Abstract
Glucagon and α-cell dysfunction are critical in the development of hyperglycemia during diabetes both in humans and rodents. We hypothesized that α-cell dysfunction leading to dysregulated glucagon secretion in diabetes is due to both a lack of insulin and intrinsic defects.
To characterize α-cell dysfunction in diabetes, we used Glucagon-Venus transgenic male mice and induced insulinopenic hyperglycemia by streptozotocin administration leading to alterations of glucagon secretion. We investigated the in vivo impact of insulinopenic hyperglycemia on glucagon-producing cells using Facs-sorted α cells from control and diabetic mice. We demonstrate that increased glucagonemia in diabetic mice is mainly due to increases of glucagon release and biosynthesis per cell compared to controls without changes in α-cell mass. We identified genes coding for proteins involved in glucagon biosynthesis and secretion, α-cell differentiation and potential stress markers such as the glucagon, Arx, MafB, cMaf, Brain4, Foxa1, Foxa3, HNF4α, CF7L2, Glut1, Sglt2, Cav2.1, Cav2.2, Nav1.7, Kir6.2/Sur1, Pten, insulin receptor, NeuroD1, GPR40 and Sumo1 genes, which were abnormally regulated in diabetic mice. Importantly insulin treatment partially corrected α-cell function and expression of genes coding for proglucagon, or involved in glucagon secretion, glucose transport and insulin signaling but not those coding for c-Maf, Foxa1 and α-cell differentiation markers as well as GPR40, NeuroD1, Cav2.1 and Sumo1.
Our results indicate that insulinopenic diabetes induce marked α cell dysfunction and moleculer alteration which are only partially corrected by in vivo insulin treatment.
Keywords: Hyperglycemia, glucagon secretion, streptozotocin, insulin treatment, Facs-sorted alpha cell
Introduction
The pathophysiology of diabetes has been attributed for many decades to insulin resistance and decreased insulin production and secretion as well as an excess of glucagon (1). Indeed, plasma glucagon levels are increased in diabetes and particularly in poorly controlled type 1 diabetes and diabetic ketoacidosis; these levels have also been reported to be increased in glucose-intolerant and type 2 diabetic patients (2). In diabetic patients glucagon release is not suppressed by increased glucose levels, and thus contributes further to postprandial hyperglycemia in both type 1 and type 2 diabetes (3,4). Furthermore, the secretory response of α cells to low glucose concentrations is impaired in “long-standing” diabetes, increasing the risk of severe hypoglycemia, especially in patients treated with insulin (5,6). Overall, plasma glucagon levels are inappropriate in the context of hyperglycemia, which normally suppress glucagon secretion. The consequences of the unsuppressed glucagon secretion are an increased rate of hepatic glucose production contributing to fasting hyperglycemia. Thus dysregulated α-cells hypersecrete glucagon which contribute in a major way to hyperglycemia.
Whether α-cell dysfunction in diabetes, particularly in response to glucose, comes from an intrinsic defect of impaired glucose sensing and/or from insulin deficiency, α-cell insulin resistance or dysfunction β cells is unclear. A large number of studies have examined the consequences of diabetes on islet functions using different animal models among them chemical β-cell ablation (7). Whereas the effects of diabetes on β cells have been extensively studied, consequences on α cells remain limited to plasma glucagon levels and α-cell mass with contradictory results.
In order to better characterize the functional and molecular defects of α cells in diabetes, we used the transgenic mouse strain Glucagon-Venus and induced diabetes by streptozotocin (STZ) administration which led to drastic β-cell ablation, severe hyperglycemia and hyperglucagonemia. In this model glucagon mRNA levels, pancreatic glucagon content and basal glucagonemia were increased in the absence of α-cell mass changes. In addition, glucose did not regulate glucagon secretion compared to control animals. To investigate whether alterations of glucagon secretion were due to intrinsic α-cell defects, we collected islets and purified Venus-α cells from control and STZ-diabetic mice and assessed α-cell secretion. We observed that basal release was upregulated and glucagon secretion was not regulated by low glucose compared to controls, similarly to what we observed in pancreatic perfusion experiments.
We then assessed mRNA levels of specific genes important for α-cell function from control and STZ sorted-α cells and revealed that glucose transporters as well as α-cell markers were decreased in STZ-diabetic mice compared to controls suggesting that the identity and glucose sensing of pancreatic α cells are altered in hypoinsulinemic hyperglycemic conditions. We also observed that Foxa1 and cMaf mRNA levels coding for two transcription factors involved in glucagon gene expression were upregulated in α cells from diabetic mice compared to controls; in agreement with mRNA levels FOXA1 protein was also increased in STZ islets compared to controls.
Whereas insulin treatment partially corrected α-cell function, it normalized glucagon, Foxa3, HNF4α, TCF7L2, Glut1, Sglt2, Cav2.2, Nav1.7, Kir6.2, Sur1, Pten and insulin receptor mRNA levels, it did not correct those coding for Arx, MafB, Brain4, Foxa1, cMaf, NeuroD1, Cav2.1, GPR40 and Sumo1.
Our results indicate that streptozotocin-induced diabetes leads to specific alterations in α cells, independently of α-cell mass, including decreases in the expression of genes coding for glucose transport as well as for α-cell identity and upregulation of genes coding for transcription factors involved in glucagon gene expression. Although many defects are corrected by insulin treatment, some of the defects remain altered and may be intrinsic to the α-cells or require intra-islet insulin or other factors to be normalized.
Materials and Methods
mGlu124 Gcg-Venus Transgenic mouse strain
The transgenic mouse strain Glucagon-Venus express specifically the Venus fluorochrome in glucagon-producing cells (8). This mouse strain was obtained through collaboration with Dr Fiona Gribble (Cambridge University, UK). The genetic background for Glucagon-Venus strain is C57Bl6J. Mice were bred in conventional housing according to ethical approbation by Swiss federal committee. Of note, all of Venus+ α cells express glucagon and 92+/% of glucagon-producing cells expressed Venus (Supplemented data, Figure 1).
Streptozotocin-induced diabetes (STZ)
20 weeks-old male Glucagon-Venus mice were separated into 3 groups (control, streptozotocin (STZ) and insulin-treated STZ-induced diabetic mice (STZ+insulin implant)) fed with chow diet. Animals of the STZ groups were injected (intraperitoneal) with a single high-dose of STZ (200mg/kg). Maximal effect of STZ is observed about 1 week after injection (9). The control group was injected with the resuspension buffer of STZ (Na-citrate/Citric acid). 7 days after STZ injection, Insulin implants (LINBIT) were placed subcutaneously in STZ-induced diabetic mice. Animals from each group were monitored each day for weight and glycemia during 4 weeks until sacrifice and pancreases collection. Weight, glycemia and glycated hemoglobin (Siemens DCA systems Hemoglobin A1c) were analyzed from blood samples.
Morphometric analyses of pancreatic α-cell mass in STZ-induced diabetic mice
The pancreases were removed after sacrifice and fixed overnight in paraformaldehyde 4%; after embedding in paraffin, 5 μm thick sections were cut. Immunofluorescence stainings were performed with the following primary antibodies (1/500): Rabbit anti-Glucagon (Millipore) and Guinea-pig anti-insulin (ThermoFischer Scientific) and with the following secondary antibodies: Alexa-Fluor anti-Rabbit 488 (1/1000) and Alexa-Fluor anti Guinea-pig 568 (1/500) as well as DAPI (10μg/ml).
Morphometric analysis was performed on 3 independent sections spaced at least 250 μm for each animal. Whole pancreas sections with glucagon, insulin, and DAPI staining were scanned using Mirax scanner. The images obtained were displayed on a large screen to localize each area with insulin and/or glucagon staining. Each islet was analyzed with Metamorph and Pannoramic Viewer softwares to measure pancreatic section area, islet area, glucagon and insulin staining area and glucagon and insulin positive cell number.
Primary Venus+ alpha-cell preparation
Islets of Langerhans were isolated using standard procedure as described (10). Primary Venus+ α cells were separated from non-Venus and exocrine cells by fluorescence-activated sorting using FACS Biorad S3 (Facs-profiles for CTRL, STZ and STZ+ groups:Supplemented data, Figures 2-4). The percentage of glucagon positive cells (99% +/-1%) was evaluated by immunohistochemistry using specific glucagon and insulin antibodies using at least 10 male mice. The purity was also assessed by PCR analysis using 6 control mice (Supplemented data, Figure 5).
Ex vivo measurements of glucagon release, secretion and contents
Glucagon contents were measured on collected mouse sorted Venus+ α cells (1000 cells) from control, STZ and STZ-treated mice in Acid/Ethanol mixture (1.5%HCl / 75% Ethanol).
For ex vivo short-term primary culture of pancreatic α cells, FACS-sorted Venus cells (1000 cells per condition) were seeded in 50μl drops using laminin pre-coated culture dishes (35mm Easy-grip Tissue Culture Dish, Falcon) as previously described (10). Cells were next incubated overnight in DMEM (#11926-025, Gibco) supplemented by 5.6mM Glucose, 1%FBS, 20mM Hepes as well as antibiotics. Glucagon release over 8h-incubation and acute 30min glucagon secreation from primary α cells of control and diabetic mice (STZ and STZ+insulin implant) were then analysed. The effect of glucose (1-5.6mM D-glucose) was assessed as described (11). Glucagon was quantified in cell contents and supernatants by Elisa kit (#10-1271-01, Mercodia).
Pancreatic perfusion experiments
16 weeks-old control and STZ-induced diabetic mice were anesthetized before experiments. Briefly, after laparotomy, pancreases were isolated to the rest of body through specific ligatures as described (12). Pancreatic perfusion samples from control and diabetic mice were next analyzed by glucagon and mouse insulin Elisa (Mercodia) kits following the manufacturer's recommendations.
RNA preparation and RT-PCR analysis
Total RNA was isolated from mouse sorted Venus+ α cells using RNeasy Plus Micro kit (Qiagen). After specific quantification (Qubit RNA High-sensitivity Assay Kit, life technologies) 10ng of total RNA were reverse-transcribed (PrimeScript RT reagent Kit, Takara) and preamplified (cDNA Pre-Amp Master kit, Roche Diagnostics) following the manufacturer’s instructions. Through candidate gene approach, specific mRNA levels were analyzed by real-time qPCR using Light-cycler technology (Roche Diagnostics). The expression of each candidate gene expression was evaluated using specific primers (Supplemented data, Table 1-3).
FOXA1 immunodetection in the pancreas of Control and STZ-treated mice
After sacrifice, pancreases were fixed overnight in 4% paraformaldehyde and embedded in paraffin. Immunofluorescence was performed on deparaffinized 5 μm thick pancreatic sections after antigen retrieval in TEG buffer. Chicken anti-Venus (1/100; ThermoFischer Scientific) and Rabbit anti-Foxa1 (1/100; Gift by Dr R. Costa) were used as primary antibodies (overnight, 4°C) following by 1h incubation with Alexa-Fluor Anti-Rabbit 568 and Anti-Chicken 488 (1/500; ThermoFischer Scientific).
Data analysis
Data are presented as means ± SE and analyzed by two-tailed Student-t test and ANOVA analysis. A p value of less than 0.05 was considered to be statistically significant. All tests were performed using the SPSS software.
Results
Functional alterations of pancreatic alpha cells in streptozotocin-induced diabetic mice
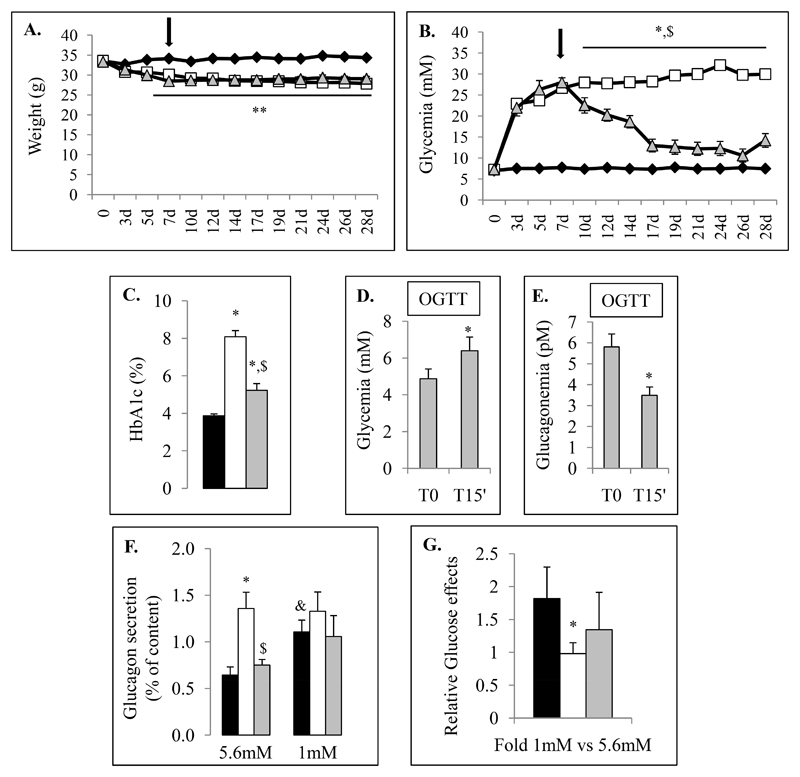
To better understand why and by which mechanisms pancreatic α cells are affected in diabetes, we used an insulin-deficient model in the mouse transgenic strain where proglucagon-producing cells are labeled by the Venus fluorescent protein. We generated streptozotocin-induced diabetic mice using a single high-dose streptozotocin injection. Control (CTRL) and streptozotocin-diabetic (STZ) animals were kept during 28 days. We first characterized the in vivo model including weight, glycemia, glycated hemoglobin measurements (HbA1c) and performed oral glucose tolerance tests (OGTT) (Fig.1).
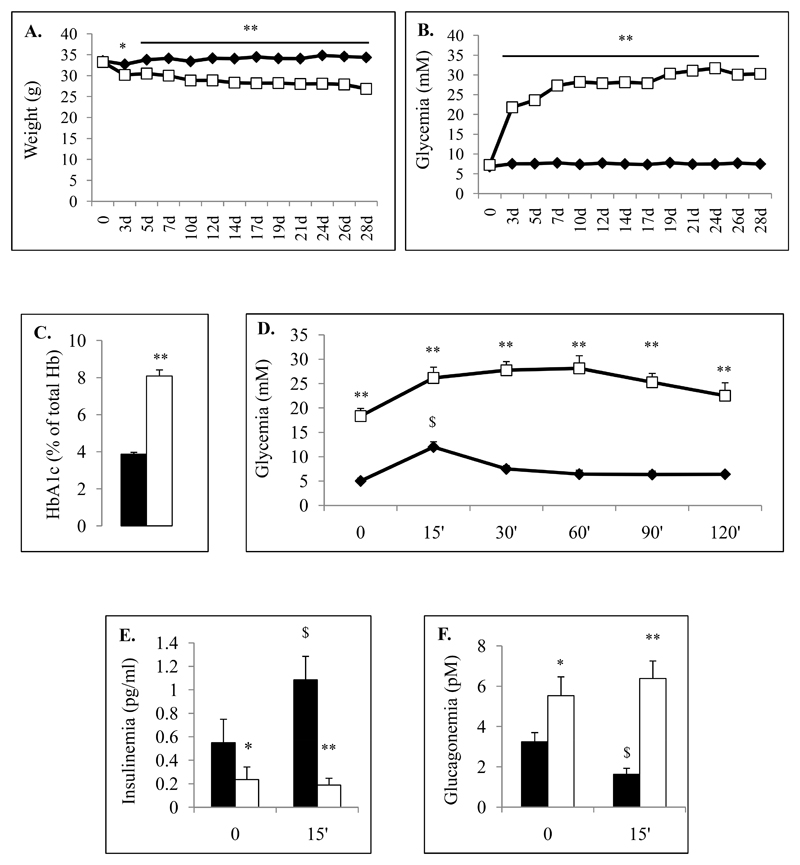
Figure 1. Physical and biological characteristics of the control and streptozotocin-induced diabetic mice.
Data corresponding to control chow-diet (CTRL) and diabetic mice (STZ) are illustrated respectively by black and white colors. Weight (A) and random glycemia (B) were monitored during 28 days for CTRL (Black Diamond) and STZ-induced diabetic mice (White Square). Glycated hemoglobin (HbA1c) were measured from CTRL (black bar) and STZ (white bar) animals at 28 days (C). Glucose tolerance test (OGTT) (D-F) were performed on control (black) and STZ (white) using oral gavage of D-Glucose solution (1g/kg) one week before sacrifice (3 weeks). Glycemia were measured before and 15, 30, 60, 90 and 120 min after glucose administration. Circulating blood insulin (E) and glucagon (F) levels were also measured from control (black bars) and STZ (white bars) serum mice before and 15 min after glucose load. Physiological parameters were evaluated on each group including 15 mice at least.
* and ** indicate statistical significance (respectively p value ≤0.05 and p value ≤0.01) compared to control mice. $ indicates statistical significance compared to T0.
We observed that streptozotocin-treated mice presented rapid weight-loss and a marked increase of glycemia from day 3 after streptozotocin injection (Fig.1A-B). At the end of protocol, STZ-induced diabetic mice exhibited 16.9% (+/-1.4) weight-loss and 415% (+/-20) increase in glycemia compared to controls. Glycated hemoglobin (HbA1c) were 3.78+/-0.08% (IFCC: 18mmol/mol) and 8+/-0.25% (IFCC: 64mmol/mol) in control and STZ-treated mice respectively (Fig.1C).
We next assessed glycemia, glucagonemia and insulinemia during an OGTT and observed that after an 8h-fasting period, STZ-induced diabetic mice exhibited hyperglycemia (18.3+/-1.6 mM for STZ vs 5+/-0.6 mM for CTRL) associated with hypoinsulinemia (0.24+/-0.11 ng/ml for STZ vs 0.55+/-0.07 ng/ml for CTRL) and hyperglucagonemia (5.53+/-0.94 pM for STZ vs 3.24+/-0.45 pM for CTRL) compared to controls (Fig.1D-F). At 15 minutes after glucose administration, insulin levels remained low and glucagon levels did not decrease in diabetic mice (T15 glycemia: 26.2+/-2.2 mM for STZ vs 12+/-1.1 mM for CTRL; insulinemia: 0.19+/-0.06 ng/ml for STZ vs 1.09+/-0.14 ng/ml for CTRL; glucagonemia: 6.38+/-0.87 pM for STZ vs 1.62+/-0.31 pM for CTRL) indicating that basal glucagon secretion was increased and not inhibited by glucose.
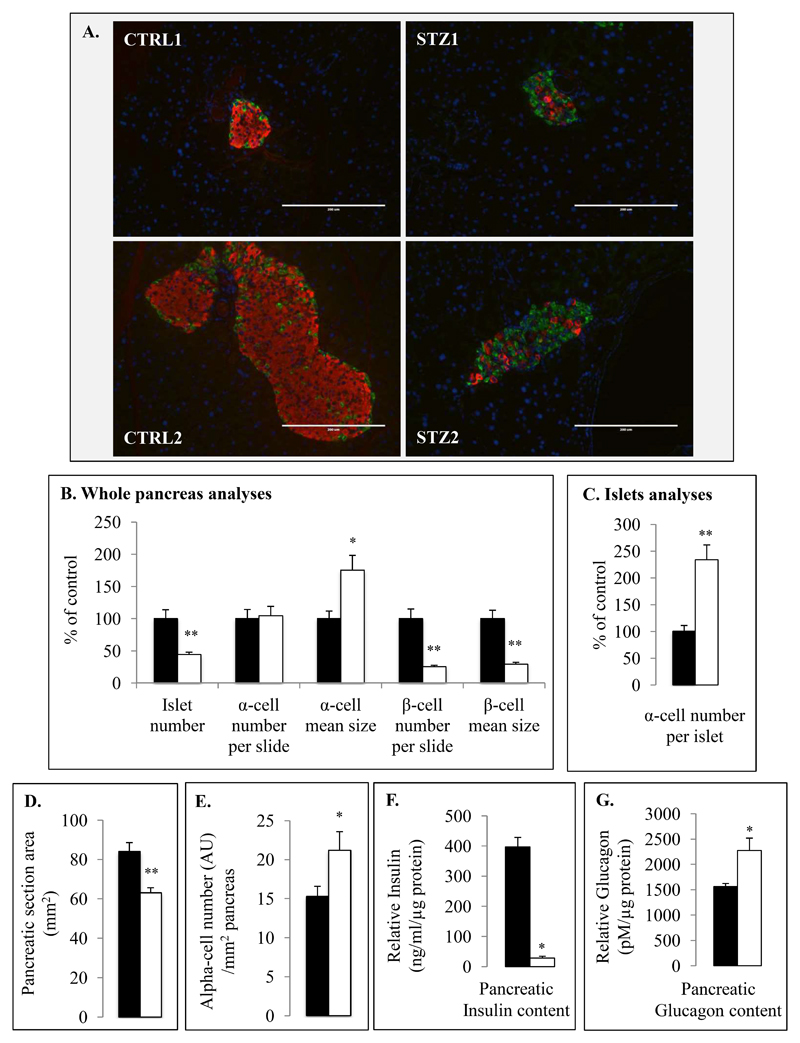
STZ-induced β-cell ablation was analyzed by pancreatic insulin content measurements as well as immunohistochemistry and morphometric analyses; STZ-induced diabetic mice presented a 74.9% (+/-2.6) decrease of β-cell mass (Fig.2A-B) as well as a 92% (+/-4) decrease of pancreatic insulin content compared to controls (Fig.2F). Remaining insulin-positive cells were 70.9% (+/-3.1) smaller compared to control β cells (Fig.2B). Furthermore, we also observed that pancreatic islet number was decreased by 55.9+/-4% in STZ mice compared to controls.
Figure 2. Morphometric analysis of pancreatic α and β cells in control and STZ-induced diabetic mice.
Illustrative images corresponding to pancreatic sections of CTRL and STZ-treated (A) mice were performed using anti-glucagon (green) and anti-insulin (red) immunostaining as well as DAPI. Seven different mice were used for quantitative and statistical analysis using Mirax scanner and Metamorph software (B-C) in each group (Control (black bars) and Streptozotocin (white bars)). Islets number, α- and β-cell numbers as well as sizes were evaluated for each animal in 3 different pancreatic sections containing islets (B). α-cell numbers were also evaluated relative to islet for CTRL and STZ groups (C). Pancreatic area (D) and relative alpha cell number per surface unit (E) were analyzed in each group (21 pancreatic sections corresponding to 7 mice) using Mirax scans and Pannoramic Viewer software.
Total pancreatic insulin (F) and glucagon (G) contents were measured from homogenized pancreas of CTRL and STZ-induced diabetic mice. Glucagon and insulin contents were expressed relative to total protein amounts. * (p≤0.05) and ** (p≤0.01) indicate statistical significance compared to control.
Importantly, pancreatic glucagon contents were 47.4% (+/-18.3) higher in STZ-induced diabetic mice compared to controls (Fig.2G). To determine if this increase was due to an increase in α-cell number we investigated pancreatic α-cell mass in islets and total pancreas and observed that there was no significant difference in the total number of glucagon-positive cells 28 days after STZ injection between diabetic and control mice. There were increases of α-cell number per islet (233.9+/-28.2% of controls, Fig.2C) and of α-cell number relative to pancreatic area (138.7+/-11.3 % of controls, Fig.2E) in STZ mice compared to controls that were compensated by the decreases of islet number in total pancreas and of pancreatic area (Fig.2B,D). Interestingly, we also observed an increase of α-cell size in pancreases of STZ mice (175.1-/23.3% of CTRL) suggesting α-cell hypertrophy.
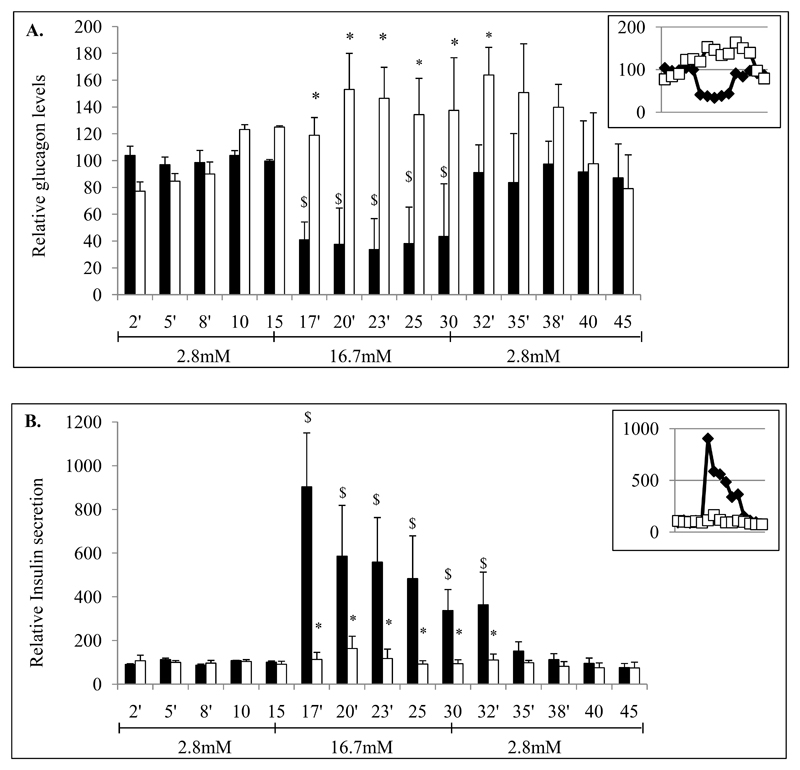
To further characterize alterations of glucose-regulated glucagon secretion, we performed pancreatic perfusion experiments using low (2.8mM D-Glucose) and high glucose (16.7mM) concentrations. In STZ-diabetic mice glucagon secretion did not decrease with high glucose as it was observed for controls (Fig.3A). These observations were associated with the absence of glucose-stimulated insulin secretion in STZ-induced diabetic mice (Fig.3B).
Figure 3. Alterations of glucagon and insulin secretions in STZ-induced diabetic mice.
Glucagon and insulin secretions were investigated from control and STZ-induced diabetic mice after 4 weeks. After an initial 30min-stabilization step, pancreases were perfused successively with 2.8mM, 16.8mM and 2.8mM glucose-Krebs solutions during 15min-periods each. Pancreatic secretions were collected every minute. Glucagon (A) and insulin (B) levels were monitored in samples collected from perfused pancreas of control (black bars) and STZ (white bars) mice. Pancreatic perfusion experiments include 5 mice per group. * indicate statistical significance compared to control. $ significance compared to first 2.8mM incubation period.
To further analyze the characteristics of the dysfunctional α cells in STZ-diabetic mice we specifically investigated α-cell glucagon contents as well as glucagon secretion from Facs-sorted α cells at the end of protocol. We observed that glucagon contents were 31% (+/-4) higher per cell in Facs-sorted α cells from STZ-induced diabetic mice compared to controls (Fig.4A) suggesting that the increase of pancreatic glucagon content was mainly due to an increase in glucagon biosynthesis per cell.
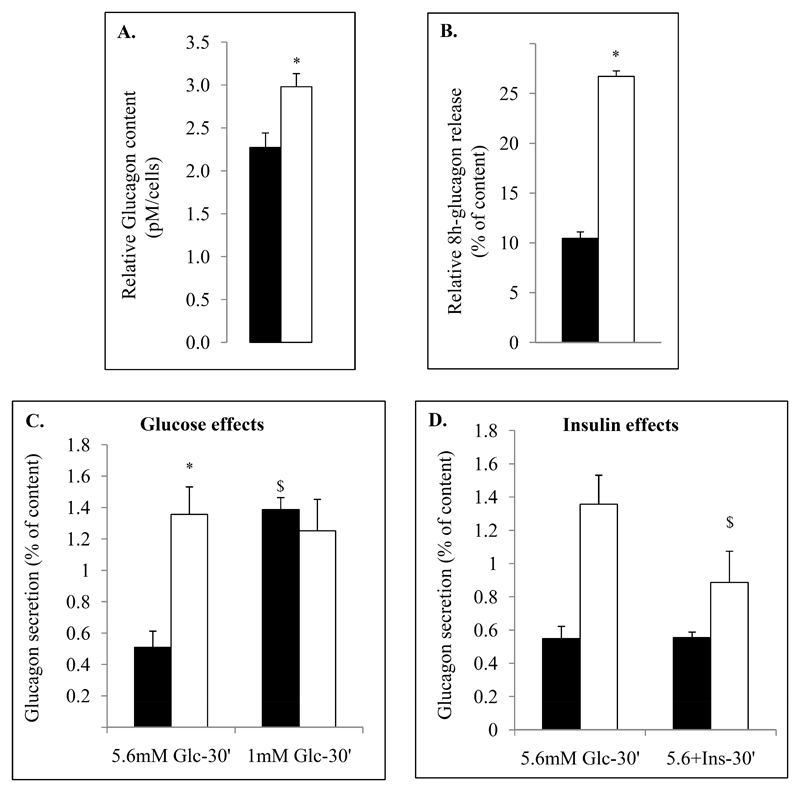
Figure 4. Glucagon secretion from Facs-sorted α-cells from control and STZ-induced diabetic mice.
After islets isolation and Facs-sorting, glucagon contents were evaluated from Facs-sorted α cells of controls (black bars) and STZ (white bars) mice (A). Venus+ cells from control (black) and diabetic mice (white) were plated in 5.6mM DMEM-1%FBS medium for the night (recovery medium). 24h after glucagon secretory capacities of cells were measured from control (CTRL, black bars) and diabetic mice (STZ, white bars) for 8h in recovery medium (continuous release, B) or after 1 hour depletion in 5.6mM glucose Krebs buffer (KRB-0.5% BSA) for acute secretion assays (C,D). Primary cells were then incubated for 30min in KRB medium supplemented by 1 or 5.6mM glucose and insulin (100ng/ml). Histograms (B-D) represent percentage of glucagon measured from the supernatant of primary control and STZ sorted α cells relative to glucagon contents; basal glucagon during 8h (B), glucose (C) and insulin effects (D). Glucose and insulin effects were evaluated during 30min incubation periods. Ex vivo secretion assays were performed on 5 different samples of Facs-sorted α cells per condition and group.
* indicate statistical significance compared to control. $ significance compared to 5.6mM conditions.
We then analyzed glucagon secretion of α cells from control and STZ-diabetic mice using Facs-sorted α cells at the end of protocol. We observed that α cells from STZ-induced diabetic mice exhibited respectively 2.48- and 2.32-fold higher basal glucagon release compared to control cells in 8h-continuous release experiment (DMEM-5.6mM Glucose-0.5%BSA) and in acute 30 minutes secretion assays (Krebs buffer-5.6mM Glucose-0.5%BSA). However, whereas glucagon release in control α cells was regulated negatively by glucose, it was not in α cells from STZ-induced diabetic mice (Fig.4C). By contrast to the absence of glucose effects on α cells from STZ-induced diabetic mice, these cells responded to insulin by decreasing basal glucagon secretion without complete normalization (Fig.4D).
Taken together these data clearly demonstrate that β-cell ablation and subsequent hyperglycemia leads to hyperglucagonemia secondary to increases of glucagon biosynthesis and secretion per cell. Importantly, isolated α cells from STZ-induced diabetic mice exhibit alterations of basal and glucose-regulated glucagon secretion.
Characterization of the molecular alterations of pancreatic alpha cells in streptozotocin-induced diabetic mice
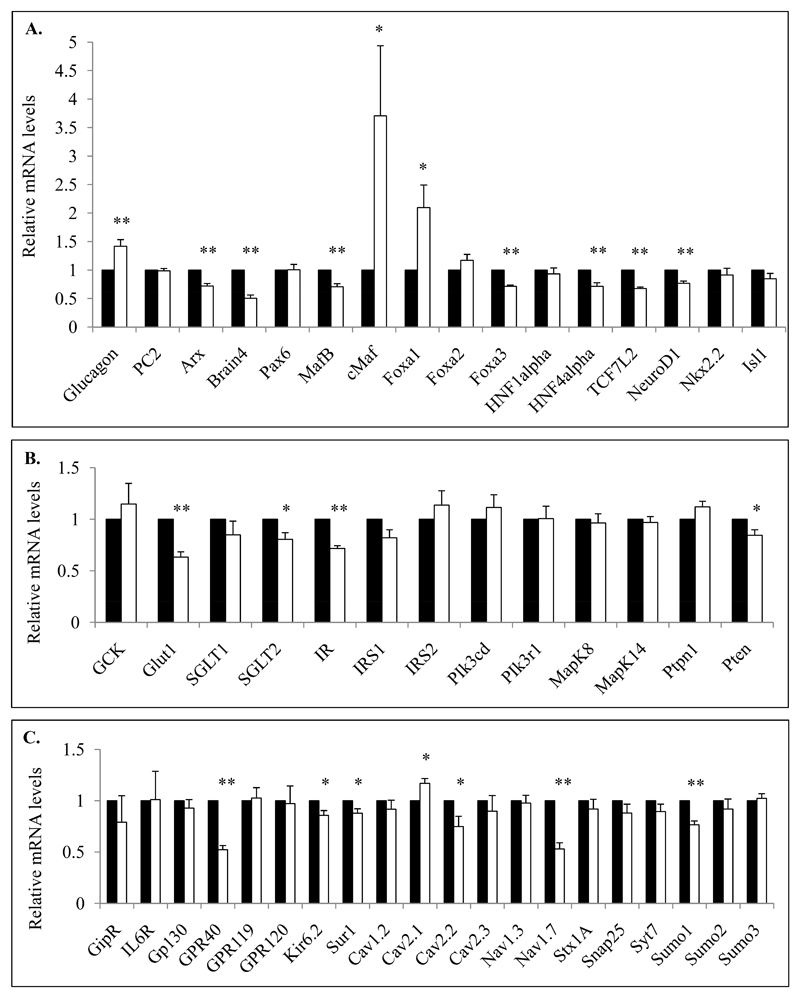
To further characterize functional alterations of α cells we searched for altered gene expression potentially involved in diabetes through a candidate gene approach. We determined a list of 49 genes which are all involved in α-cell identity and function including specific gene targets which code for proteins involved in glucagon biosynthesis and secretion as well as α-cell differentiation and maturation. We thus quantified mRNA levels of targeted genes from Facs-sorted α cells of control and STZ-diabetic mice after 28 days protocol period (Fig.5). We first observed that proglucagon mRNA levels were significantly increased in STZ-induced diabetic mice (1.42+/-0.12 fold induction) compared to controls whereas Arx, Brain4 (Pou3f4), MafB, Foxa3 and NeuroD1 were reduced suggesting that α-cell identity is modified in STZ-induced diabetes (Fig.5A). Interestingly, we also observed robust increases of Foxa1 and cMaf (v-Maf) mRNA levels (2.1+/-0.4 and 3.71+/-1.2 -fold induction for Foxa1 and cMaf respectively) and decreases of Foxa3 and TCF7L2 mRNAs coding for transcription factors in diabetic mice compared to controls. We then analyzed genes coding for factors involved in glucose metabolism and insulin signaling and identified that the glucose transporters Glut1 and Sglt2 as well as the insulin receptor (IR) and pten mRNAs were decreased in diabetic mice but not those for Irs1 and 2 (Fig.5B), suggesting alterations in glucose transport and insulin signaling in insulinopenic diabetes.
Figure 5. Molecular analyses of α-cell specific gene transcripts involved in glucagon biosynthesis and secretion as well as α-cell differentiation from control and STZ-induced mice.
FACS-sorted Venus+ α cells from control and STZ-induced diabetic mice were collected and analyzed for mRNA quantification of specific genes coding for proteins involved in α-cell identity and glucagon biosynthesis (A) or glucose metabolism and insulin signalling (B) and mRNAs coding for receptors and ions channels as well as general components of exocytosis (C). mRNA levels of each tested genes are relative to RPS9, HPRT, Actin Beta, Tubulin Beta and cyclophilin mRNA values. Data are presented as the means (% of control) ± SE (%) for 4 different experiments including at least 11 mice in each group. Relative mRNA levels of STZ-diabetic mice are analyzed compared to their own littermate controls.
* and ** indicate statistical significance (respectively p value ≤0.05 and p value ≤0.01) compared to control mice.
We finally investigated the mRNA levels of genes involved in glucagon secretion and observed that Nav1.7 (Scn9a), Cav2.2 (Cacna1B), Kir6.2 (Kcnj11), Sur1 (Abcc8) and Sumo1 were decreased in STZ-diabetic mice compared to controls whereas Cav2.1 (Cacna1A) mRNA levels were slightly but significantly increased (Fig.5C). The fatty-acid receptor GPR40, but not GPR119/120, was also affected. Of note, gene expression of the IL6 receptor (IL6R) and transducer (GP130) were not affected in STZ-induced diabetic mice. Critical genes also involved in α-cell functions such as PC2 (Pcsk2), Pax6, Foxa2, Gck, Nkx2.2, Isl1 as well as general exocytosis components such as Nav1.3 (Scn3a), Stx1A (syntaxin1A), SNAP25 and Syt7 (synaptotagmin7) were not affected in STZ-induced diabetic mice.
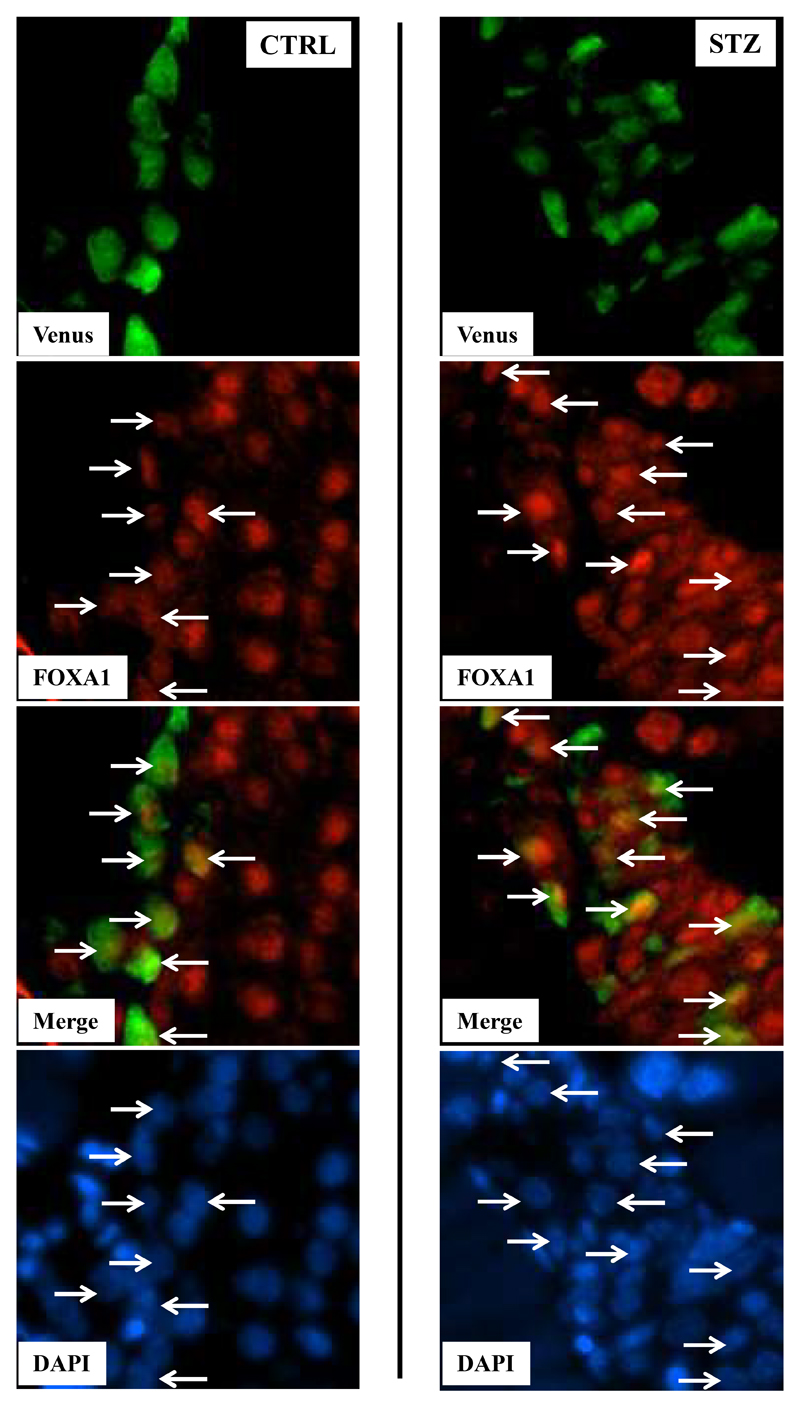
To confirm that molecular alterations of identified target genes were translated at the protein levels we performed immunofluorescences using pancreatic sections of CTRL and STZ mice. FOXA1 was upregulated in Venus+ α cells of STZ mice compared to controls (Fig.6) whereas cMaf expressed at low levels in α cells was not detected in both groups (data not shown).
Figure 6. FOXAI expression in α cells from control and STZ islets.
Illustrative images corresponding to pancreatic sections of CTRL and STZ mice were performed using anti-Venus (green) and anti-Foxa1 (red) as well as DAPI staining (blue). Merge images represent DAPI, Venus and Foxa1 signals. White arrows target Foxa1/Venus positive cells in control and STZ islets.
These experiments clearly show that α cells from STZ-diabetic mice exhibit specific molecular alterations including critical genes involved in glucagon biosynthesis (glucagon itself, Foxa1, cMaf and NeuroD1), α-cell identity (Arx, MafB and Brain4), glucose transport (Glut1 and Sglt2), insulin signaling (IR and pten) and secretion (Cav2.1/2.2, Kir6.2/Sur1, Nav1.7, GPR40 and Sumo1).
In vivo insulin treatment partially corrects functional and molecular alterations of pancreatic alpha cells in streptozotocin-induced diabetic mice
To evaluate the potential reversibility of α-cell alterations in STZ-induced diabetes we evaluated the impact of chronic insulin treatment using subcutaneous insulin implants 7 days after STZ injection for 21 days (Fig.7). As expected, insulin treatment improved glycemia (Fig.7B) and HbA1c levels of STZ-diabetic mice (IFCC: 36mmol/mol (HbA1c 5.48+/-0.21%) for STZ+insulin implant vs 64mmol/mol (HbA1c 8%) for STZ; Fig.7C) although these mice were still mildly diabetic (Fig.7A). First we observed that in vivo glucagon secretion in response to glucose loading (Fig.7D) was corrected by insulin treatment whereas fasting glucagonemia of treated diabetic mice were still elevated (5.8+/-0.6pM, Fig.7E) compared to control mice (3.2+/-0.5, Fig.1F).
Figure 7. Insulin treatment of STZ-induced diabetic mice partially corrects α-cell function.
Data corresponding to control chow-diet (CTRL), diabetic mice (STZ) and treated diabetic mice with insulin implant (STZ+Insulin implant) are illustrated respectively by black, white and grey colors. Black arrow indicates the begining of insulin treatment (insulin implant input). Weight (A) and random glycemia (B) were monitored during 28 days whereas glycated hemoglobin (HbA1c; C) was measured at the end of treatment. OGTT was performed in insulin-treated STZ-induced diabetic mice and evaluated for glycemia (D) and glucagonemia before and 15min after glucose loading (E). Due to the fact that insulin-treated STZ-induced diabetic mice do not tolerate fasting, glucose (1mg/kg of D-glucose) was administered during the fasting period when glycemia reached 5 to 6mM (T0). α-cell secretion was evaluated from Facs-sorted Venus+ α cells of control (CTRL, black), STZ-diabetic (STZ, white) and insulin-treated STZ (STZ+Insulin implant, grey) mice (F-G). Acute glucagon secretion was evaluated during 30min periods in 5.6 and 1mM glucose solutions and expressed relative to glucagon content in each condition (F). The effect of glucose was evaluated by the ratio of glucagon secretion measured at 1mM and 5.6mM glucose (G). Each parameter was evaluated from 11 mice except for ex vivo secretion assays which included Facs-sorted α cells from 5 mice.
* indicate statistical significance compared to control. $ significance compared to STZ group. & significance compared to 5.6mM conditions
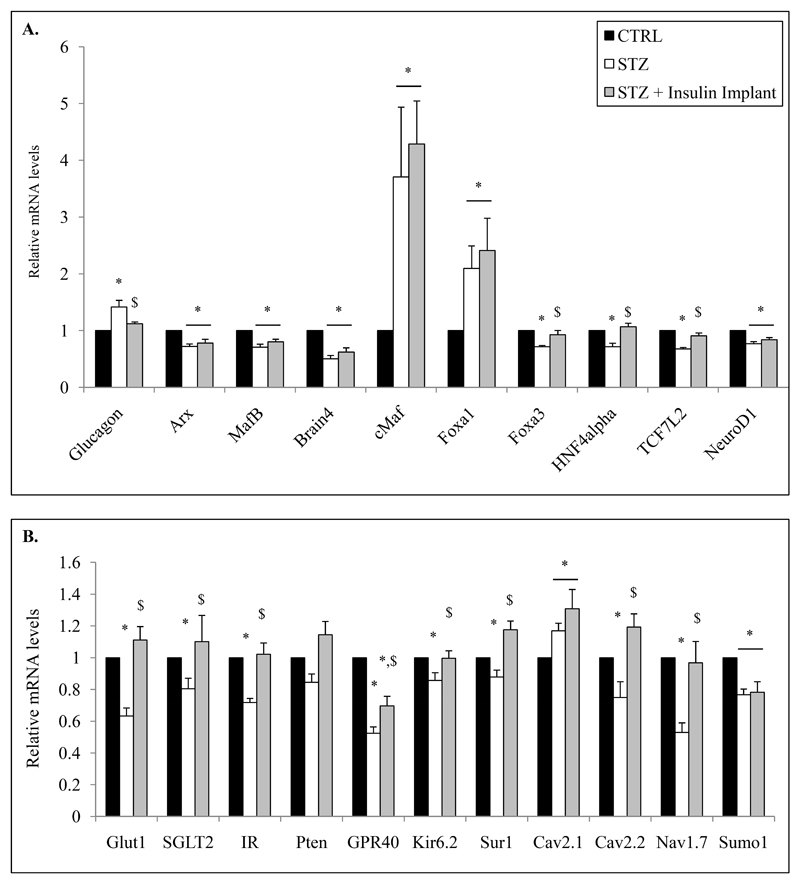
We then specifically analyzed Facs-sorted α cells from control, STZ-diabetic and insulin-treated STZ-diabetic mice and observed that insulin treatment partially corrected altered glucagon secretion (Fig.6F). Indeed, basal glucagon secretion (5.6mM glucose condition) of sorted α cells from STZ mice was normalized by insulin whereas stimulation by low glucose was not fully corrected (Fig.7F-G). We then investigated the effects of insulin treatment on α-cell gene expression. Increased glucagon mRNA levels observed in diabetic mice were corrected by insulin (Fig.8A). Similarly Foxa3, HNF4alpha, TCF7L2, Glut1 and Sglt2, Cav2.2, Nav1.7, Kir6.2, Sur1, Pten and IR mRNA levels were normalized by insulin treatment whereas those coding for Arx, MafB, Brain4, Foxa1, cMaf, NeuroD1, Cav2.1 and Sumo1 were not (Fig.8A-B). GPR40 mRNA levels were partially corrected but still remained significantly different compared to controls (Fig.8B).
Figure 8. Insulin treatment of STZ-induced diabetic mice partially corrects molecular alterations of α-cell.
mRNA quantification of specific genes from FACS-sorted α cells from control, STZ and STZ+insulin implant mice (A-B). mRNA levels of each tested genes are relative to RPS9, HPRT, Actin Beta, Tubulin Beta and cyclophilin mRNA values. Data are presented as the means (relative to control) ± SE (%) for 3 different experiments including 10 animals in each group. Relative mRNA levels of STZ-diabetic mice (+/- Insulin implant) are analyzed compared to their own littermate controls.
* indicate statistical significance compared to control. $ significance compared to STZ group.
Our results thus suggest that insulin treatment can reverse specific alterations of α cells observed in diabetes such as glucagon biosynthesis and expression of genes involved in glucose transport and insulin signaling but is not sufficient or unable to completely correct dysregulated glucagon secretion and genes involved in α-cell differentiation and maturation.
Discussion
Alterations of the glucagon secretory responses observed in diabetes have been attributed to several defects in α-cells including defective glucose sensing, loss of β-cell function, insulin resistance or autonomic malfunction (13,14). Despite a paucity of informations concerning potential intrinsic defects of pancreatic α cells in diabetes, recent data suggest that α-cell dysfunction might be due to both glucotoxicity and insulin deficiency (15). Furthermore, in vitro studies mainly performed on rodent glucagon-producing cell lines have illustrated the effects of glucose and palmitate excess on α-cell function including insulin resistance, altered glucagon secretion and increased glucagon biosynthesis (16,17).
Whether α-cell dysfunction, particularly in response to glucose, is an intrinsic defect of impaired glucose sensing and/or of insulin action or an extrinsic defect due to absence of functional β cells has still to be clearly determined. To characterize α-cell alterations in diabetes, we used insulinopenic high dose STZ-induced diabetes male mice which are characterized by specific functional α-cell alterations including increases of basal and dysregulated glucagon secretion as well as a transient increase of α-cell mass (18,19). Our data confirm basal hyperglucagonemia during fasting and increased glucagon secretion after glucose ingestion with a similar α-cell mass compared to control mice. Despite controversial data, several studies have clearly shown a transient increase of α-cell mass in STZ-induced diabetes mainly due to α-cell proliferation a few days after STZ injection (20–22). By contrast when focusing on α-cell mass 4 weeks after STZ-induced hyperglycemia, no major variation was observed anymore (15,19). Our data clearly indicate that alpha cell number per islet and relative to pancreatic area were increased with a significant decreased of islet number and pancreatic area leading to eventually no change in the total pancreatic α-cell number 28 days after STZ administration. Furthermore we also observed that α-cell size as well as cellular glucagon content was increased in STZ mice suggesting that α cells exhibit hypertrophy with cellular accumulation of glucagon in STZ-induced diabetes as previously described (15).
Recent studies using transgenic mice with specific labeling of glucagon-producing cells have clearly highlighted that α cells from STZ-induced diabetic mice exhibited increases of glucagon content and release (15). Our ex vivo studies using isolated α cells from control and STZ-diabetic mice indicate that the increase of glucagonemia is secondary to increases in glucagon biosynthesis and release per cell with 42% increases of glucagon mRNA levels and 2.5-fold higher basal glucagon secretion without glucose regulation suggesting that these cells are potentially locked in a hypersecretory state.
We demonstrate for the first time that isolated α cells from STZ-diabetic mice exhibit chronic molecular alterations of genes coding for proteins involved in glucagon biosynthesis and secretion as well as α-cell identity. The mRNA levels coding for Arx, MafB, Brain4 and NeuroD1, specific α-cell markers and transcription factors linked to the α-cell lineage, differentiation and maturation (23–26), are decreased in α cells from STZ-diabetic mice suggesting that α-cell identity could be altered as previously proposed in STZ-treated rats (27). Interestingly, insulin treatment which partially corrects α-cell function did not correct these alterations suggesting that chronic insulinopenic hyperglycemic in vivo situation leads to persistent α-cell defects.
Regulated glucagon secretion is a complex process involving many factors among them glucose and insulin. Whereas several components such as glucose transporters, ion channels and signaling pathways are identified as modulators of glucagon secretion few of them have been shown to be altered in diabetes (13,14). Among them sodium glucose cotransporters Sglt2 as well as its potential regulators HNF4alpha emerge as potential key factors linked to diabetes and alterations in glucagon biosynthesis and secretion (28). Recent work showed indeed that the specific Sglt2 antagonist dapaglifozin led to increases of glucagon mRNA levels and secretion in human islets as it was observed after specific silencing of Sglt2 and HNF4alpha using siRNAs indicating the critical importance of SGLT2 in glucagon secretion. Furthermore expression of these 2 genes were found to be affected in human islets from obese and type 2 diabetic patients and decreased after prolonged exposure to high-glucose concentrations in α-TC1.9 glucagon-producing cells. Similarly we observed that Sglt2 as well as HNF4alpha are also decreased in α cells from STZ-diabetic mice as well as the expression of the Glut1 gene suggesting that glucose influx in α cells could be affected in diabetes and potentially lead to blindness of the α cells to glucose and thus hypersecretion of glucagon. Of importance, insulin treatment normalized both Sglt2 and HNF4α as as well as Glut1 gene expression and hypersecretion of glucagon.
The expression of genes coding for Kir6.2 and Sur1 subunits of K-ATP channel, Nav1.7 sodium channel and Cav2.2 calcium channel were also decreased whereas expression of the Cav2.1 gene was increased in α cells from STZ-induced diabetic mice. These channels were previously identified as regulators/effectors of glucagon secretion (29–32). Indeed, mutation of Kir6.2 as well as Sur1 knock-out led to alterations of glucose effects on glucagon secretion evidencing their contribution in the regulated secretion process (33–35). Nevertheless, except for the Nav1.7 gene which exhibited 47% decreased expression in α cells from diabetic mice, alterations of Kir6.2/Sur1 and Cav2.1/2.2 expression were modest and unlikely to fully explain the unresponsiveness to glucose. Dysregulation of these genes except Cav2.1 were also normalized by insulin treatment.
Finally, we observed significant and substantial increases of Foxa1 and cMaf expression, two transcription factors involved in glucagon gene expression (36,37), in diabetic α cells suggesting a potential link with the increase of glucagon biosynthesis and secretion. However, we observed that in vivo insulin treatment reversed the increase of mRNA levels of glucagon but not those coding for Foxa1 and cMaf indicating that these 2 transcription factors are not causal in the increase of glucagon biosynthesis in diabetes.
Chronic but not short-term insulin treatment of diabetic mice has been shown to correct α-cell dysfunctions (19,38). Our data indicate that chronic insulin treatment of STZ-diabetic mice with non-regulated insulin implant which partially improve glycemia and glucagon secretion, is able to normalize the expression of multiple genes. However Foxa1, cMaf, GPR40, Sumo1 and Cav2.1 as well as Arx, MafB, Brain4 and NeuroD1 gene expression were not corrected by insulin suggesting that these targets may reflect intrinsic α-cell defects in diabetes, need intra-islet insulin or other additional factors to be corrected.
The changes in α-cell gene expression we observed might be implicated in the alterations of α-cell function or be secondary or adaptative to diabetes and reflect protective mechanisms. STZ-diabetic mice as well as type 1 diabetic patients are characterized by hypoinsulinemia, hyperglycemia and elevated circulating FFA (39,40). During long-term exposure with high glucose and/or FFA concentrations as well as insulin deficiency adaptative responses as it was previously described in several organs including placenta, retina, muscle, kidney and pancreatic β cells. Indeed, in vitro exposure to high concentration of glucose led to a 50% decrease of Glut1 mRNA levels in human placental trophoblast (41) as well as in the retina and its microvessels of STZ-diabetic rats (42). Similarly inhibition of fatty-acids receptor GPR40 gene expression, which is known to be involved in glucagon secretion, protects insulin-producing cells to palmitate-induced ER stress and apoptosis (43). Our in vivo results showing decreased Sglt2, Glut1 and GPR40 gene expression in α cells in response to high concentration of glucose or palmitate go along with an adaptative response which then may lead to α-cell dysfunction.
Recent data suggest that pancreatic α cells are resistant to metabolic stress-induced apoptosis in diabetes involving a detoxification mechanism with Bcl2l1 overexpression (44). Interestingly cMaf was previously identified as a protective factor in the kidney under ischemic and oxidative-stress conditions (45). Recent data also mentioned that large-Mafs transcription factors could be involved in the control of autophagic activity and protection of pancreatic cells for ER stress (46). In this context, our observation on the increase of cMaf gene expression (unregulated by insulin treatment) and decreases of Glut1, Sglt2 and GPR40 mRNA levels in STZ-diabetic mice could bring crucial and new informations concerining potential resistance of pancreatic α cells to the toxic environment in diabetes and assimilated to protective mechanisms.
In conclusion, hyperglycemia and hypoinsulinemia lead to chronic α-cell dysregulation involving increases of glucagon biosynthesis and secretion with changes in gene expression which are partially reversed by insulin treatment in male mice. We suggest that excess glucose and FFA in insulinopenic conditions lead to constitutive alterations of key genes and that the combination of these defects engage pancreatic α cells on its own protection in response to the toxic environment as well as in a dedifferentiation process developing subsequent alterations of glucagon biosynthesis and secretion.
Supplementary Material
Acknowledgments
This work was supported by the Swiss National Science Foundation, Fondation romande pour la recherche, Insuleman, Desirée and Niels Yde Fundation and Fondation pour la lutte contre le cancer et pour les études médico-biologiques and Novo Nordisk Pharmaceutical company.
We thank Pr. Pedro Herrera and his team (Department of Genetic Medicine and Development, University Medical Center, University of Geneva Medical School, Geneva, Switzerland) for transmission of mGlu124 Gcg-Venus Transgenic mouse strain.
YG and JP are the guarantors of this work and, as such, had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
1. Disclosure Statement: YG, RD, SH, MHM, FV, CV, FG and JP have nothing to declare. JP received grant support from Swiss National Fund (Main recipient, N°31003A-149749).
2. Disclosure Statement: The authors have nothing to disclose.
There is no conflict of interest. The authors have nothing to declare. The authors have nothing to disclose.
Authors Contribution
R.D, SH, MHM, FV and CV: researched data; F.G. contributed to reviewed/edited manuscript; J.P. contributed discussion/reviewed/edited manuscript; Y.G. wrote manuscript, researched data.
Footnotes
The abbreviations used are: IR : Insulin Receptor, HbA1c : glycated hemoglobin (%), CTRL : control(s), STZ : streptozotocin, OGTT : Oral Glucose Tolerance Test, RPS9 : ribosomic protein 9S, SE: Standard Error of the Mean (=Standard Deviation/√(sample size). FFA: Free Fatty-Acids.
References
- 1.Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975;1:14–16. doi: 10.1016/s0140-6736(75)92375-2. [DOI] [PubMed] [Google Scholar]
- 2.Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocrine reviews. 2007;28:253–283. doi: 10.1210/er.2006-0026. [DOI] [PubMed] [Google Scholar]
- 3.Dinneen S, Alzaid A, Turk D, Rizza R. Failure of glucagon suppression contributes to postprandial hyperglycaemia in IDDM. Diabetologia. 1995;38:337–343. doi: 10.1007/BF00400639. [DOI] [PubMed] [Google Scholar]
- 4.Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. The Journal of clinical endocrinology and metabolism. 2000;85:4053–4059. doi: 10.1210/jcem.85.11.6993. [DOI] [PubMed] [Google Scholar]
- 5.Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182:171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- 6.Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia. 2002;45:937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- 7.King AJ. The use of animal models in diabetes research. Br J Pharmacol. 2012;166:877–894. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell metabolism. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes. 2011;60:391–397. doi: 10.2337/db10-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gosmain Y, Marthinet E, Cheyssac C, Guerardel A, Mamin A, Katz LS, Bouzakri K, Philippe J. Pax6 controls the expression of critical genes involved in pancreatic {alpha} cell differentiation and function. J Biol Chem. 2010;285:33381–33393. doi: 10.1074/jbc.M110.147215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gosmain Y, Katz LS, Masson MH, Cheyssac C, Poisson C, Philippe J. Pax6 is crucial for beta-cell function, insulin biosynthesis, and glucose-induced insulin secretion. Mol Endocrinol. 2012;26:696–709. doi: 10.1210/me.2011-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maechler P, Gjinovci A, Wollheim CB. Implication of glutamate in the kinetics of insulin secretion in rat and mouse perfused pancreas. Diabetes. 2002;51(Suppl 1):S99–102. doi: 10.2337/diabetes.51.2007.s99. [DOI] [PubMed] [Google Scholar]
- 13.Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocrine reviews. 2007;28:84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- 14.Quesada I, Tuduri E, Ripoll C, Nadal A. Physiology of the pancreatic alpha-cell and glucagon secretion: role in glucose homeostasis and diabetes. The Journal of endocrinology. 2008;199:5–19. doi: 10.1677/JOE-08-0290. [DOI] [PubMed] [Google Scholar]
- 15.Huang YC, Rupnik MS, Karimian N, Herrera PL, Gilon P, Feng ZP, Gaisano HY. In situ electrophysiological examination of pancreatic alpha cells in the streptozotocin-induced diabetes model, revealing the cellular basis of glucagon hypersecretion. Diabetes. 2013;62:519–530. doi: 10.2337/db11-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen XX, Li HL, Pan L, Hong J, Xiao J, Hermansen K, Jeppesen PB, Li GW. Glucotoxicity and alpha cell dysfunction: involvement of the PI3K/Akt pathway in glucose-induced insulin resistance in rat islets and clonal alphaTC1-6 cells. Endocrine research. 2012;37:12–24. doi: 10.3109/07435800.2011.610855. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Hermansen K, Xiao J, Bystrup SK, O'Driscoll L, Jeppesen PB. Isosteviol has beneficial effects on palmitate-induced alpha-cell dysfunction and gene expression. PloS one. 2012;7:e34361. doi: 10.1371/journal.pone.0034361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Karlsson FA, Sandler S. Islet loss and alpha cell expansion in type 1 diabetes induced by multiple low-dose streptozotocin administration in mice. The Journal of endocrinology. 2000;165:93–99. doi: 10.1677/joe.0.1650093. [DOI] [PubMed] [Google Scholar]
- 19.Meier JJ, Ueberberg S, Korbas S, Schneider S. Diminished glucagon suppression after beta-cell reduction is due to impaired alpha-cell function rather than an expansion of alpha-cell mass. American journal of physiology Endocrinology and metabolism. 2011;300:E717–723. doi: 10.1152/ajpendo.00315.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Zhang Y, Bone RN, Cui W, Peng JB, Siegal GP, Wang H, Wu H. Regeneration of pancreatic non-beta endocrine cells in adult mice following a single diabetes-inducing dose of streptozotocin. PloS one. 2012;7:e36675. doi: 10.1371/journal.pone.0036675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeda Y, Fujita Y, Honjo J, Yanagimachi T, Sakagami H, Takiyama Y, Makino Y, Abiko A, Kieffer TJ, Haneda M. Reduction of both beta cell death and alpha cell proliferation by dipeptidyl peptidase-4 inhibition in a streptozotocin-induced model of diabetes in mice. Diabetologia. 2012;55:404–412. doi: 10.1007/s00125-011-2365-4. [DOI] [PubMed] [Google Scholar]
- 22.Thyssen S, Arany E, Hill DJ. Ontogeny of regeneration of beta-cells in the neonatal rat after treatment with streptozotocin. Endocrinology. 2006;147:2346–2356. doi: 10.1210/en.2005-0396. [DOI] [PubMed] [Google Scholar]
- 23.Wilcox CL, Terry NA, Walp ER, Lee RA, May CL. Pancreatic alpha-cell specific deletion of mouse Arx leads to alpha-cell identity loss. PloS one. 2013;8:e66214. doi: 10.1371/journal.pone.0066214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Artner I, Blanchi B, Raum JC, Guo M, Kaneko T, Cordes S, Sieweke M, Stein R. MafB is required for islet beta cell maturation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3853–3858. doi: 10.1073/pnas.0700013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heller RS, Stoffers DA, Liu A, Schedl A, Crenshaw EB, 3rd, Madsen OD, Serup P. The role of Brn4/Pou3f4 and Pax6 in forming the pancreatic glucagon cell identity. Developmental biology. 2004;268:123–134. doi: 10.1016/j.ydbio.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes & development. 1997;11:2323–2334. doi: 10.1101/gad.11.18.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang XD, Guo YY, Sun M, Ding Y, Wang N, Yuan L, De W. Streptozotocin-induced expression of Ngn3 and Pax4 in neonatal rat pancreatic alpha-cells. World journal of gastroenterology : WJG. 2011;17:2812–2820. doi: 10.3748/wjg.v17.i23.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thevenet J, Beaucamps C, Delalleau N, Popescu I, Malaisse WJ, Sener A, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nature medicine. 2015;21:512–517. doi: 10.1038/nm.3828. [DOI] [PubMed] [Google Scholar]
- 29.Rorsman P, Salehi SA, Abdulkader F, Braun M, MacDonald PE. K(ATP)-channels and glucose-regulated glucagon secretion. Trends in endocrinology and metabolism: TEM. 2008;19:277–284. doi: 10.1016/j.tem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Rorsman P, Braun M, Zhang Q. Regulation of calcium in pancreatic alpha- and beta-cells in health and disease. Cell calcium. 2012;51:300–308. doi: 10.1016/j.ceca.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vignali S, Leiss V, Karl R, Hofmann F, Welling A. Characterization of voltage-dependent sodium and calcium channels in mouse pancreatic A- and B-cells. The Journal of physiology. 2006;572:691–706. doi: 10.1113/jphysiol.2005.102368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Q, Chibalina MV, Bengtsson M, Groschner LN, Ramracheya R, Rorsman NJ, Leiss V, Nassar MA, Welling A, Gribble FM, Reimann F, et al. Na+ current properties in islet alpha- and beta-cells reflect cell-specific Scn3a and Scn9a expression. The Journal of physiology. 2014;592:4677–4696. doi: 10.1113/jphysiol.2014.274209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tschritter O, Stumvoll M, Machicao F, Holzwarth M, Weisser M, Maerker E, Teigeler A, Haring H, Fritsche A. The prevalent Glu23Lys polymorphism in the potassium inward rectifier 6.2 (KIR6.2) gene is associated with impaired glucagon suppression in response to hyperglycemia. Diabetes. 2002;51:2854–2860. doi: 10.2337/diabetes.51.9.2854. [DOI] [PubMed] [Google Scholar]
- 34.Gromada J, Ma X, Hoy M, Bokvist K, Salehi A, Berggren PO, Rorsman P. ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1-/- mouse alpha-cells. Diabetes. 2004;53(Suppl 3):S181–189. doi: 10.2337/diabetes.53.suppl_3.s181. [DOI] [PubMed] [Google Scholar]
- 35.Cheng-Xue R, Gomez-Ruiz A, Antoine N, Noel LA, Chae HY, Ravier MA, Chimienti F, Schuit FC, Gilon P. Tolbutamide controls glucagon release from mouse islets differently than glucose: involvement of K(ATP) channels from both alpha-cells and delta-cells. Diabetes. 2013;62:1612–1622. doi: 10.2337/db12-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heddad Masson M, Poisson C, Guerardel A, Mamin A, Philippe J, Gosmain Y. Foxa1 and Foxa2 regulate alpha-cell differentiation, glucagon biosynthesis, and secretion. Endocrinology. 2014;155:3781–3792. doi: 10.1210/en.2013-1843. [DOI] [PubMed] [Google Scholar]
- 37.Gosmain Y, Avril I, Mamin A, Philippe J. Pax-6 and c-Maf functionally interact with the alpha-cell-specific DNA element G1 in vivo to promote glucagon gene expression. The Journal of biological chemistry. 2007;282:35024–35034. doi: 10.1074/jbc.M702795200. [DOI] [PubMed] [Google Scholar]
- 38.Dufrane D, Maillart JF, Aouassar N, Goebbels RM, Guiot Y, Gianello P. Native pancreatic alpha-cell adaptation in streptozotocin-induced diabetic primates: importance for pig islet xenotransplantation. Xenotransplantation. 2009;16:152–163. doi: 10.1111/j.1399-3089.2009.00530.x. [DOI] [PubMed] [Google Scholar]
- 39.Ito M, Kondo Y, Nakatani A, Naruse A. New model of progressive non-insulin-dependent diabetes mellitus in mice induced by streptozotocin. Biological & pharmaceutical bulletin. 1999;22:988–989. doi: 10.1248/bpb.22.988. [DOI] [PubMed] [Google Scholar]
- 40.Murray AJ, Panagia M, Hauton D, Gibbons GF, Clarke K. Plasma free fatty acids and peroxisome proliferator-activated receptor alpha in the control of myocardial uncoupling protein levels. Diabetes. 2005;54:3496–3502. doi: 10.2337/diabetes.54.12.3496. [DOI] [PubMed] [Google Scholar]
- 41.Hahn T, Barth S, Weiss U, Mosgoeller W, Desoye G. Sustained hyperglycemia in vitro down-regulates the GLUT1 glucose transport system of cultured human term placental trophoblast: a mechanism to protect fetal development? FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1998;12:1221–1231. doi: 10.1096/fasebj.12.12.1221. [DOI] [PubMed] [Google Scholar]
- 42.Badr GA, Tang J, Ismail-Beigi F, Kern TS. Diabetes downregulates GLUT1 expression in the retina and its microvessels but not in the cerebral cortex or its microvessels. Diabetes. 2000;49:1016–1021. doi: 10.2337/diabetes.49.6.1016. [DOI] [PubMed] [Google Scholar]
- 43.Wu J, Sun P, Zhang X, Liu H, Jiang H, Zhu W, Wang H. Inhibition of GPR40 protects MIN6 beta cells from palmitate-induced ER stress and apoptosis. Journal of cellular biochemistry. 2012;113:1152–1158. doi: 10.1002/jcb.23450. [DOI] [PubMed] [Google Scholar]
- 44.Marroqui L, Masini M, Merino B, Grieco FA, Millard I, Dubois C, Quesada I, Marchetti P, Cnop M, Eizirik DL. Pancreatic alpha Cells are Resistant to Metabolic Stress-induced Apoptosis in Type 2 Diabetes. EBioMedicine. 2015;2:378–385. doi: 10.1016/j.ebiom.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shirota S, Yoshida T, Sakai M, Kim JI, Sugiura H, Oishi T, Nitta K, Tsuchiya K. Correlation between the expression level of c-maf and glutathione peroxidase-3 in c-maf -/- mice kidney and c-maf overexpressed renal tubular cells. Biochemical and biophysical research communications. 2006;348:501–506. doi: 10.1016/j.bbrc.2006.07.111. [DOI] [PubMed] [Google Scholar]
- 46.Tsuchiya M, Misaka R, Nitta K, Tsuchiya K. Large Mafs modulate autophagic activity and ER stress in diabetic pancreas. ADA congress. 2015 (poster communication #2279-P); Unpublished data. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.