Abstract
Long-term risks of macro- and microvascular complications may be reduced in people with type 2 diabetes who achieve early and sustained glycemic control. Delays in attaining A1C goals are associated with poor long-term cardiovascular (CV) outcomes. Glucagon-like peptide 1 (GLP-1) receptor agonists and sodium–glucose cotransporter 2 (SGLT2) inhibitors are glucose-lowering therapies that act through complementary mechanisms of action with regard to the pathophysiologic defects of type 2 diabetes. Trials of agents in both drug classes have demonstrated improvements in CV and renal outcomes. This review discusses the rationale for combination therapy with a GLP-1 receptor agonist and an SGLT2 inhibitor, including early initiation of this combination in newly diagnosed patients. This combination may lead to timely glycemic control and potentially additive CV and renal benefits. Clinical studies of the combination have shown partially additive effects on A1C reduction, additive effects on weight reduction, and potentially synergistic effects on blood pressure reduction. Long-term studies are needed to determine whether the combination provides an additional effect on CV and renal outcomes compared with agents from either drug class when used alone.
Type 2 diabetes is a chronic, progressive disease characterized by impaired insulin secretion or insulin resistance (1). Patients with type 2 diabetes have an increased risk of macrovascular and microvascular complications, which can lead to high rates of morbidity and mortality. Early and sustained achievement of glycemic control may reduce the long-term risk of macrovascular and microvascular complications of diabetes (2), whereas delays in achieving A1C goals are often associated with poor long-term cardiovascular (CV) outcomes (3,4). In addition, patients with newly diagnosed type 2 diabetes often already have several comorbidities associated with an increased risk of cardiovascular disease (CVD), including obesity, hypertension, and dyslipidemia (5). Therefore, it is important that A1C goals are achieved as early as possible to halt the progression of diabetes complications.
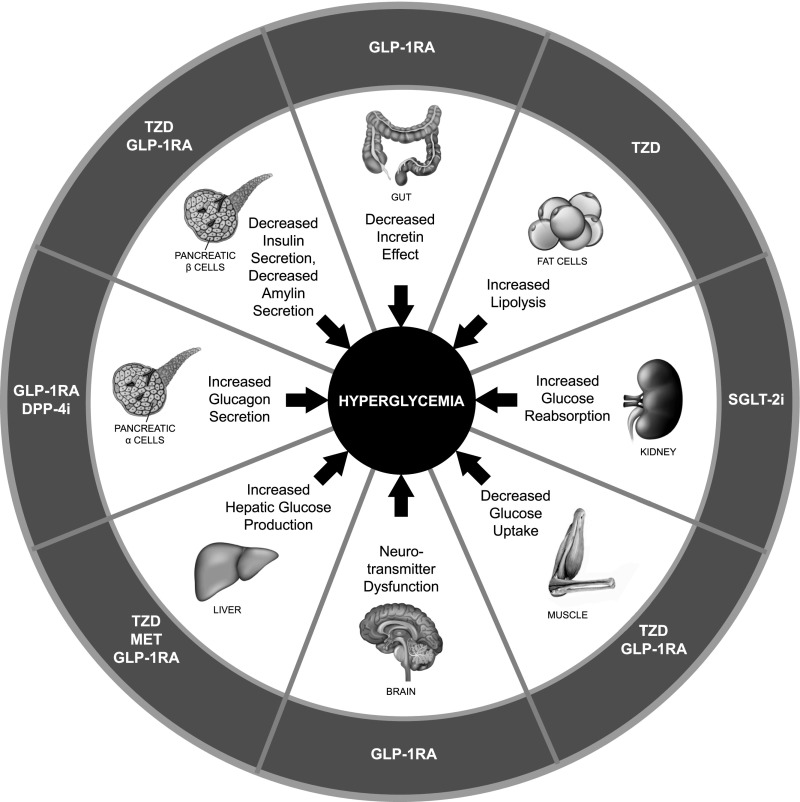
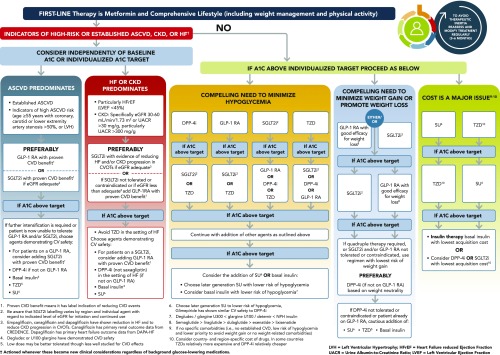
Current treatment guidelines recommend regular assessment (e.g., every 3 months) and adjustment of treatment, including the introduction of combination therapy, if A1C remains above the target level (2,6,7). Given the multifactorial pathophysiology of type 2 diabetes, disease management should include consideration of the multiple underlying defects of type 2 diabetes and which drug classes address these (Figure 1) (8–11). Clinical guidelines therefore recommend the stepwise addition of different classes of glucose-lowering therapies with complementary mechanisms of action to initial metformin therapy (2,6,7). In addition to achieving glycemic control (an A1C target of <7% in most patients), clinical guidelines recommend that treatment choice should also take into account relevant patient comorbidities (including CVD, heart failure [HF], and chronic kidney disease [CKD]) and avoidance of hypoglycemia and weight gain (2,6,7). A patient-centered approach to treatment selection, which includes considering risk factors for CVD, hypoglycemia, and weight gain, as well as cost, route of administration, and patient preferences (6,7), is recommended (Figure 2).
FIGURE 1.
Pathophysiology of type 2 diabetes with therapeutic targets of glucose-lowering therapies. Adapted from ref. 8 in ref. 9; reprinted with permission from refs. 8 and 9. DPP-4i, dipeptidyl peptidase 4 inhibitor; MET, metformin; TZD, thiazolidinedione.
FIGURE 2.
Recommendations for glucose-lowering therapy for type 2 diabetes. Reprinted with permission from ref. 6. ASCVD, atherosclerotic CVD; DPP-4i, dipeptidyl peptidase 4 inhibitor; eGFR, estimated glomerular filtration rate; GLP-1RA, glucagon-like peptide receptor agonist; HbA1C, hemoglobin A1C; SGLT2i, sodium-glucose cotransporter-2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione.
Despite the guideline recommendations for combination therapy to achieve A1C goals in patients with type 2 diabetes, there remains an urgent need to overcome clinical inertia with regard to its use (12–14). More than half of patients do not receive a change or intensification of their therapy within 12 months of follow-up despite not being at their target A1C (14). An examination of prescribing patterns in patients with newly diagnosed type 2 diabetes found that regimen changes from initial metformin therapy occurred after >2.5 years (12), and in a population-based study, patients had a mean A1C of 9.2% before combination therapy was initiated (13).
Recommendations for combination therapy include the use of glucagon-like peptide 1 (GLP-1) receptor agonists and sodium–glucose cotransporter-2 (SGLT2) inhibitors—two classes of glucose-lowering therapies with a low risk of hypoglycemia. These drug classes are also associated with important nonglycemic benefits such as reductions in CV risk factors and improvements in CV and renal outcomes in patients with type 2 diabetes and established CVD or multiple CV risk factors (15–21). In addition to reducing hospitalization for HF in cardiovascular outcomes trials (CVOTs) (22), SGLT2 inhibitors have been shown to improve HF-related outcomes in patients with HF with reduced ejection fraction with or without type 2 diabetes (23).
GLP-1 receptor agonists and SGLT2 inhibitors act through complementary mechanisms of action with regard to glycemic control and the pathophysiologic defects of type 2 diabetes. Therefore, combining the agents from these two classes may yield timely achievement of glycemic control and potentially additive CV and renal benefits (24).
Mechanism of Action and Clinical Outcomes
GLP-1 Receptor Agonists
GLP-1 receptor agonists stimulate GLP-1 receptors in many tissues of the body, including the pancreas, liver, gastrointestinal tract, and brain (Figure 1) (5,25). This drug class promotes insulin secretion and suppresses glucagon release by the pancreas, resulting in glucose-dependent reductions in plasma glucose as well as reductions in postprandial glucose levels through inhibition of hepatic glucose production and delayed gastric emptying (5,24,25). In addition to delaying gastric emptying, GLP-1 receptor agonists act in regions of the brain associated with appetite and reward to induce satiety, which reduces food intake and promotes weight loss (25,26). These drugs may also stimulate anti-inflammatory pathways by reducing oxidative stress, expression of inflammatory cytokines, and nuclear factor-κB binding of mononuclear cells and by increasing adiponectin (a cytokine that can decrease insulin resistance) (27).
GLP-1 receptor agonists have been shown to reduce A1C, postprandial glucose fluctuations, weight, and some CV risk factors and are associated with a low risk of hypoglycemia (7). Seven drugs in this class have been approved for the treatment of type 2 diabetes in the United States, including short-acting (exenatide twice daily and lixisenatide) and long-acting (albiglutide, dulaglutide, exenatide once weekly [QW], liraglutide, and semaglutide) injectable agents (25), and oral semaglutide (28); all but albiglutide are currently available in the United States (6,28).
In CVOTs of liraglutide (15), semaglutide (16), albiglutide (17), and dulaglutide (18), there were significant reductions in the risk of a three-point composite end point of major adverse CV events (MACE) (CV death, nonfatal myocardial infarction [MI], or nonfatal stroke) with GLP-1 receptor agonist therapy (range of reduction in risk across trials of 12–26%) compared with placebo in patients with type 2 diabetes and multiple CVD risk factors or established CVD. There were also significant reductions versus placebo in the risk of nephropathy events by 22% with liraglutide (15), in the risk of new or worsening nephropathy by 36% with semaglutide (16), and in the risk of the composite renal end point (new macroalbuminuria, a sustained ≥30% decrease in estimated glomerular filtration rate, or chronic renal replacement therapy) by 15% with dulaglutide (18). In the CVOT of exenatide QW (29), among patients with type 2 diabetes and a wide range of CV risk, the risk of MACE was not significantly different between the exenatide QW and placebo groups. However, in a subgroup analysis of patients in this study who had established CVD at baseline, exenatide QW was associated with a 10% reduction in the risk of MACE compared with placebo (P = 0.047) (30). In the lixisenatide CVOT (31), the addition of lixisenatide to standard care did not significantly reduce the risk of the three-point MACE composite end point or hospitalization for unstable angina in patients with type 2 diabetes and a recent acute coronary event. However, a meta-analysis (32) of data from GLP-1 receptor agonist CVOTs (15–18,29,31,33) found that, as a class, GLP-1 receptor agonists reduce the risk of three-point MACE compared with placebo.
SGLT2 Inhibitors
SGLT2 inhibitors decrease plasma glucose levels through inhibition of renal glucose reabsorption in the proximal tubule, which results in increased glucose excretion by the kidneys (34). These reductions in plasma glucose lead to improvements in insulin sensitivity and β-cell function (24). The increased glucose excretion also leads to reductions in weight and adiposity (35). Because inhibition of SGLT2 transporters also reduces sodium reabsorption, SGLT2 inhibitors are associated with increased sodium excretion (natriuresis) and antihypertensive effects (35). Through this mechanism, drugs in this class are believed to restore solute delivery to the macula densa and reactivate tubuloglomerular feedback, which leads to a reduction in glomerular hyperfiltration (36). Furthermore, because of their glucose-dependent mechanism of action, SGLT2 inhibitors have a low risk of hypoglycemia (34).
Four SGLT2 inhibitors are currently available in the United States for the treatment of type 2 diabetes: canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin (37–40). Across clinical studies in patients with type 2 diabetes, these agents reduced A1C and fasting plasma glucose and were associated with improvements in CV risk factors, including reductions in blood pressure, weight, waist circumference, and triglycerides and an increase in HDL cholesterol (41).
In CVOTs of SGLT2 inhibitors, significant reductions in the primary composite end point of three-point MACE were associated with empagliflozin (19) and canagliflozin (20) treatment. Significant reductions in hospitalization for HF (secondary and exploratory end points, respectively, in these trials) versus placebo were also observed with empagliflozin (19) and canagliflozin (20). In its CVOT (21), dapagliflozin was noninferior to placebo with regard to the risk of three-point MACE and significantly reduced the risk of CV death or hospitalization for HF compared with placebo. A subgroup analysis of the dapagliflozin CVOT found that MACE were significantly reduced with dapagliflozin versus placebo among patients with a history of MI but not those without (42). A meta-analysis of SGLT2 inhibitor CVOTs found that drugs in this class reduced the risk of three-point MACE only in patients with established CVD, but that the risk of the composite outcome of CV death or hospitalization for HF was reduced in patients with or without CVD at baseline (22).
In a separate study involving patients with HF (23), dapagliflozin was also found to significantly reduce the risk of the composite primary end point of CV death or worsening HF (HF hospitalization or urgent HF visit requiring intravenous therapy) compared with standard care alone in patients with HF and reduced ejection fraction, both with and without type 2 diabetes. Ongoing clinical trials of SGLT2 inhibition in patients with HF with or without type 2 diabetes are expected to provide further evidence on the potential benefits of this drug class with respect to HF-related outcomes.
Trials have also found that SGLT2 inhibitors are also associated with significant improvements in composite renal end points (21,22,43–45). Furthermore, a study of canagliflozin in patients with type 2 diabetes and CKD (46), which was stopped early after achievement of its prespecified efficacy outcomes, showed a 30% reduction in the risk of the composite of end-stage kidney disease, doubling of serum creatinine, or death from renal or CV causes (primary outcome) with canagliflozin versus placebo.
Of note, in a real-world study of patients who newly initiated SGLT2 inhibitor therapy in routine clinical practice (47), the proportion of patients with established CVD was ∼13% compared with >99% in the empagliflozin CVOT (19), 66% in the canagliflozin CVOT (20), and 41% in the dapagliflozin CVOT (21). This finding indicates that most patients in clinical practice have multiple CVD risk factors rather than established CVD. In the aforementioned real-world study, SGLT2 inhibition was associated with a reduced risk of MACE, hospitalization for HF, and CV and all-cause mortality compared with other glucose-lowering therapies (47,48).
A CVOT of ertugliflozin (49) is currently investigating long-term CV and renal outcomes in patients with type 2 diabetes and established CVD.
Combination Therapy With a GLP-1 Receptor Agonist Plus an SGLT2 Inhibitor
Rationale
Given the complementary mechanisms of action and improved clinical outcomes associated with these two drug classes, therapy combining agents from each may result in potentially greater beneficial outcomes in patients with type 2 diabetes (5,10,11) When used in combination, a GLP-1 receptor agonist and an SGLT2 inhibitor can potentially correct seven of the eight pathophysiologic defects of type 2 diabetes (Figure 1) (9,24).
Early initiation of such a combination may allow for timely achievement of A1C goals, thereby lowering the risks of diabetes-related morbidity and mortality in patients with early-stage type 2 diabetes. In a real-world study of patients with newly diagnosed type 2 diabetes (3), those with early sustained glycemic control (A1C 6.5–7.0%) had a reduced risk of CV events. Furthermore, an A1C ≥6.5% in the year after diagnosis was associated with an increased risk of microvascular and macrovascular complications, and an A1C ≥7.0% was linked to an increased risk of mortality (4).
Clinical Outcomes
The efficacy and safety of combination therapy with a GLP-1 receptor agonist and an SGLT2 inhibitor have been investigated in randomized controlled trials (50,51), as well as in nonrandomized trials (52,53) and real-world observational studies (54–56) (Table 1). Significant reductions in A1C have been observed with the combination versus either drug class alone or baseline A1C levels (Table 1) (50–56). Because GLP-1 receptor agonists and SGLT2 inhibitors reduce A1C through different mechanisms, combination therapy theoretically would be expected to have an additive effect with regard to A1C reduction (24). In general, these studies showed a partially additive effect with the combination of a GLP-1 receptor agonist and an SGLT2 inhibitor (25,50).
TABLE 1.
Summary of Studies Investigating Combination Therapy With a GLP-1 Receptor Agonist and an SGLT2 Inhibitor in Patients With Type 2 Diabetes
| Study, Duration | Design | Treatment | Clinical Outcomes With Combination Therapy* | |||
|---|---|---|---|---|---|---|
| ↓ A1C | ↓ W | ↓ SBP | Hypoglycemia | |||
| Simultaneous start | ||||||
| Jabbour et al. (50), 52 weeks, and Hardy et al. (65), 104 weeks | R, DB | Exenatide QW + dapagliflozin; exenatide QW + placebo; | ✓ | ✓ | ✓ | No major episodes; few minor/other episodes (more common with combination) |
| dapagliflozin + placebo | ||||||
| Sequential start | ||||||
| Ludvik et al. (51), 24 weeks | R, DB | Dulaglutide vs. placebo added to SGLT2 inhibitor | ✓ | ✓ | ✓ | 1 severe episode |
| Curtis et al. (54), 48 weeks | Ret, Obs | Dapagliflozin added to GLP-1 receptor agonist | ✓ | ✓ | NR | NR |
| Deol et al. (55), 3–6 months | Ret, Obs | SGLT2 inhibitor added to GLP-1 receptor agonist | ✓ | ✓ | NR | NR |
| Saroka et al. (56), mean 10.7 months | Obs | Canagliflozin added to GLP-1 receptor agonist | ✓ | ✓ | ✗ | NR |
↓ indicates reduction; ✓ indicates significant reduction with combination therapy versus comparator (or versus baseline in observational studies); ✗ indicates no significant reduction. DB, double-blind; Obs, observational; NR, not reported, R, randomized; Ret, retrospective; SBP, systolic blood pressure; W, weight.
Endogenous glucose production is increased in response to reduction in plasma glucose with SGLT2 inhibition, and this increase may not be completely reversed by the GLP-1 receptor agonist, resulting in a less-than-additive effect on the A1C response (57). This less-than-additive A1C response with combination therapy is a common observation when combining two classes of glucose-lowering therapies and may also be because the potential A1C reduction is dependent on baseline glycemic control (50). Combination therapy with exenatide QW plus dapagliflozin in the DURATION-8 trial (50) led to a greater proportion of patients achieving A1C targets of <7.0 and ≤6.5% at 52 weeks than with exenatide QW plus placebo or dapagliflozin plus placebo.
SGLT2 inhibitor−induced glucosuria is believed to cause appetite stimulation, which may partially offset weight reductions (58), whereas GLP-1 receptor agonists are associated with appetite suppression (26). However, the combination appears to have an almost additive effect on weight reduction, indicating that reduced food intake with a GLP-1 receptor agonist is not limited by glucosuria-induced weight loss with an SGLT2 inhibitor (25). DURATION-8 showed greater reductions in weight from baseline to week 52 with the combination (3.31 kg) versus exenatide QW plus placebo (1.51 kg, P <0.001) or dapagliflozin plus placebo (2.28 kg, P = 0.057) (50).
Combination therapy with a GLP-1 receptor agonist plus a SGLT2 inhibitor has slightly greater-than-additive effects on blood pressure reduction, most likely because of the different mechanisms of action (24,25). Exenatide QW plus dapagliflozin was associated with a significantly greater reduction in systolic blood pressure from baseline to week 52 than exenatide QW plus placebo, but the reduction was not significantly greater than dapagliflozin plus placebo (50). Addition of dulaglutide to stable SGLT2 inhibitor therapy was associated with a greater reduction from baseline to week 24 in systolic blood pressure with dulaglutide 1.5 mg but not with dulaglutide 0.75 mg versus placebo in the AWARD-10 study (51). In a post hoc analysis of the canagliflozin CVOT (59), patients who received canagliflozin in addition to a GLP-1 receptor agonist showed a reduction from baseline in systolic and diastolic blood pressure of –7.0 and –2.6 mmHg, respectively, after 18 weeks.
Because both drug classes are associated with small decreases in plasma triglycerides, combination therapy may further reduce plasma triglycerides (24). In DURATION-8, there was a numerically greater change in triglyceride levels after 52 weeks with exenatide QW plus dapagliflozin (−0.22 mmol/L) versus exenatide QW plus placebo (–0.06 mmol/L) or dapagliflozin plus placebo (+0.01 mmol/L), although the between-group differences were not statistically significant (50). Agents from both drug classes are associated with modest improvements in insulin sensitivity through enhancement of β-cell function and weight reduction (24). However, it is not yet known whether the combined use of agents from these classes results in an additive effect with regard to β-cell function.
On the basis of current evidence from clinical trials, combination therapy with a GLP-1 receptor agonist plus an SGLT2 inhibitor is associated with significantly greater reductions in A1C and weight versus a drug from either class alone and potentially synergistic reductions in systolic blood pressure and triglycerides. Considering the improvements in CV and renal end points observed in CVOTs of drugs in both classes, combination therapy could potentially provide additional benefits for metabolic, CV, and renal outcomes in patients with type 2 diabetes. However, long-term studies are needed to substantiate these benefits.
Safety Considerations
GLP-1 receptor agonists and SGLT2 inhibitors are both generally well tolerated when used individually (Table 2), with a minimal risk of hypoglycemia (60). In CVOTs, the rate of severe hypoglycemia, or hypoglycemia requiring assistance, with GLP-1 receptor agonists (15–18,29) and SGLT2 inhibitors (19–21) therapy was either similar to or reduced versus that with placebo. Regarding the risk of thyroid cancer with GLP-1 receptor agonists (Table 2), there were few or no reports of medullary thyroid cancer in CVOTs (15–18,29). The overall rates of malignant neoplasms with semaglutide and exenatide QW were similar to rates observed with placebo (16,29), whereas liraglutide was associated with a non–statistically significant increase in the incidence of benign or malignant neoplasms and pancreatic cancer (15).
TABLE 2.
Summary of the Safety Profiles of GLP-1 Receptor Agonists and SGLT2 Inhibitors
| Type of AE | GLP-1 Receptor Agonists | SGLT2 Inhibitors |
|---|---|---|
| Most common AEs | Mild or moderate gastrointestinal disorders (higher incidence with exenatide BID than exenatide QW) (28,71–76), injection site nodules (76) | Female genital mycotic infections (37–40), urinary tract infections (37–39) |
| AEs of special interest | Thyroid C-cell tumors: boxed warning in prescribing information. Based on animal studies, these agents are contraindicated in patients with a personal or family history of medullary thyroid carcinoma or MEN-2 syndrome (28,73–76) | Lower-extremity amputations: boxed warning in prescribing information for canagliflozin (38); included in warnings and precautions for ertugliflozin (40) |
| Acute pancreatitis: included in warnings and precautions in prescribing information (28,71–76) | Fractures: included in the warnings and precautions in prescribing information for canagliflozin (38) | |
| Diabetic ketoacidosis: included in the warnings and precautions in prescribing information (37–40) | ||
| Events consistent with volume depletion: may occur as SGLT2 inhibitors cause intravascular volume contraction (37–40) | ||
| Acute kidney injury: included in warnings and precautions in prescribing information (37–40). No warnings or precautions for pancreatitis, malignancies, or thromboembolic events in prescribing information (37–40) |
BID, twice daily; MEN-2, multiple endocrine neoplasia.
The SGLT2 inhibitor canagliflozin was associated with a risk of lower-extremity amputations in patients with type 2 diabetes in its CVOT (20), but there was no significant increase in risk in the trial of canagliflozin in patients with CKD (Table 2) (43). Some real-world studies have indicated an increased risk of lower-extremity amputation with SGLT2 inhibitors (61,62); however, the results of another real-world study (63) and a meta-analysis of observational databases (64) have suggested that there is no consistent increase in the risk of lower-extremity amputations with agents in this drug class. Because of the limited number of prospective studies and inherent limitations of observational and pharmacovigilance studies, additional research is needed to determine the risk of amputation associated with SGLT2 inhibition (61–64). Canagliflozin, but not empagliflozin or dapagliflozin, may also be associated with an increased risk of fractures on the basis of observations in CVOTs (19–21) (Table 2), although no increased risk was seen in trial of canagliflozin in patients with CKD (46). Similarly, volume depletion occurred at a significantly higher rate with canagliflozin (20), but not with empagliflozin or dapagliflozin (19,21).
In clinical studies of GLP-1 receptor agonist plus SGLT2 inhibitor use, the safety profile of the combination therapy was consistent with those of the individual agents, with no unexpected findings (50–53,56). The risk of hypoglycemia was low across studies of the combination therapy (50–53); there were no reports of major hypoglycemia and few reports of minor hypoglycemia or other hypoglycemic events over 104 weeks with exenatide QW plus dapagliflozin in DURATION-8 (65), and only one severe episode was reported with dulaglutide plus an SGLT2 inhibitor over 24 weeks in AWARD-10 (51) (Table 1).
Regarding other adverse events (AEs) of special interest, incidences of pancreatitis, volume depletion, acute renal failure, and marked hematocrit abnormality were low in all treatment groups in DURATION-8 (50). Acute pancreatitis, C-cell hyperplasia, medullary thyroid cancer, amputations, diabetic ketoacidosis, and acute kidney injury were not reported with dulaglutide plus an SGLT2 inhibitor in AWARD-10, although a few patients experienced possibly hypotension-related AEs (51).
Clinical Implications
The clinical presentation of type 2 diabetes at the time of diagnosis ranges from patients who are asymptomatic to those with severe hyperglycemia or ketoacidosis, and many patients may already have an increased risk of developing microvascular complications (1). Because of the complex nature of type 2 diabetes pathophysiology and the increased risk of diabetes complications, most patients require combination glucose-lowering therapy that targets multiple metabolic defects to achieve effective glycemic control (to prevent microvascular complications) and correct CV risk factors (to prevent macrovascular complications) (1).
Guidelines from the American Association of Clinical Endocrinologists (AACE)/American College of Endocrinology (ACE) provide a suggested hierarchy for the use of glucose-lowering therapies that takes into account the properties of each drug class, including the risk of hypoglycemia and weight gain, and note that combination therapy is usually required to achieve glycemic control (2). GLP-1 receptor agonists and SGLT2 inhibitors are preferred as add-on therapy to metformin over other drug classes in dual- and triple-combination therapy regimens (2). Guidelines from AACE/ACE, the American Diabetes Association (ADA), and the European Association for the Study of Diabetes recommend that the choice of glucose-lowering therapy should include consideration of individual patients’ CV, cerebrovascular, and renal status in addition to the glycemic efficacy, hypoglycemia risk, effects on weight, AEs, and cost of different treatments (2,6,7). These guidelines favor the use of GLP-1 receptor agonists and SGLT2 inhibitors with proven CV or renal benefits in patients with CVD and CKD (2,6,7).
The AACE/ACE and ADA guidelines also recognize that concomitant medications for control of blood pressure and lipids are needed in most patients to reduce the risk of CVD (2,6). Simultaneous management of multiple CV risk factors is associated with clinical benefits in patients with type 2 diabetes, including reductions in CVD morbidity and mortality (7,66). Given the importance of achieving A1C goals early to avoid macrovascular and microvascular complications (2), patients with early-stage type 2 diabetes may be candidates for combination therapy (1).
There are some potential challenges with using a combination of GLP-1 receptor agonist and SGLT2 inhibitor therapy, including adherence to different routes of administration (subcutaneous and oral), the lack of a fixed combination product, and the associated costs of combination therapy. Furthermore, some patients may be averse to using injectable therapy (67). However, when considering injectable glucose-lowering therapy, patients have the option of weekly GLP-1 receptor agonist injections (68).
The early initiation of combination glucose-lowering therapy could potentially provide timely achievement of glycemic control in patients with type 2 diabetes (69). Long-term evidence suggests that the use of a GLP-1 receptor agonist and an SGLT2 inhibitor, when added to metformin, could delay the start of insulin therapy by 5–6 years (70). Because drugs from both of these classes have a low risk of weight gain and hypoglycemia and most GLP-1 receptor agonists offer a decreased injection burden compared with insulin (QW or once daily versus once or twice daily), their combined use may address both physician- and patient-related barriers to effective glycemic control (69).
Conclusion
Because of complementary mechanisms of action, combination therapy with a GLP-1 receptor agonist plus an SGLT2 inhibitor provides effective and durable glycemic control in patients with type 2 diabetes and carries a low risk of hypoglycemia. Evidence from clinical studies of the combination’s effects on CVD risk factors, as well as evidence of CV benefits reported in the CVOTs of the individual agents suggests that using this combination could be a good option to overcome some of the clinical barriers to achieving timely and effective glycemic control in patients with type 2 diabetes. Further long-term studies are needed to demonstrate that improvements in CV risk factors with such a combination have a significant effect on CV and renal outcomes in patients with type 2 diabetes and either established CVD or high CV risk.
Article Information
Acknowledgments
Sarah Greig, PhD, of inScience Communications, Springer Healthcare (Auckland, New Zealand), provided medical writing support in accordance with Good Publication Practice (GPP3). Ultimate responsibility for opinions, conclusions, and data interpretation lies with the author.
Funding
Medical writing support was funded by AstraZeneca.
Duality of Interest
J.E.A. has served on advisory boards for or as a consultant to Abbott Diabetes Care, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Mannkind, Merck, Novo Nordisk, and Sanofi and has served on speaker bureaus for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk, and Sanofi.
Author Contributions
J.E.A. contributed to the development and review of the manuscript prepared by the medical writer and approved the final version. He is the guarantor of this work and, as such, had full access to all the data presented and takes responsibility for the integrity of the analysis and the accuracy of the review.
References
- 1.DeFronzo RA, Ferrannini E, Groop L, et al. . Type 2 diabetes mellitus. Nat Rev Dis Primers 2015;1:15019. [DOI] [PubMed] [Google Scholar]
- 2.Garber AJ, Abrahamson MJ, Barzilay JI, et al. . Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2019 executive summary. Endocr Pract 2019;25:69–100 [DOI] [PubMed] [Google Scholar]
- 3.Alatorre CI, Hoogwerf BJ, Deeg MA, et al. . Factors associated with stroke, myocardial infarction, ischemic heart disease, unstable angina, or mortality in patients from real world clinical practice with newly-diagnosed type 2 diabetes and early glycemic control. Curr Med Res Opin 2018;34:337–343 [DOI] [PubMed] [Google Scholar]
- 4.Laiteerapong N, Ham SA, Gao Y, et al. . The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the Diabetes & Aging Study). Diabetes Care 2019;42:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busch RS, Kane MP. Combination SGLT2 inhibitor and GLP-1 receptor agonist therapy: a complementary approach to the treatment of type 2 diabetes. Postgrad Med 2017;129:686–697 [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S98–S110 [DOI] [PubMed] [Google Scholar]
- 7.Davies MJ, D’Alessio DA, Fradkin J, et al. . Management of hyperglycemia in type 2 diabetes, 2018: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Triplitt CL, Abdul-Ghani M, Cersosimo E. Novel agents for the treatment of type 2 diabetes. Diabetes Spectr 2014;27:100–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunton S. Pathophysiology of type 2 diabetes: the evolution of our understanding. J Fam Pract 2016;65(Suppl. 4):supp_az_0416 [PubMed] [Google Scholar]
- 10.DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care 2013;36(Suppl. 2):S127–S138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thrasher J. Pharmacologic management of type 2 diabetes mellitus: available therapies. Am J Med 2017;130(Suppl. 6):S4–S17 [DOI] [PubMed] [Google Scholar]
- 12.Brouwer ES, West SL, Kluckman M, et al. . Initial and subsequent therapy for newly diagnosed type 2 diabetes patients treated in primary care using data from a vendor-based electronic health record. Pharmacoepidemiol Drug Saf 2012;21:920–928 [DOI] [PubMed] [Google Scholar]
- 13.Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care 2004;27:1535–1540 [DOI] [PubMed] [Google Scholar]
- 14.Khunti K, Gomes MB, Pocock S, et al. . Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab 2018;20:427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 17.Hernandez AF, Green JB, Janmohamed S, et al.; Harmony Outcomes Committees and Investigators . Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519–1529 [DOI] [PubMed] [Google Scholar]
- 18.Gerstein HC, Colhoun HM, Dagenais GR, et al.; REWIND Investigators . Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121–130 [DOI] [PubMed] [Google Scholar]
- 19.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 20.Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 21.Wiviott SD, Raz I, Bonaca MP, et al.; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 22.Zelniker TA, Wiviott SD, Raz I, et al. . SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31–39 [DOI] [PubMed] [Google Scholar]
- 23.McMurray JJV, Solomon SD, Inzucchi SE, et al.; DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008 [DOI] [PubMed] [Google Scholar]
- 24.DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab 2017;19:1353–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Baar MJB, van Ruiten CC, Muskiet MHA, van Bloemendaal L, IJzerman RG, van Raalte DH. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care 2018;41:1543–1556 [DOI] [PubMed] [Google Scholar]
- 26.van Bloemendaal L, IJzerman RG, Ten Kulve JS, et al. . GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes 2014;63:4186–4196 [DOI] [PubMed] [Google Scholar]
- 27.Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation 2017;136:849–870 [DOI] [PubMed] [Google Scholar]
- 28.Rybelsus 2020. [package insert]. Plainsboro, NJ, Novo Nordisk,
- 29.Holman RR, Bethel MA, Mentz RJ, et al.; EXSCEL Study Group . Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mentz RJ, Thompson VP, Aguilar D, et al. . Effects of once-weekly exenatide on clinical outcomes in patients with preexisting cardiovascular disease. Circulation 2018;138:2576–2578 [DOI] [PubMed] [Google Scholar]
- 31.Pfeffer MA, Claggett B, Diaz R, et al.; ELIXA Investigators . Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–2257 [DOI] [PubMed] [Google Scholar]
- 32.Giugliano D, Maiorino MI, Bellastella G, Longo M, Chiodini P, Esposito K. GLP-1 receptor agonists for prevention of cardiorenal outcomes in type 2 diabetes: an updated meta-analysis including the REWIND and PIONEER 6 trials. Diabetes Obes Metab 2019;21:2576–2580 [DOI] [PubMed] [Google Scholar]
- 33.Husain M, Birkenfeld AL, Donsmark M, et al.; PIONEER 6 Investigators . Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019;381:841–851 [DOI] [PubMed] [Google Scholar]
- 34.Abdul-Ghani MA, DeFronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30–50% of filtered glucose load in humans. Diabetes 2013;62:3324–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia 2017;60:215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alicic RZ, Johnson EJ, Tuttle KR. SGLT2 inhibition for the prevention and treatment of diabetic kidney disease: a review. Am J Kidney Dis 2018;72:267–277 [DOI] [PubMed] [Google Scholar]
- 37.Farxiga 2020. [package insert]. Wilmington, DE, AstraZeneca Pharmaceuticals,
- 38.Invokana 2020. [package insert]. Titusville, NJ, Janssen Pharmaceuticals,
- 39.Jardiance 2020. [package insert]. Ridgefield, CT, Boehringer Ingelheim Pharmaceuticals,
- 40.Steglatro 2020. [package insert]. Whitehouse Station, NJ, Merck & Co.,
- 41.Mazidi M, Rezaie P, Gao HK, Kengne AP. Effect of sodium-glucose cotransport-2 inhibitors on blood pressure in people with type 2 diabetes mellitus: a systematic review and meta-analysis of 43 randomized control trials with 22 528 patients. J Am Heart Assoc 2017;6:e004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furtado RHM, Bonaca MP, Raz I, et al. . Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation 2019;139:2516–2527 [DOI] [PubMed] [Google Scholar]
- 43.Perkovic V, de Zeeuw D, Mahaffey KW, et al. . Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol 2018;6:691–704 [DOI] [PubMed] [Google Scholar]
- 44.Wanner C, Inzucchi SE, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334 [DOI] [PubMed] [Google Scholar]
- 45.Mosenzon O, Wiviott SD, Cahn A, et al. . Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol 2019;7:606–617 [DOI] [PubMed] [Google Scholar]
- 46.Perkovic V, Jardine MJ, Neal B, et al.; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 47.Kosiborod M, Cavender MA, Fu AZ, et al.; CVD-REAL Investigators and Study Group . Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation 2017;136:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Birkeland KI, Jørgensen ME, Carstensen B, et al. . Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol 2017;5:709–717 [DOI] [PubMed] [Google Scholar]
- 49.Cannon CP, McGuire DK, Pratley R, et al.; VERTIS-CV Investigators . Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV). Am Heart J 2018;206:11–23 [DOI] [PubMed] [Google Scholar]
- 50.Jabbour SA, Frías JP, Hardy E, et al. . Safety and efficacy of exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy: 52-week results of the DURATION-8 randomized controlled trial. Diabetes Care 2018;41:2136–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ludvik B, Frías JP, Tinahones FJ, et al. . Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2018;6:370–381 [DOI] [PubMed] [Google Scholar]
- 52.Harashima SI, Inagaki N, Kondo K, et al. . Efficacy and safety of canagliflozin as add-on therapy to a glucagon-like peptide-1 receptor agonist in Japanese patients with type 2 diabetes mellitus: a 52-week, open-label, phase IV study. Diabetes Obes Metab 2018;20:1770–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seino Y, Yabe D, Sasaki T, et al. . Sodium-glucose cotransporter-2 inhibitor luseogliflozin added to glucagon-like peptide 1 receptor agonist liraglutide improves glycemic control with bodyweight and fat mass reductions in Japanese patients with type 2 diabetes: a 52-week, open-label, single-arm study. J Diabetes Investig 2018;9:332–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Curtis L, Humayan MA, Walker J, Hampton K, Partridge H. Addition of SGLT2 inhibitor to GLP-1 agonist therapy in people with type 2 diabetes and suboptimal glycaemic control. Practical Diabetes 2016;33:129–132 [Google Scholar]
- 55.Deol H, Lekkakou L, Viswanath AK, Pappachan JM. Combination therapy with GLP-1 analogues and SGLT-2 inhibitors in the management of diabesity: the real world experience. Endocrine 2017;55:173–178 [DOI] [PubMed] [Google Scholar]
- 56.Saroka RM, Kane MP, Busch RS, Watsky J, Hamilton RA. SGLT-2 inhibitor therapy added to GLP-1 agonist therapy in the management of T2DM. Endocr Pract 2015;21:1315–1322 [DOI] [PubMed] [Google Scholar]
- 57.Martinez R, Al-Jobori H, Ali AM, et al. . Endogenous glucose production and hormonal changes in response to canagliflozin and liraglutide combination therapy. Diabetes 2018;67:1182–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium–glucose cotransporter 2 inhibition. Diabetes Care 2015;38:1730–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fulcher G, Matthews DR, Perkovic V, et al.; CANVAS Trial Collaborative Group . Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab 2016;18:82–91 [DOI] [PubMed] [Google Scholar]
- 60.Consoli A, Formoso G, Baldassarre MPA, Febo F. A comparative safety review between GLP-1 receptor agonists and SGLT2 inhibitors for diabetes treatment. Expert Opin Drug Saf 2018;17:293–302 [DOI] [PubMed] [Google Scholar]
- 61.Chang HY, Singh S, Mansour O, Baksh S, Alexander GC. Association between sodium-glucose cotransporter 2 inhibitors and lower extremity amputation among patients with type 2 diabetes. JAMA Intern Med 2018;178:1190–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khouri C, Cracowski JL, Roustit M. SGLT-2 inhibitors and the risk of lower-limb amputation: is this a class effect? Diabetes Obes Metab 2018;20:1531–1534 [DOI] [PubMed] [Google Scholar]
- 63.Yuan Z, DeFalco FJ, Ryan PB, et al. . Risk of lower extremity amputations in people with type 2 diabetes mellitus treated with sodium-glucose co-transporter-2 inhibitors in the USA: a retrospective cohort study. Diabetes Obes Metab 2018;20:582–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ryan PB, Buse JB, Schuemie MJ, et al. . Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes Obes Metab 2018;20:2585–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hardy E, Öhman P, Jabbour S, Guja C, Frías J, Bhattacharya S. DURATION-8 randomised controlled trial 104-week results: efficacy and safety of once-weekly exenatide (ExQW) plus once-daily dapagliflozin (DAPA) vs ExQW or DAPA alone. Diabetologia 2018;61(Suppl.):S20 [Google Scholar]
- 66.American Diabetes Association 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl 1):S111–S134 [DOI] [PubMed] [Google Scholar]
- 67.Polonsky WH, Jackson RA. What’s so tough about taking insulin? Addressing the problem of psychological insulin resistance in type 2 diabetes. Clin Diabetes 2004;22:147–150 [Google Scholar]
- 68.Hauber AB, Nguyen H, Posner J, Kalsekar I, Ruggles J. A discrete-choice experiment to quantify patient preferences for frequency of glucagon-like peptide-1 receptor agonist injections in the treatment of type 2 diabetes. Curr Med Res Opin 2016;32:251–262 [DOI] [PubMed] [Google Scholar]
- 69.Reach G, Pechtner V, Gentilella R, Corcos A, Ceriello A. Clinical inertia and its impact on treatment intensification in people with type 2 diabetes mellitus. Diabetes Metab 2017;43:501–511 [DOI] [PubMed] [Google Scholar]
- 70.Charokopou M, Vioix H, Verheggen BG, Maddocks D, Bratt T, Franks D. Economic assessment of delaying insulin treatment through the use of newer anti-diabetic agents, dapagliflozin (Forxiga) and exenatide (Bydureon), both as add-on to metformin; a cost-effectiveness analysis from a UK NHS perspective. Value Health 2014;17:A344–A345 [DOI] [PubMed] [Google Scholar]
- 71.Adlyxin 2019. [package insert]. Bridgewater, NJ, Sanofi,
- 72.Byetta [package insert]. Wilmington, DE, AstraZeneca Pharmaceuticals,2018.
- 73.Victoza 2019. [package insert]. Plainsboro, NJ, Novo Nordisk,
- 74.Ozempic 2020. [package insert]. Plainsboro, NJ, Novo Nordisk,
- 75.Trulicity 2019. [package insert]. Indianapolis, IN, Eli Lilly and Co.,
- 76.Bydureon BCise 2019. [package insert]. Wilmington, DE, AstraZeneca Pharmaceuticals,