Abstract
Intermittent fasting has been suggested as an option for managing overweight and obesity. The purpose of this article is to present a balanced review of the practice of intermittent fasting and its impact on glycemic control in people with diabetes.
Medical nutritional therapy is an essential component of the management of patients with diabetes, hypertension, cardiovascular disease (CVD), and other metabolic disorders (1). The increasing prevalence rates of diabetes and other metabolic disorders are linked to overweight and obesity. Dietary factors and physical inactivity underlie the increasing rates of overweight and obesity. Consequently, interventions involving calorie restriction and increased physical activity have been promoted as adjuncts to the management of chronic metabolic disorders.
An observational analysis (2) of 5,145 participants in the Look AHEAD (Action for Health in Diabetes) study explored the association between changes in cardiovascular (CV) risk factors and the amount of weight loss in people with type 2 diabetes. The study showed that participants who lost 2–5% of their body weight had increased odds of having significant improvements in A1C of 1.80% (95% CI 1.44–2.24). Participants who lost at least 5–10% of their body weight also experienced improvements in systolic blood pressure, diastolic blood pressure, HDL cholesterol, and triglycerides (P <0.0001). This study showed that weight loss is the key component of treatment to reduce A1C in patients with type 2 diabetes.
Given the strong association between overweight/obesity and type 2 diabetes, lifestyle intervention to induce weight loss has become a primary objective of diabetes prevention and management efforts (3,4). Key components of lifestyle intervention include maintenance of calorie-reduced healthy eating patterns and increased physical activity.
Intermittent fasting (IF) is an emerging practice in dietary modification. The purpose of this article is to present a balanced review of the practice of IF and its impact on glycemic control in people with diabetes.
IF Definition
Although approaches differ, IF generally refers to a practice in which individuals fast for 24 hours every other day or for 16 hours every day. The most common IF method is an approach in which people take the last meal of the day at 8:00 p.m., begin fasting upon waking the next morning, and continue fasting until noon that day. Total sleeping hours plus morning fasting hours yields 16 hours of fasting. The typical feeding period in this plan is between noon and 8:00 p.m. This approach is easy to follow, especially for beginners, and has been reported to achieve more significant weight loss than alternative IF schedules (5,6). During the fasting period, people are allowed to drink water and zero-calorie beverages. During the feeding period, there is no restriction on eating or food choices.
Unlike the postprandial state, fasting results in decreased levels of blood glucose and insulin leading to increased glucagon secretion, which in turn promotes glycogenolysis and the release of free glucose to prevent hypoglycemia. IF is not a novel concept; it has a long history in human cultural and religious traditions (7–9). What is novel is the modern application of IF as a tool for achieving clinical outcomes (10,11).
The long-term effects of IF, including benefits and harms, and its underlying mechanisms, have not been well studied. Detailed knowledge of the risks and benefits of IF must be understood before its widescale adoption in the population.
Literature Review
Search Strategy
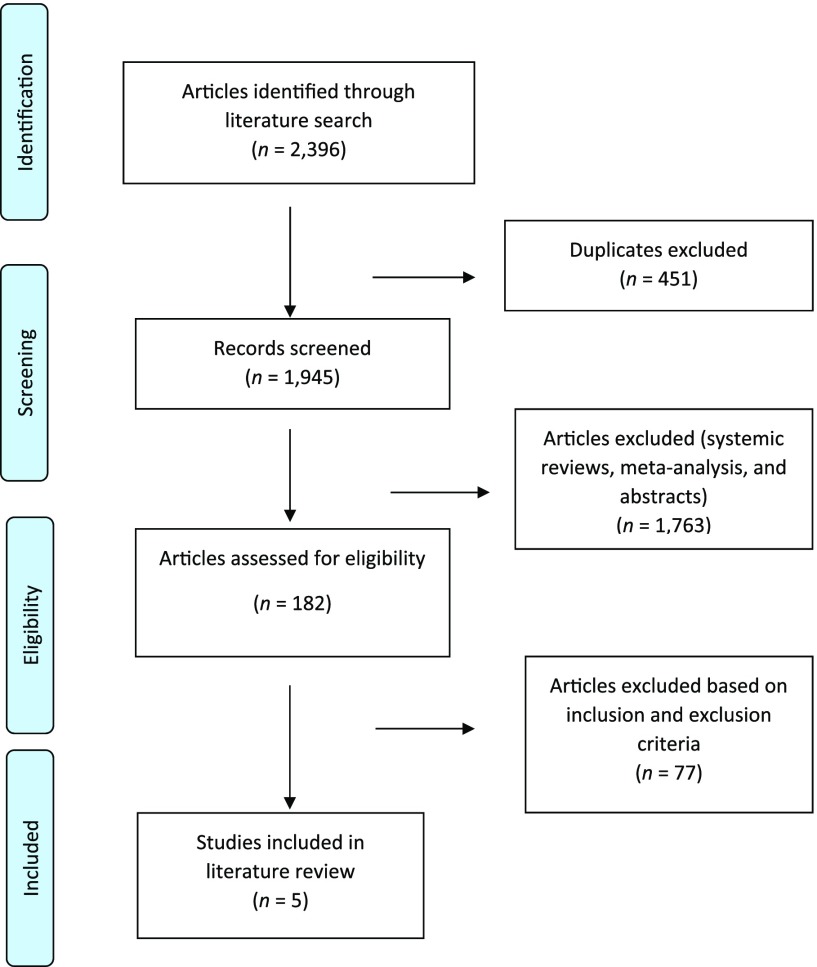
Our search strategy was based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Figure 1), using the search engines Clinical Key, PubMed, Medline, Ovid, and Google Scholar and the search terms “intermittent fasting,” “fasting,” “diabetes,” “glycemic control,” “HbA1c,” and “blood glucose.” All identified publications between 1998 and 2019 were included for further processing (Figure 1).
FIGURE 1.
PRISMA flow diagram of literature search strategy.
Selected for inclusion in our literature review were randomized controlled trials (RCTs) that tested any types of IF among adults with type 2 diabetes that lasted at least 3 months and reported change in A1C as one of the prespecified clinical outcomes. We excluded studies in which participants were <18 years of age, that had designs other than an RCT, and that did not specify A1C as an outcome. We also excluded studies reported in languages other than English, those published before 1998, and nonhuman studies.
We identified 2,396 studies in the initial literature search and excluded 451 studies that were duplicates. Among the 1,945 articles screened, we excluded abstracts, studies in progress, systematic reviews, and meta-analyses, leaving 182 potentially eligible articles. We then applied our inclusion and exclusion criteria, which yielded five articles to include in our literature review.
Review of Included Studies
Table 1 summarizes the baseline characteristics of participants, durations, and outcomes of the studies selected for review.
TABLE 1.
Studies Showing the Effects of IF on A1C and Weight Loss in Adults With Type 2 Diabetes
| Groups.. | Design.. | n.. | Mean Age, years | Study Duration, months. | Primary Outcome.. | Mean Weight Change From Baseline, kg | Mean A1C Change From Baseline, % | P for Change in A1C | |
|---|---|---|---|---|---|---|---|---|---|
| Sundfør et al. (12) | Intermittent | RCT | 54 | 49.9 | 12 | Weight loss | −9.1 | −0.3 | 0.2 |
| Continuous | 58 | 47.5 | −9.4 | −0.2 | 0.2 | ||||
| Carter et al. (13) | Intermittent | RCT | 70 | 61.0 | 12 | A1C | −6.8 | −0.3 | <0.001 |
| Continuous | 67 | 61.0 | −5.0 | −0.5 | <0.001 | ||||
| Corley et al. (14) | Consecutive | RCT | 19 | 62.0 | 3 | Hypoglycemia | −3.6 | −0.6 | 0.53 |
| Nonconsecutive | 22 | 58.0 | 3 | −3.1 | −0.7 | 0.53 | |||
| Li et al. (15) | Fasting | RCT | 23 | 64.7 | 4 | Weight loss | −3.5 | −0.2 | 0.30 |
| Control | 23 | 65.4 | −2.0 | −0.2 | 0.28 | ||||
| Williams et al. (17) | 1-day VLCD | RCT | 18 | 51.4 | 4 | Weight loss and glycemic control | −16.5 | −0.71 | 0.38 |
| 5-day VLCD | 18 | 50.3 | 4 | −10.4 | −0.97 | 0.38 | |||
| Control | 18 | 54.1 | 4 | −5.4 | −0.23 | 0.38 |
In a randomized nonblinded clinical trial by Sundfør et al. (12), participants were assigned to an intermittent energy restriction (IER) group (n = 54, mean age 49.9 ± 10.1 years) or a continuous energy restriction (CER) group (n = 58, mean age 47.5 ± 11.6 years) for 12 months. The primary outcome was change in weight. The IER group had a daily intake of 400 kcal for women and 600 kcal for men for 2 nonconsecutive days per week and consumed their usual meals on 5 days per week. The CER group reduced their food intake evenly throughout the week but achieved the same weekly reduction in energy intake as the IER group. The mean change in A1C was 0.3% in the IER group versus 0.2% in the CER (P = 0.2). The authors concluded that IER was as effective as CER at inducing weight reduction and improving metabolic outcomes.
In a parallel randomized trial by Carter et al. (13), 137 participants with type 2 diabetes were randomized into two groups. The IER group (n = 70, mean age 61 ± 9.0 years) alternately consumed 500–600 kcal/day for 2 days per week, followed by their usual diet for 5 days per week for 3 months. The CER group (n = 67, mean age 61 ± 9.2 years) consumed a continuous calorie-restricted diet (1,200–1,500 kcal/day) for 3 months. The primary outcome of change in A1C was 0.3% in the IER group versus 0.5% in the CER group (P <0.001). Hypoglycemia events were similar between groups. This study showed that IF can be an effective diet to improve A1C in patients with type 2 diabetes (13).
In a nonblinded study by Corley et al. (14), 41 participants with type 2 diabetes (baseline A1C 6.7–10.0%) receiving metformin and other antidiabetic medications were randomized into two groups, one adhering to consecutive fasting days and the other to nonconsecutive fasting days. Participants in the consecutive-day arm fasted for 2 consecutive days per week, whereas those in the nonconsecutive-day arm fasted on any 2 days of their choice per week for 12 weeks. Energy was restricted on fasting days to 2,092–2,510 kJ (men could consume ∼400 kJ/day more than women). The primary outcome of hypoglycemia events was 35 in the consecutive-day arm versus 20 in the nonconsecutive-day arm. The authors observed that the fasting intervention was associated with a twofold increase in the relative risk of hypoglycemia; however, there was no difference in the risk of having a hypoglycemic event between the consecutive-day and nonconsecutive-day fasting groups (14). The study also showed A1C reductions of 0.6% in the consecutive-day arm and 0.7% in the nonconsecutive-day arm (P = 0.53 for mean difference between groups).
In a pilot study by Li et al. (15), 46 participants with type 2 diabetes were randomized into two groups (a fasting group and a control group). The fasting group (n = 23, mean age 64.7 ± 7.0 years) had two prefasting days during which they consumed ∼1,200 kcal of a low-salt diet including only vegetables and plain cooked rice, followed by a fasting period of ∼7 days, during which they consumed 300 kcal/day (vegetable soup, 200 mL fruit juice, herbal tea, and unrestricted water). The fast was followed by 3 days of a low-calorie diet with solid foods reintroduced in a stepwise manner, after which participants were advised to follow a normocaloric Mediterranean diet (16) for 4 months. The control group (n = 23, mean age 65.4 ± 5.7 years) consumed a Mediterranean diet for 4 months. The primary outcome of change in weight was significantly greater on the fasting group than in the control group (Table 1). Both groups experienced improved glycemic control; change in A1C (a secondary outcome) was similar between groups.
Finally, in a study by Williams et al. (17), 54 overweight participants with type 2 diabetes were randomized into three groups. Group 1 consumed a very-low-calorie diet (VLCD; 400–600 kcal/day) on 1 day per week for 20 weeks. Group 2 consumed the VLCD for 5 consecutive days every 5 weeks throughout the 20-week study period. Group 3 was a standard behavioral therapy group in which participants consumed 1,500–1,800 kcal/day throughout the study period. During nonfasting days, participants in groups 1 and 2 consumed the same energy as those in group 3. Compared with the standard behavioral therapy group, participants in both VLCD groups showed significant weight loss (Table 1). Mean change in A1C was also greater in both VLCD groups (0.71 and 0.97%) compared with the nonfasting group (0.23%) (Table 1).
Discussion
We were surprised to find how few RCTs had been conducted to test the effects of IF on glucoregulation in patients with type 2 diabetes. Based on results of the few available trials, IF appears to offer some metabolic benefits. Caloric reduction through IF can be expected to lead to weight loss if maintained over time. Thus, the chronic benefits of IF on glucoregulation may be explained by improved insulin sensitivity after weight loss (18–20).
It is also possible that acute metabolic effects can result from IF or from skipping a meal that would have delivered a carbohydrate and fat load. Reduction in meal-delivered carbohydrate and fat load can be expected to have beneficial effects on glycemia. Because IF strategies tend to involve skipping breakfast and subsequent meals and snacks, the question of potential adverse effects of skipping breakfast becomes pertinent. There are conflicting reports on the effects of skipping breakfast, including a risk of possible weight gain (21–24). However, a recent systematic review of 11 studies on the subject concluded that skipping breakfast is not associated with weight gain (25).
There are several potential mechanisms that link IF to metabolic benefits. These include modulation of macronutrient fluxes (e.g., free fatty acids, amino acids, and carbohydrates) (26), inflammatory cytokines (27), the gut microbiome (28,29), and sirtuin 1 gene activation (30), among others.
Studies by Boden et al. (31) and Krebs et al. (32) showed that a low-carbohydrate diet is associated with improvement in insulin sensitivity, blood glucose levels, and A1C. Such a diet also leads to a decrease in total cholesterol and triglycerides (31,32). Intermittent exclusion of fat intake through IF also can be expected to improve insulin sensitivity. Obese patients with diabetes treated with acipimox (which reduced free fatty acids) showed enhanced insulin-mediated glucose disposal and suppression of gluconeogenesis (33). Similarly, specific branched-chain and aromatic amino acids have been associated with insulin resistance, and reducing their circulating levels can be expected to improve insulin signaling (34–37).
There is emerging evidence that restricting feeding times and extending fasting (or interprandial) intervals can promote weight loss, possibly via modulation of the gut microbiome (28,29). Furthermore, calorie restriction is a potent activator of sirtuin, the mammalian ortholog of the highly conserved nicotinamide adenine dinucleotide-dependent protein deacetylase that has emerged as a key metabolic and longevity mediator (38). In experimental models, calorie restriction has prolonged life span and vigorous healthy life (39,40). Theoretically, IF that is not overcompensated for by excessive food intake on feeding days could potentially lead to the consumption of fewer calories overall, which in turn could lead to weight loss (41). Sustained weight loss would then predict improved glucose and lipid levels.
Neuroendocrine Adaptations to Fasting
Fasting, especially if prolonged, is associated with several neuroendocrine adaptations, including increased secretion of cortisol, glucagon, adiponectin, growth hormone, and melatonin and decreased secretion of insulin, leptin, and thyroid hormones (42,43). Of the latter adaptations, the downregulation of leptin secretion exerts the most pertinent effect on energy balance. Leptin is a polypeptide hormone derived from adipocytes that bind to specific receptors in the hypothalamus to signal satiety and decrease energy intake. Leptin levels decrease dramatically during fasting, and such a drop is associated with decreased energy expenditure and increased appetite, which could hinder the desired weight loss (44). Leptin supplementation has been shown to prevent the impulse of weight regain in the post-obese state (45). Thus, there is a need to study the effect of IF on leptin expression to determine whether persistent leptin suppression might be a factor weighing against the sustained efficacy of IF as a weight loss strategy.
Pitfalls of IF
Based on current practice, people who follow an IF regimen are not restricted with regard to the quantity or quality of foods consumed during feeding periods. IF has also been linked to pathological eating patterns and increased stress response (46–48). The consumption of unwholesome diets during feeding days could expose practitioners of IF to foods that are proinflammatory and increase oxidative stress. Thus, it is advisable for people who practice IF to receive counseling on the need for healthy eating patterns during feeding days.
Conclusion
Our interpretation and synthesis of the pertinent studies (12–15,17) showed that IF improves glycemic control. However, not all studies were concordant regarding the metabolic benefits that can be expected from IF. Variable responses have been reported, which may be explained by possible nonadherence to IF among trial participants. Nonetheless, the weight of the evidence to date suggests that IF might have a role to play in the management of chronic metabolic disorders such as obesity and type 2 diabetes. The challenges with IF approaches appear to be the risk of unwholesome feeding behavior on nonfasting days and hunger management in general. In addition to behavioral intervention and nutrition counseling, pharmacologic agents that inhibit hunger could potentially be used with IF. To date, however, no studies have reported on such a strategy.
Article Information
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
K.G. researched data and wrote the manuscript. Y.H. assisted with data research. S.D.-J. reviewed and edited the manuscript. K.G. is the guarantor of this work and, as such, had full access to all the data reported and takes responsibility for the integrity of the data and the accuracy of the review.
References
- 1.Dagogo-Jack S, Egbuonu N, Edeoga C. Principles and practice of nonpharmacological interventions to reduce cardiometabolic risk. Med Princ Pract 2010;19:167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wing RR, Lang W, Wadden TA, et al. ; Look AHEAD Research Group . Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011;34:1481–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wadden TA, West DS, Delahanty L, et al. ; Look AHEAD Research Group . The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horne BD, Muhlestein JB, Anderson JL. Health effects of intermittent fasting: hormesis or harm? A systematic review. Am J Clin Nutr 2015;102:464–470 [DOI] [PubMed] [Google Scholar]
- 6.Gabel K, Hoddy KK, Haggerty N, et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging 2018;4:345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibrahim M, Abu Al Magd M, Annabi FA, et al. Recommendations for management of diabetes during Ramadan: update 2015. BMJ Open Diabetes Res Care 2015;3:e000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalra S, Bajaj S, Gupta Y, et al. Fasts, feasts and festivals in diabetes: 1. glycemic management during Hindu fasts. Indian J Endocrinol Metab 2015;19:198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarri KO, Tzanakis NE, Linardakis MK, Mamalakis GD, Kafatos AG. Effects of Greek Orthodox Christian Church fasting on serum lipids and obesity. BMC Public Health 2003;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem 2005;16:129–137 [DOI] [PubMed] [Google Scholar]
- 11.Anson RM, Guo Z, de Cabo R, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A 2003;100:6216–6220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundfør TM, Svendsen M, Tonstad S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: a randomized 1-year trial. Nutr Metab Cardiovasc Dis 2018;28:698–706 [DOI] [PubMed] [Google Scholar]
- 13.Carter S, Clifton PM, Keogh JB. Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes: a randomized noninferiority trial. JAMA Netw Open 2018;1:e180756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corley BT, Carroll RW, Hall RM, Weatherall M, Parry-Strong A, Krebs JD. Intermittent fasting in type 2 diabetes mellitus and the risk of hypoglycaemia: a randomized controlled trial. Diabet Med 2018;35:588–594 [DOI] [PubMed] [Google Scholar]
- 15.Li C, Sadraie B, Steckhan N, et al. Effects of a one-week fasting therapy in patients with type-2 diabetes mellitus and metabolic syndrome: a randomized controlled explorative study. Exp Clin Endocrinol Diabetes 2017;125:618–624 [DOI] [PubMed] [Google Scholar]
- 16.Wilhelmi de Toledo F, Buchinger A, Burggrabe H, et al. ; Medical Association for Fasting and Nutrition [Ärztegesellschaft für Heilfasten und Ernährung, ÄGHE] . Fasting therapy: an expert panel update of the 2002 consensus guidelines. Forsch Komplementmed 2013;20:434–443 [DOI] [PubMed] [Google Scholar]
- 17.Williams KV, Mullen ML, Kelley DE, Wing RR. The effect of short periods of caloric restriction on weight loss and glycemic control in type 2 diabetes. Diabetes Care 1998;21:2–8 [DOI] [PubMed] [Google Scholar]
- 18.Clamp LD, Hume DJ, Lambert EV, Kroff J. Enhanced insulin sensitivity in successful, long-term weight loss maintainers compared with matched controls with no weight loss history. Nutr Diabetes 2017;7:e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houmard JA, Tanner CJ, Yu C, et al. Effect of weight loss on insulin sensitivity and intramuscular long-chain fatty acyl-CoAs in morbidly obese subjects. Diabetes 2002;51:2959–2963 [DOI] [PubMed] [Google Scholar]
- 20.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab 2018;27:1212–1221.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timlin MT, Pereira MA, Story M, Neumark-Sztainer D. Breakfast eating and weight change in a 5-year prospective analysis of adolescents: Project EAT (Eating Among Teens). Pediatrics 2008;121:e638–e645 [DOI] [PubMed] [Google Scholar]
- 22.Betts JA, Chowdhury EA, Gonzalez JT, Richardson JD, Tsintzas K, Thompson D. Is breakfast the most important meal of the day? Proc Nutr Soc 2016;75:464–474 [DOI] [PubMed] [Google Scholar]
- 23.Spence C. Breakfast: the most important meal of the day? Int J Gastron Food Sci 2017;8:1–6 [Google Scholar]
- 24.Zhang L, Cordeiro LS, Liu J, Ma Y. The association between breakfast skipping and body weight, nutrient intake, and metabolic measures among participants with metabolic syndrome. Nutrients 2017;9:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sievert K, Hussain SM, Page MJ, et al. Effect of breakfast on weight and energy intake: systematic review and meta-analysis of randomised controlled trials. BMJ 2019;364:l42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stentz FB, Kitabchi AE. Transcriptome and proteome expressions involved in insulin resistance in muscle and activated T-lymphocytes of patients with type 2 diabetes. Genomics Proteomics Bioinformatics 2007;5:216–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes 2004;53:2079–2086 [DOI] [PubMed] [Google Scholar]
- 28.Lee CJ, Sears CL, Maruthur N. Gut microbiome and its role in obesity and insulin resistance. Ann N Y Acad Sci 2020;1461:37–52 [DOI] [PubMed] [Google Scholar]
- 29.Jiao N, Baker SS, Nugent CA, et al. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: a meta-analysis. Physiol Genomics 2018;50:244–254 [DOI] [PubMed] [Google Scholar]
- 30.Crujeiras AB, Parra D, Goyenechea E, Martínez JA. Sirtuin gene expression in human mononuclear cells is modulated by caloric restriction. Eur J Clin Invest 2008;38:672–678 [DOI] [PubMed] [Google Scholar]
- 31.Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med 2005;142:403–411 [DOI] [PubMed] [Google Scholar]
- 32.Krebs JD, Bell D, Hall R, et al. Improvements in glucose metabolism and insulin sensitivity with a low-carbohydrate diet in obese patients with type 2 diabetes. J Am Coll Nutr 2013;32:11–17 [DOI] [PubMed] [Google Scholar]
- 33.DeFronzo RA. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidaemia and atherosclerosis. Neth J Med 1997;50:191–197 [DOI] [PubMed] [Google Scholar]
- 34.Owei I, Umekwe N, Stentz F, Wan J, Dagogo-Jack S. Amino acid signature predictive of incident prediabetes: a case-control study nested within the longitudinal pathobiology of prediabetes in a biracial cohort. Metabolism 2019;98:76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng S, Rhee EP, Larson MG, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012;125:2222–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karusheva Y, Koessler T, Strassburger K, et al. Short-term dietary reduction of branched-chain amino acids reduces meal-induced insulin secretion and modifies microbiome composition in type 2 diabetes: a randomized controlled crossover trial. Am J Clin Nutr 2019;110:1098–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol 2016;17:679–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amano H, Chaudhury A, Rodriguez-Aguayo C, et al. Telomere dysfunction induces sirtuin repression that drives telomere-dependent disease. Cell Metab 2019;29:1274–1290.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meynet O, Ricci JE. Caloric restriction and cancer: molecular mechanisms and clinical implications. Trends Mol Med 2014;20:419–427 [DOI] [PubMed] [Google Scholar]
- 41.Field AE, Austin SB, Taylor CB, et al. Relation between dieting and weight change among preadolescents and adolescents. Pediatrics 2003;112:900–906 [DOI] [PubMed] [Google Scholar]
- 42.Dagogo-Jack S, Umamaheswaran I, Askari H, Tykodi G. Leptin response to glucocorticoid occurs at physiological doses and is abolished by fasting. Obes Res 2003;11:232–237 [DOI] [PubMed] [Google Scholar]
- 43.Washburn RL, Cox JE, Muhlestein JB, et al. Pilot study of novel intermittent fasting effects on metabolomic and trimethylamine N-oxide changes during 24-hour water-only fasting in the FEELGOOD trial. Nutrients 2019;11:E246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinha MK, Caro JF. Clinical aspects of leptin. Vitam Horm 1998;54:1–30 [DOI] [PubMed] [Google Scholar]
- 45.Skowronski AA, Ravussin Y, Leibel RL, LeDuc CA. Energy homeostasis in leptin deficient Lepob/ob mice. PLoS One 2017;12:e0189784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaumberg K, Anderson DA, Reilly EE, Anderson LM. Does short-term fasting promote pathological eating patterns? Eat Behav 2015;19:168–172 [DOI] [PubMed] [Google Scholar]
- 47.Stice E, Davis K, Miller NP, Marti CN. Fasting increases risk for onset of binge eating and bulimic pathology: a 5-year prospective study. J Abnorm Psychol 2008;117:941–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laessle RG, Platte P, Schweiger U, Pirke KM. Biological and psychological correlates of intermittent dieting behavior in young women: a model for bulimia nervosa. Physiol Behav 1996;60:1–5 [DOI] [PubMed] [Google Scholar]