makers might have to consider the appropriateness of embarking on a tracheostomy, with the associated health-care resources, in the context of shortages of staff, equipment, medications, and facilities. An independent triage or ethics committee could help to guide decisions, communicate with patients and their relatives, and reduce the burden on frontline staff.11
Timing of tracheostomy
Emerging virological data
A systematic review comparing health-care workers who did an aerosol-generating procedure with those who did not during the 2003 SARS outbreak found an increased risk of contracting SARS in those who did a tracheal intubation (odds ratio 6·6 [95% CI 2·3–18·9]) and tracheostomy (4·2 [1·5–11·5]), and those who put patients on non-invasive ventilation (3·1 [1·4–6·8]) and manual ventilation before intubation (2·8 [1·3–6·4]).2 Although data on SARS-CoV-2 infectivity are scarce, infection and death among health-care workers have been reported.3, 12
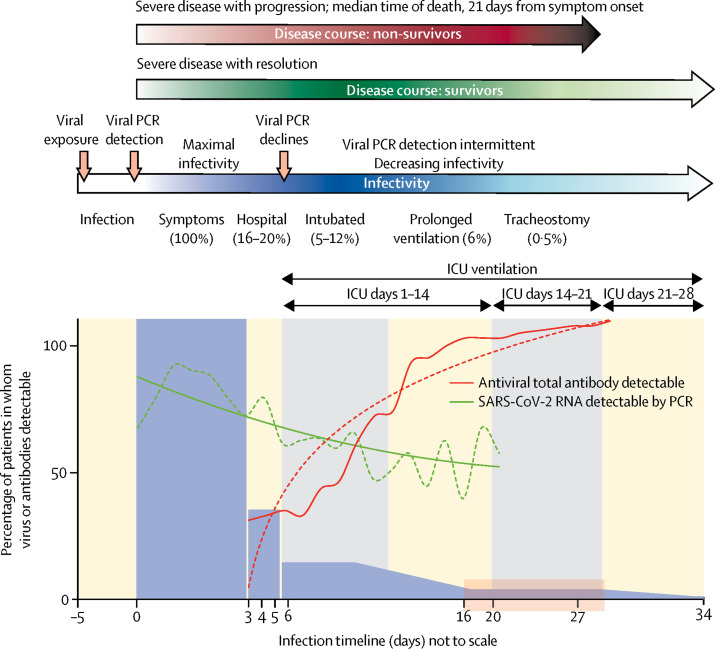
The median time from SARS-CoV-2 exposure to onset of symptoms (incubation period) is approximately 5 days (range 4–14).13, 14 SARS-CoV-2 is normally most abundant around the time of symptom onset, as determined by PCR of viral RNA from mucosal samples from the upper respiratory tract. After symptom onset, viral load typically decreases over the following 3–4 days.15 In most patients, samples from the lower respiratory tract remained PCR-positive for SARS-CoV-2 after samples from the upper respiratory tract had become negative, for up to 39 days.16 In patients with severe disease, the viral RNA load is significantly higher and decreases more slowly than in those with mild disease.17, 18
The immune response (antiviral antibody) typically appears both in the respiratory secretions and in the blood around 7 days after symptom onset, and is detectable in 90% of patients by 12 days after symptom onset.17 The presence of antibody inhibits the infectivity of detectable virus. The presence of viral RNA detected by PCR (so-called viral shedding), does not necessarily indicate infectivity, especially in the presence of antiviral antibodies. True infectivity can only be assessed by viral culture in cells in vitro, or be inferred from clinical or epidemiological data.
Detailed analysis of nine individuals who developed COVID-19 established virus replication culture at several anatomical sites.19 Pharyngeal viral RNA peaked during the first week of symptoms, reaching 7 × 108 copies per throat swab on day 4, persisting beyond the duration of symptoms. By cell culture, infectious viruses were present in samples from the throat and lungs, but not from stool (despite high viral RNA concentrations in faeces); infectious virus was never detected in blood or urine. Serum antibodies were detected after 7 days in half of cases, and in all individuals by day 14. All individuals had mild disease courses. The authors of this study predicted little residual risk of infectivity beyond 10 days after symptom onset, when the patient had less than 100 000 viral RNA copies per mL of sputum.19
A timeline of the typical clinical course of severe SARS-CoV-2 infection is shown in figure 1 , based on authors' local data and published case series.13, 17, 19, 20, 21, 22, 23 Further studies are required to define the immune response to SARS-CoV-2 in critically ill patients and in those with comorbidities and those who are immunocompromised, and to establish the viral burden that various aerosol-generating procedures generate in these patients.
Figure 1.
Typical clinical course, viral PCR, and antiviral antibody detection and infectivity of severe SARS-CoV-2 infection
The transparent red box shows the suggested window for tracheostomy, on ICU days 10–21, which corresponds with 16–30 days from symptom onset. The solid bars and curves represent the proportion of all cases. Time zero is symptom onset (the x-axis is not to scale). Timeline data are from authors' local data and published case series.13, 17, 19, 20, 21, 22 Pooled data from two studies describing SARS-CoV-2 detection by PCR and antiviral antibody were used to generate stylised curves.16, 18 181 patients were included in the pooled viral and antibody data, of whom 32 (18%) were defined as critically ill and 72 (40%) were estimated to have severe disease on the basis of overlapping case series.17 These data are representative of the population of interest, 16–20% of whom are likely to need admission to the ICU.19 ICU=intensive care unit. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Tracheostomy during the COVID-19 pandemic
Outside the context of the COVID-19 pandemic, controversy exists about the timing of tracheostomy.24, 25 Although several guidelines support early tracheostomy in select groups of patients, such as those with traumatic brain injury and patients with trauma-related injuries in general, most tracheostomies are done on a case-by-case basis.26, 27
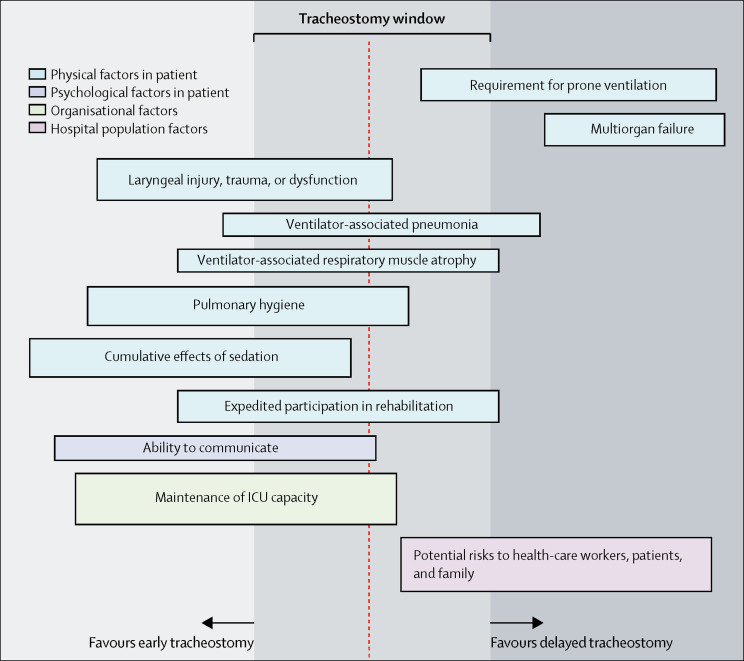
Although delaying tracheostomy for patients with COVID-19 might reduce risks for staff, extended duration of translaryngeal intubation, sedation, mechanical ventilation, and ICU stay associated with such delays can lead to complications. We suggest that decision making during the COVID-19 pandemic reflect the range of applicable considerations (figure 2 ; appendix pp 2–7). Patient selection for attempted primary extubation should be based on established practice—ie, in those with improving cardiovascular and respiratory physiological parameters, reducing markers of infection and inflammation, and a successful spontaneous breathing trial.28 However, premature extubation exposes patients and staff to the risks associated with urgent rescue oxygenation strategies and re-intubation. We recommend a conservative approach to attempted extubation, limited to those predicted to have a high chance of success.
Figure 2.
Considerations for tracheostomy after intubation for COVID-19-associated pneumonitis
The window for tracheostomy is 10–21 days after intubation. Bar heights represent relative weights of the factors. Bar heights and positions were proposed and agreed by the consensus working group. ICU=intensive care unit.
Considerations for tracheostomy during the COVID-19 pandemic should continue to emphasise current best practice. When elective tracheostomy is done, an inflated tracheostomy tube cuff via which pressure support ventilation can be delivered affords a closed system for controlled weaning of respiratory support. Although tracheostomy is associated with risks of infectious transmission, a primary extubation strategy in patients for whom the likelihood of success is low also carries risk. Several members of the consensus working group have personally been involved with or are aware of challenging re-intubations in patients with COVID-19, which suggests a role for mitigating risks of urgent or emergency re-intubation in difficult circumstances. Data are needed to understand attendant risks of infectious exposure to staff and other patients. Therapies for unsuccessful extubation, such as high-flow oxygenation, continuous positive airway pressure, or non-invasive ventilation, generate hazardous aerosols to varying degrees and might not be appropriate; they also impose a strain on the capacity of hospital wards to deliver oxygen.29 Recovering patients who continue to require ventilatory support via a tracheostomy can be managed with minimal sedation, which might simplify care and facilitate transfer to lower-acuity facilities, thus creating capacity for more acute patients.
Although tracheostomy is of benefit for carefully selected patients recovering from COVID-19-associated pneumonitis, we do not recommend the procedure in recovering patients who still need high fractions of inspired oxygen (FiO2), have high ventilator requirements, and might require prone positioning as part of their ventilatory strategy. Patients with tracheostomy can be managed in the prone position, but the airway cannot be visualised, risking displacement and pressure damage. Clear decision making is important in patients who are recovering, with the options including primary extubation with or without a plan for re-intubation, a tracheostomy or, in some cases, palliation. Ensuring adequate PPE, to maintain the safety of the health-care team, is among the foremost considerations in decisions around the management of patients with COVID-19 in the ICU. Notably, COVID-19-associated acute respiratory distress syndrome can be characterised by vascular insult and disrupted vasoregulation, which can affect the critical care strategy, and the need for tracheostomy might be reduced with high-quality ICU care that considers the characteristic pathophysiology.30 Based on case series and expert opinion, we suggest that tracheostomy be delayed until at least day 10 of mechanical ventilation and considered only when patients are showing signs of clinical improvement.
Performance of tracheostomy
Optimal setting for tracheostomy insertion
A variety of logistical and practical considerations influence the optimal location for a tracheostomy procedure in patients who are critically ill, including operator training and expertise; hospital infrastructure, including provision of side rooms and negative pressure air flows; availability of staff and equipment; and the ability to transfer a patient who is critically ill to another setting.31 Tracheostomy can be done in the ICU (often with suboptimal equipment and lighting, restricted availability of assistants, and suboptimal positioning on wide ICU beds) or in the operating room (requiring transfer from the ICU, exposure risks to multiple staff, and associated logistics). Percutaneous, surgical, or hybrid approaches can be used in either location. Because tracheostomy is an aerosol-generating procedure,2 we recommend a hierarchical approach to choosing the operative location to balance patient and staff risks (appendix p 1),32 recognising that many health-care facilities lack negative-pressure rooms.1 Portable high-efficiency particulate air filtration systems might be an acceptable alternative. We suggest that ICU and surgical teams review the optimal location for tracheostomy insertion during the pandemic, balancing the risks to patients and staff and appraising local facilities and expertise.1
Optimal tracheostomy procedure
Health-care workers who do tracheostomies must take into account additional considerations associated with the infectivity of SARS-CoV-2. Of the published cases of tracheostomies done in Singapore, Hong Kong, and Canada during the SARS outbreak, hospitals used enhanced PPE measures in addition to standard PPE, ranging from face shields to powered air-purifying respirators (PAPRs).1 On the basis of these reports, we suggest the use of enhanced PPE, with PAPRs, eye protection, fluid-repellent disposable surgical gown, and gloves. If a PAPR is not available, we advise the use of a fit-tested filtering face piece 3 (FFP3) or N95 mask with an additional fluid shield. The number of personnel present should be kept to an absolute minimum, with the most experienced operator and anaesthetist present. The team should prepare, rehearse, simulate, and communicate effectively.
The tracheostomy technique is determined by local expertise and resources. We suggest that operators continue to do tracheostomies using the techniques and equipment with which they are familiar, and confident and experienced in using. Strategies to minimise aerosol generation are well documented.1 Percutaneous tracheostomy usually involves opening the ventilator circuit more frequently than does surgical tracheostomy, and although ultrasound and bronchoscopy guidance might improve safety, the likelihood of aerosol generation is increased with percutaneous tracheostomy compared with surgical approaches. Surgical tracheostomies were generally favoured over percutaneous tracheostomies during the SARS outbreak;33, 34 however, percutaneous techniques have subsequently advanced and no data are available to establish superiority of one approach over the other from the standpoint of infectious transmission or safety. Single-use bronchoscopes with a sealed ventilator circuit are preferable when doing percutaneous tracheostomies.
When using an open approach, we suggest maintenance of a bloodless field, minimal use of diathermy, and use of a smoke evacuator. No definitive evidence exists regarding the relative superiority of any technique in minimising infectivity or complications in patients on anticoagulants, including those given extracorporeal membrane oxygenation support.35 Whichever technique is used, careful choice of tracheostomy tube and meticulous assessment of position once inserted are essential to minimise risks of later displacement, especially in patients who are obese.36
Paralysis with neuromuscular blocking drugs eliminates patient movement and coughing, but tachyphylaxis in response to such drugs can occur in patients who are critically ill.37, 38 Neuromuscular monitoring is useful to ensure adequate paralysis during tracheostomy.
Pausing ventilation during insertion of the tracheostomy tube minimises aerosol spread. Adjunctive manoeuvres such as placing the inflated cuff of the endotracheal tube well below the tracheostomy site in surgical tracheostomy can minimise the duration of apnoea. Because apnoea can cause rapid and substantial hypoxia in patients who are critically ill and dependent on a ventilator, we suggest preoxygenation, followed by a trial of apnoea in the ICU, with an FiO2 of 1·0 and positive end expiratory pressure of 5 cm H2O, in a patient in a supine position before tracheostomy. Rapid desaturation during these trials predicts a similar response during tracheostomy, indicating risks to the patient (and also to staff who might be required to do unplanned or additional airway interventions) and tracheostomy should be deferred. The ability to conduct or tolerate an apnoea trial should not replace multidisciplinary clinical judgment regarding the risks and benefits of undertaking tracheostomy in a given patient.
Optimal management after tracheostomy
Care of patients with COVID-19 after tracheostomy extends from the same fundamental principles of tracheostomy care in all patients. Safe and high-quality care can be provided in various settings.39, 40 However, because of the risk of viral transmission, the concept of patient-centred care must be balanced against the safety of health-care workers. Patients need to be managed by experienced staff who are trained in tracheostomy care and management.41 Key principles include a focus on essential care and avoidance of unnecessary interventions (especially those that generate aerosols), early recognition of deterioration, and timely response to emergencies.
Airway interventions should be planned in advance, as far as possible, to allow appropriate PPE to be applied. The use of PPE by staff remains a priority even in emergencies, and we suggest preparing systems to summon help from adjacent areas where staff might already be wearing PPE. Standard approaches to managing tracheostomy emergencies should be followed.
Patients with existing tracheostomies who do not have COVID-19 are at unknown risk of SARS-CoV-2 infection from visitors and staff. Safe locations need to be identified to manage patients with COVID-19 and those without COVID-19 with differing care needs, and consideration of novel options beyond standard hospital ward settings might be necessary, such as maximising use of telemedicine where applicable.
Tracheostomy to facilitate an extended period of ventilation support or weaning from ventilation initially requires a closed system to deliver pressure to the lungs. Strategies to minimise ventilator circuit disconnection and aerosol risks to staff include use of a cuffed non-fenestrated tube, use of in-line suction, and avoidance of unnecessary airway interventions.42, 43 Cuffs should remain initially inflated and pressures checked every 12 h, maintained at 20–30 cm H2O (or above ventilator peak inflation pressures). Water-filled cuffed tubes should be avoided and few leak tests should be done (to prevent aerosolisation) unless staff are wearing full PPE.44 Tube changes should be delayed ideally until patients are considered non-infectious.43
Humidification and disposable inner cannulae are common strategies to safeguard against tube occlusion from respiratory secretions and reduce suctioning requirements.45 If an inner cannula is used, we suggest reducing the frequency of changing, and reviewing the situation daily. We recommend a simple heat and moisture exchange (HME) filter, which provides adequate humidification and does not generate aerosols.46 Active water-based humidification might be required if secretions are thick, but their use should be assessed on an individual basis. Nebulisers can improve secretion clearance but require additional handling of ventilator circuits and can waterlog HME filters; they should therefore be used only after careful consideration.
Weaning from mechanical ventilation is generally managed through gradual reductions in pressure support, periods of cuff deflation, use of vocalisation strategies, promotion of coughing, and rehabilitation of swallowing. Many of these activities involve the generation of aerosols, including ventilator-adjusted leak speech, above-cuff vocalisation strategies, and the use of one-way valves.47 The aerosol-generating potential of a patient with a tracheostomy and deflated cuff who is receiving positive-pressure ventilator support is probably similar to that of a patient receiving continuous positive airway pressure via non-invasive ventilation, although different ventilators generate different maximal flows.2 We suggest the use of face masks and tracheostomy shields to mitigate the risks from aerosols. An excessively cautious approach might disadvantage patients, slowing their recovery and limiting their ability to communicate. Creative methods of augmentative and alternative communication must be implemented to reduce potential feelings of isolation among patients and increase safety during the COVID-19 pandemic.48 Patients who are infectious and clinically ready to commence cuff deflation trials should be managed in locations dedicated to treatment of patients with COVID-19 by experienced staff who are protected with the appropriate PPE. Swallowing assessments should rely on clinical skills rather than the use of instrumental swallowing examinations. Decannulation should be considered as soon as safely possible, and managed by a multidisciplinary tracheostomy team.
Conclusions and future directions
Our recommendations on the use of tracheostomy during the COVID-19 pandemic are presented in the panel . A defining feature of this pandemic is its pervasive and variable character. Increases in the number of patients with COVID-19 who are critically ill can swiftly overwhelm hospitals, particularly because many require extended periods of ventilator support, and many will require tracheostomy to facilitate recovery. Globally, studies of the COVID-19 pandemic have a recurring theme: foresight and planning save lives, whereas inadequate preparedness is associated with overwhelmed health systems. Because tracheostomy is at the intersection of health-care worker safety, resource allocation, and patient-centred care, sound guidance is crucial.
Panel. Summary of recommendations for tracheostomy during the COVID-19 pandemic.
Tracheostomy has a continuing role in managing weaning from extended periods of mechanical ventilation during the COVID-19 pandemic, but the procedure might not always provide benefit, and tracheostomy and subsequent care pose risks to health-care workers. The consensus working group recommendations are expert opinion (denoted by “we suggest” throughout the text) informed by best available evidence, or published supporting statements (denoted by “we recommend”).
Patient selection for tracheostomy
-
•
We suggest that standard decision making be adapted for the COVID-19 pandemic, taking into account a range of considerations, including potential risks and benefits for the individual patient; risks posed to health-care workers, other patients, and family; and available health-care resources
Timing of tracheostomy
-
•
We suggest that tracheostomy be delayed until at least day 10 of mechanical ventilation and considered only when patients are showing signs of clinical improvement
-
•
We recommend a conservative approach to attempted extubation, limited to patients who are predicted to have a high chance of success
Performance of tracheostomy
-
•
We suggest that ICU and surgical teams review the optimal location for tracheostomy during the pandemic, balancing the risks to patients and staff and taking into account local facilities and expertise
-
•
We recommend a hierarchical approach to operative location, balancing patient and staff risks (appendix p 1)
-
•
We suggest use of enhanced PPE, using powered air-purifying respirators, eye protection, fluid-repellent disposable surgical gown, and gloves
-
•
We suggest that operators continue to do tracheostomies using the techniques and equipment with which they are familiar, and confident and experienced in using
-
•
We suggest maintenance of a bloodless field, minimal use of diathermy, and use of a smoke evacuator when using an open surgical approach for tracheostomy
-
•
We suggest preoxygenation, followed by a trial of apnoea in the ICU, with an FiO2 of 1·0 and positive end expiratory pressure of 5 cm H2O, in patients who are supine before tracheostomy to show physiological readiness to tolerate the procedure, with strategies to mitigate aerosolisation
Optimal management after tracheostomy
-
•
Key principles include a focus on essential care and avoidance of unnecessary interventions (especially those that generate aerosols), early recognition of deterioration, and timely responses to emergencies
-
•
We suggest that systems be planned to summon help from adjacent clinical areas during a tracheostomy emergency (staff might already be wearing PPE in preparation for such emergencies)
-
•
We suggest reducing the frequency of changing an inner cannula (if used) and cuff pressure checks; these decisions should be made on an individual basis and reviewed daily
-
•
We recommend commencing care after tracheostomy with a simple heat and moisture exchange filter to provide humidification; the requirement for heated, water-based humidification or adjuncts, such as saline or hypertonic saline nebulisers, should be made on an individual basis and reviewed daily
-
•
We suggest that facemasks and tracheostomy shields be used by patients undergoing trials of tracheostomy cuff deflation to mitigate risks of aerosols
ICU=intensive care unit. PPE=personal protective equipment. FiO2=fractional concentration of oxygen in inspired air.
Many questions remain unanswered, and prospective data are needed to answer pressing questions around tracheostomy in the setting of the COVID-19 pandemic. Data on infectivity and persistence of viral RNA in patients who are critically ill are sparse, particularly at timepoints beyond 20 days after symptom onset, when tracheostomy is typically considered. The predictive value of peak viral load and antibody response for gauging infectivity and transmission risks associated with different strategies for tracheostomy insertion require further study. The experiences of European countries show that preparedness is a powerful determinant of patient outcomes. Delayed intubation and lack of ventilator availability contribute to poor outcomes. Robust data collection, analysis, and benchmarking are essential, such as the approach taken by the Global Tracheostomy Collaborative.39 If unconventional approaches to care (eg, shared ventilators or early tracheostomy) are used in a crisis, a moral imperative exists to study these interventions to assess their safety and efficacy.
Search strategy and selection criteria
We searched PubMed, Embase, MEDLINE, and the internet (Google and Google Scholar from a UK-based connection), including grey literature, for papers published between Jan 1, and April 4, 2020, with no language restriction, using combinations of the search terms “coronavirus”, “COVID-19”, “tracheostomy”, and “tracheotomy”. Sources identified included statements and guidance retrieved from websites of hospitals or recognised national societies identified by the search strategy, or known by the authors, many of whom were either involved in or aware of the development of national, local, or specialty-specific guidance. When several overlapping data sources were retrieved, we prioritised selection of sources representing national groups, or the larger case series. The final reference list was generated on the basis of relevance to the topics covered in this Health-care Development paper, with the aim of providing a contemporaneous review of emerging data on COVID-19 with direct relevance to the planning, conduct, and subsequent management of tracheostomy during the pandemic.
Acknowledgments
Acknowledgments
No funding was received for this Health-care Development paper. We thank Klaus Ott, Eddy Wong Wai Yeung, Elliott R Haut, Nico Espinosa, and David W Roberson for their valued perspective and input into this paper. We also thank Qingbiao Li, Qilei Song, Wei Chen, Rongjun Chen, Daqing Ma, Nilay Shah, Wang Wei, Zhang Xinhao, and Haibo Qiu for providing detailed accounts relating to early and current experience with the COVID-19 outbreak in Wuhan, China and surrounding regions.
Contributors
BAM, MJB, and SJW contributed to conception and design of the work, data acquisition, data analysis, data interpretation, and drafting of the manuscript. BAM and MJB did the literature search. BAM, MJB, SJW, and PJMO prepared the figures. VP, RDT, SDB, and GCYL contributed to data interpretation. AA, NT, and DJF-K contributed to conception and design of the work, data acquisition, data interpretation, and drafting of the manuscript. TSC, SG, and AD contributed to data acquisition, data interpretation, and drafting of the manuscript. JMA and GHM provided data and contributed to data interpretation and drafting of the manuscript. CM and CHR provided data and contributed to data interpretation. JA, SV, PD, JZ, MA, PP, PJMO, and HM contributed to design of the work, data acquisition, data interpretation, and drafting of the manuscript. LQ provided data and contributed to design of the work, data acquisition, data interpretation, and drafting of the manuscript. BKW and EW contributed to design of the work, data interpretation, and drafting of the manuscript. YS and MS provided data and contributed to data interpretation. All authors contributed to critical revision of the manuscript for intellectual content and all are responsible for the content of this Health-care Development paper.
Declaration of interests
VP is a consultant for Medtronic. CHR and DJF-K report unrestricted grants from Cook Medical supporting medical education; CHR reports a patent pending for an intubation protective tent and method of use and disinfecting (US patent application number 63/000,106, filed March 26, 2020, and application number 63/012,746, filed April 20, 2020; patent licensee, trustees of the University of Pennsylvania). JA reports a grant from Becton-Dickinson, outside the submitted work. JZ reports consultancy fees from Karl Storz and Zeiss, outside the submitted work. MA reports grants from GE and Intersurgical, and personal fees from Orion and Intersurgical, outside the submitted work. MS reports a grant from NewB and fees to his institution from Merck Sharp & Dohme, Amormed, and Biotest, outside the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.Tay JK, Khoo ML-C, Loh WS. Surgical considerations for tracheostomy during the COVID-19 pandemic: lessons learned from the severe acute respiratory syndrome outbreak. JAMA Otolaryngol Head Neck Surg. 2020 doi: 10.1001/jamaoto.2020.0764. published online March 31. [DOI] [PubMed] [Google Scholar]
- 2.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu J, Yang N, Wei Y, et al. Clinical characteristics of 54 medical staff with COVID-19: a retrospective study in a single center in Wuhan, China. J Med Virol. 2020 doi: 10.1002/jmv.25793. published online March 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly FE, Fong K, Hirsch N, Nolan JP. Intensive care medicine is 60 years old: the history and future of the intensive care unit. Clin Med (Lond) 2014;14:376–379. doi: 10.7861/clinmedicine.14-4-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balakrishnan K, Schechtman S, Hogikyan ND, Teoh AYB, McGrath B, Brenner MJ. COVID-19 pandemic: what every otolaryngologist-head and neck surgeon needs to know for safe airway management. Otolaryngol Head Neck Surg. 2020 doi: 10.1177/0194599820919751. published online April 4. [DOI] [PubMed] [Google Scholar]
- 6.Markel H, Lipman HB, Navarro JA, et al. Nonpharmaceutical interventions implemented by US cities during the 1918-1919 influenza pandemic. JAMA. 2007;298:644–654. doi: 10.1001/jama.298.6.644. [DOI] [PubMed] [Google Scholar]
- 7.Mehta AB, Syeda SN, Bajpayee L, Cooke CR, Walkey AJ, Wiener RS. Trends in tracheostomy for mechanically ventilated patients in the United States. Am J Respir Crit Care Med. 2015;192:446–454. doi: 10.1164/rccm.201502-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGrath BA, Wallace S, Goswamy J. Laryngeal oedema associated with COVID-19 complicating airway management. Anaesthesia. 2020 doi: 10.1111/anae.15092. published online April 17. [DOI] [PubMed] [Google Scholar]
- 9.Hosokawa K, Nishimura M, Egi M, Vincent J-L. Timing of tracheotomy in ICU patients: a systematic review of randomized controlled trials. Crit Care. 2015;19:424–512. doi: 10.1186/s13054-015-1138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vargas M, Sutherasan Y, Brunetti I, et al. Mortality and long-term quality of life after percutaneous tracheotomy in intensive care unit: a prospective observational study. Minerva Anestesiol. 2018;84:1024–1031. doi: 10.23736/S0375-9393.18.12133-X. [DOI] [PubMed] [Google Scholar]
- 11.Truog RD, Mitchell C, Daley GQ. The toughest triage — allocating ventilators in a pandemic. N Engl J Med. 2020 doi: 10.1056/NEJMp2005689. published online March 23. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Zhou M, Liu F. Reasons for healthcare workers becoming infected with novel coronavirus disease 2019 (COVID-19) in China. J Hosp Infect. 2020 doi: 10.1016/j.jhin.2020.03.002. published online March 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C, Gao G, Xu Y, et al. SARS-CoV-2-positive sputum and feces after conversion of pharyngeal samples in patients with COVID-19. Ann Intern Med. 2020 doi: 10.7326/M20-0991. published online March 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa344. published online March 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 doi: 10.1038/s41586-020-2196-x. published online April 1. [DOI] [PubMed] [Google Scholar]
- 20.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323 doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 21.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang H, Li Y, Ariani F, Chen X, Lin J. Timing of tracheostomy in critically ill patients: a meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andriolo BN, Andriolo RB, Saconato H, Atallah AN, Valente O. Early versus late tracheostomy for critically ill patients. Cochrane Database Syst Rev. 2015;1 doi: 10.1002/14651858.CD007271.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elkbuli A, Narvel RI, Spano PJ, 2nd, et al. Early versus late tracheostomy: is there an outcome difference? Am Surg. 2019;85:370–375. [PubMed] [Google Scholar]
- 27.Wang R, Pan C, Wang X, Xu F, Jiang S, Li M. The impact of tracheotomy timing in critically ill patients undergoing mechanical ventilation: a meta-analysis of randomized controlled clinical trials with trial sequential analysis. Heart Lung. 2019;48:46–54. doi: 10.1016/j.hrtlng.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 29.Cook TM, El-Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID-19. Anaesthesia. 2020 doi: 10.1111/anae.15054. published online March 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020 doi: 10.1001/jama.2020.6825. published online April 24. [DOI] [PubMed] [Google Scholar]
- 31.Chao T, Braslow BM, Martin ND, et al. Tracheotomy in ventilated patients with COVID-19. Ann Surg. 2020 doi: 10.1097/SLA.0000000000003956. published online May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.NHS England and NHS Improvement; 2020. Guidance for the role and use of non-invasive respiratory support in adult patients with coronavirus (confirmed or suspected) 26 March 2020 Version 2.https://amhp.org.uk/app/uploads/2020/03/Guidance-Respiratory-Support.pdf [Google Scholar]
- 33.Tien HC, Chughtai T, Jogeklar A, Cooper AB, Brenneman F. Elective and emergency surgery in patients with severe acute respiratory syndrome (SARS) Can J Surg. 2005;48:71–74. [PMC free article] [PubMed] [Google Scholar]
- 34.Chee VW, Khoo ML, Lee SF, Lai YC, Chin NM. Infection control measures for operative procedures in severe acute respiratory syndrome-related patients. Anesthesiology. 2004;100:1394–1398. doi: 10.1097/00000542-200406000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Salna M, Tipograf Y, Liou P, et al. Tracheostomy is safe during extracorporeal membrane oxygenation support. ASAIO J. 2019 doi: 10.1097/MAT.0000000000001059. published online Aug 16. [DOI] [PubMed] [Google Scholar]
- 36.McGrath BA, Lynch K, Templeton R, et al. Assessment of scoring systems to describe the position of tracheostomy tubes within the airway – the lunar study. Br J Anaesthesia. 2017;118:132–138. doi: 10.1093/bja/aew336. [DOI] [PubMed] [Google Scholar]
- 37.Haddad S. Tachyphylaxis to cisatracurium—case reports and literature review. Middle East J Anaesthesiol. 2008;19:1079–1092. [PubMed] [Google Scholar]
- 38.Tschida SJ, Graupe KJ, Hoey LL, Vance-Bryan K. Resistance to nondepolarizing neuromuscular blocking agents. Pharmacotherapy. 1996;16:409–418. [PubMed] [Google Scholar]
- 39.Brenner MJ, Milliren CE, Graham DA, et al. Global Tracheostomy Collaborative: improving patient safety through teamwork, standardisation, and data. Br J Anaesthesia (in press). [DOI] [PubMed]
- 40.McGrath BA, Wallace S, Lynch J et al. Improving tracheostomy care in the United Kingdom: results of a guided quality improvement program in 20 diverse hospitals. Br J Anaesthesia (in press). [DOI] [PubMed]
- 41.McGrath BA, Bates L, Atkinson D, Moore JA. Multidisciplinary guidelines for the management of tracheostomy and laryngectomy airway emergencies. Anaesthesia. 2012;67:1025–1041. doi: 10.1111/j.1365-2044.2012.07217.x. [DOI] [PubMed] [Google Scholar]
- 42.Respiratory Care Committee of Chinese Thoracic Society Expert consensus on preventing nosocomial transmission during respiratory care for critically ill patients infected by 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;17:e020. doi: 10.3760/cma.j.issn.1001-0939.2020.0020. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 43.Harrison L, Ramsden J, Winter S, Rocke J, Heward E. ENTUK; London: March 19, 2020. Guidance for surgical tracheostomy and tracheostomy tube change during the COVID-19 pandemic.https://www.entuk.org/tracheostomy-guidance-during-covid-19-pandemic [Google Scholar]
- 44.Zuo MZ, Huang YG, Ma WH, et al. Expert recommendations for tracheal intubation in critically ill patients with novel coronavirus disease 2019. Chin Med Sci J. 2020 doi: 10.24920/003724. published online Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan JYK, Wong EWY, Lam W. Practical aspects of otolaryngologic clinical services during the 2019 novel coronavirus epidemic: an experience in Hong Kong. JAMA Otolaryngol Head Neck Surg. 2020 doi: 10.1001/jamaoto.2020.0488. published online March 20. [DOI] [PubMed] [Google Scholar]
- 46.Brusasco C, Corradi F, Vargas M, et al. In vitro evaluation of heat and moisture exchangers designed for spontaneously breathing tracheostomized patients. Respir Care. 2013;58:1878–1885. doi: 10.4187/respcare.02405. [DOI] [PubMed] [Google Scholar]
- 47.McGrath B, Lynch J, Wilson M, Nicholson L, Wallace S. Above cuff vocalisation: a novel technique for communication in the ventilator-dependent tracheostomy patient. J Intensive Care Soc. 2016;17:19–26. doi: 10.1177/1751143715607549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ten Hoorn S, Elbers PW, Girbes AR, Tuinman PR. Communicating with conscious and mechanically ventilated critically ill patients: a systematic review. Crit Care. 2016;20:333. doi: 10.1186/s13054-016-1483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.