Abstract
Background:
Studies have documented how levels and change in depression correspond to ART non-adherence. However, few studies have examined how levels of and change in adherence may relate to levels of and change in depression, although one might expect mental health to be related to physical health and how successful one is in managing disease. To assess the bidirectional nature of the association between these two constructs, we examined data from a prospective trial of an ART adherence intervention in Uganda that followed 143 participants over 20 months.
Methods:
Adherence was measured using electronic monitoring caps; non-adherence was defined as missing > 10% of prescribed doses; self-reported depression was measured using the Patient Health Questionnaire (PHQ-9), and PHQ-9 > 4 defined the presence of at least minor depression. Adjusted linear and logistic regression models were used to examine the longitudinal relationships between depression and non-adherence.
Results:
At baseline, 40.6% had at least minor depression and 37.1% were non-adherent. Time varying change in the classification of depression (e.g., becoming depressed) predicted change in non-adherence status (e.g., becoming non-adherent), and this association remained when examining continuous measures of the constructs. Similarly, time varying measures of increases in non-adherence predicted increases in depression, regardless of whether continuous or binary classification measures were used. A temporal trend of increased non-adherence over time was observed, and this was accelerated by an increase in depression. Furthermore, those who had at least minor depression at baseline were more likely to be non-adherent at follow-up.
Conclusions:
These findings support the potential benefits of depression care and adherence support for improving adherence and mental health, respectively, and call for further research to examine such benefits.
The trial has been registered with ClinicalTrials.gov ( NCT02503072).
Keywords: depression, adherence, ART, HIV, Uganda
INTRODUCTION
Rates of clinical depression in people living with HIV/AIDS (PLHA) in sub-Saharan Africa (SSA) range from 10–20% (1–3), and an additional 20–40% have elevated depressive symptoms (1,2). Research in both high resource settings and SSA, has found depression to have ramifications for HIV disease management as it has been associated with lower immune function or CD4 count (4,5), higher HIV viral load (6), greater likelihood of mortality (7,8), and worse immunologic and virologic response to antiretroviral therapy (ART) (9). This negative impact of depression is thought to be in part related to its association with nonadherence to antiretroviral therapy (ART) (10–12) and disengagement from HIV care (12,13).
Drawing on Social Cognitive Theory (14) and the Information, Motivation and Behavioral skills (IMB) model of health behavior (15), depression may influence HIV care adherence through its effects on self-efficacy and motivation to adhere well and attend clinic visits. Common symptoms of depression such as loss of interest, hopelessness, poor concentration, and fatigue may diminish self-efficacy and motivation to engage in healthy behaviors including taking medication every day and travelling to attend clinic visits. Depression alleviation, via improvements in motivation and self-efficacy, may translate into increased self-care behaviors including taking medication as directed and attending clinic visits. Depression treatment has been associated not only with improved mental health (16), but also ART utilization and adherence as demonstrated in our prior research in Uganda (17) as well as research elsewhere (18). Furthermore, a longitudinal analysis involving electronically measured ART adherence in a study that combined data from several longitudinal samples of HIV-infected participants in the U.S. found that change in depression over 12 months was associated with corresponding changes in adherence (19).
While depression is commonly thought of as an antecedent to nonadherence, and it is this direction that has been most studied, the relationship between depression and adherence may be bidirectional (20). Depression seems to impede health behaviors such as treatment adherence, but does adherence and associated effects on physical health relate to corresponding changes in depression? Our research in Uganda has shown that initiation of ART is associated with improvement in mental health over time as measured by a reduction in depression (21), presumably as a result of improved or stabilized physical health and increased optimism for one’s future; however, we did not evaluate whether ART adherence mediated this relationship. Few longitudinal studies have examined whether adherence interventions or improved adherence is associated with corresponding improvement in depression. A small number of studies have used cognitive-behavioral interventions to target both depression and ART adherence because of common cognitive mechanisms that influence both; results have been mixed, with some finding improvement in both adherence and mood (22) and others finding only mood effects (23).
In a prospective study that examined effects of an adherence intervention for HIV patients in Uganda who had been on ART for at least two years and exhibiting some evidence of adherence problems, we examined whether changes in adherence were associated with changes in depression, as well as whether changes in depression were related to changes in adherence, in an attempt to evaluate the bidirectionality of the relationship between these two constructs.
METHODS
Study Design
This randomized controlled trial was designed to evaluate the Rewarding Adherence Program (RAP), an intervention that uses variable rewards based on behavioral economic principles to improve ART adherence and retention in care (see a prior publication from this study for further details; 24). The program attempts to reduce present bias and increase information salience by providing small prizes allocated by a drawing at each clinic visit conditional on keeping scheduled clinic appointments (treatment group 1) and high ART adherence measured by MEMS caps (treatment group 2). Randomization used a ratio of 1:1:1 to assign participants to one of the two intervention arms or the usual care control. The study was implemented at Mildmay Uganda, an NGO in the capital Kampala that provides HIV primary care to over 11,000 patients. Recruitment took place between March and August 2013; participants completed a baseline survey before being informed of their randomized treatment assignment and before they were exposed to the intervention. Follow-up assessments were conducted at months 4, 8, 14 and 20, with participants paid 10,000 Uganda Shillings ($3 USD) after each assessment to cover costs of transportation.
Eligible participants included those who were 18 years of age or older, on ART for at least the past two years, and had adherence problems (either self-reported or otherwise indicated in the medical records data) in the last six months. Informed, written consent was obtained from all participants in the study. The study was approved by the HSPC Board at RAND, the IRB review board at Mildmay, and the Uganda National Science Council (UNCST). The trial has been registered with ClinicalTrials.gov (NCT02503072).
Measures
All measures were translated into Luganda using standard translation and back-translation methods, and were interviewer-administered by trained study coordinators.
Demographic and background characteristics included age, gender, and education level (classified as primary school or less vs. at least some secondary education). CD4 count at baseline and ART status were abstracted from the client’s medical chart.
Self-Reported Depression.
The 9-item Patient Health Questionnaire (PHQ-9) was used to measure the presence of depressive symptoms over the past 2 weeks (25), and it has established validity and reliability when used with PLHA in sub-Saharan Africa (26). The 9 items correspond to the symptoms used to diagnose depression according to the Diagnostic and Statistics Manual (27); responses to each item range from 0 ‘not at all’ to 3 ‘nearly every day’. Item scores are summed and scores of 5–9 reflect minor depression, while a score of 10 or greater has been found to correspond highly to major depression (25). In analyses, we used the PHQ-9 total score, a continuous measure, and a binary variable to represent the presence of at least minor depression (PHQ-9 > 4). One of the symptoms assessed by the PHQ-9 is suicidal ideation. Participants who reported suicidal ideation were assessed for suicidal risk (presence of a plan and means of carrying out the plan) by the study coordinator, and those determined to be at risk were immediately connected with a mental health counselor at the clinic for further evaluation, treatment, and referral as needed.
ART adherence.
Electronic data monitoring caps were used to measure adherence. These caps house an electronic chip that records the exact time that the cap is unscrewed from the bottle. Participants were instructed to remove the cap from the bottle at the time that they planned to ingest the medication and to only remove one dose at a time. Since the caps measure when the cap is unscrewed, and not when or how much medication is actually ingested, data from the caps can both underestimate (e.g., patient may take medication from a source other than the bottle with the electronic cap, resulting in a taken dose not being recorded by the cap) and overestimate adherence (e.g., patient unscrews cap but doesn’t actually ingest the medication, or takes two doses from the bottle at the same time with the plan of taking the second dose later [which will not be recorded by the cap]). To reduce measurement error associated with improper use of the electronic cap, participants were asked to report the number of occasions that they were unable to follow the cap instructions (e.g., removing multiple doses at once; opening the bottle without removing a dose; taking medication from another source); these data were then used to adjust the adherence summary scores. This strategy has been previously shown to strengthen the relationship between electronically monitored adherence and virologic treatment outcomes (28).
The continuous measure of non-adherence was operationalized as the percentage of prescribed doses not taken (continuous variable), and the binary classification of non-adherence was defined as less than 90% of prescribed doses taken—a cutoff that is commonly used in research and that represents a level of adherence needed to achieve sustained virologic response and prevent drug resistance (29, 30).
Adherence variables were calculated at each clinic visit, with data representing adherence in the prior month. We excluded the first month of adherence data, because we observed significantly higher adherence for the sample as a whole in this time period, which we believe is likely due to the novelty of being monitored by the cap and a resulting Hawthorne effect, as supported in other research (31, 32). Therefore, adherence in the second month of follow-up was used in analysis as the initial measure of adherence, and is henceforth referred to as “baseline” adherence in this paper.
Statistical Analysis
Our analytic sample includes all study participants with a baseline value for both depression and adherence. The analytic strategy employed mixed-effects regression modeling (also known as longitudinal hierarchical modeling) that allows each subject to serve as his or her own control (33–35). Using repeated measures of time-varying predictors and outcomes, we were able to examine within-individual covariance of these variables over time. Longitudinal analyses were conducted with data from five assessments at baseline and months 4, 8, 14 and 20. During modeling, we included time in months as a continuous variable, so that its coefficient estimates the effect corresponding to one-unit or monthly change in time. (In a separate specification, we included time as a series of binary indicators. However, a joint test indicated that the coefficients were not significantly different from each other). The outcomes for these analyses are continuous or binary measures of depression or non-adherence, and the primary predictors are non-adherence or depression, respectively.
In this hierarchical modeling, we decomposed the time-varying predictor into between-individual and within-individual components, with the coefficient of the within-individual component providing an estimate of the longitudinal relationship between predictor and outcome (35–36). For each participant, we calculated the deviation between the predictor’s continuous value observed at a follow-up measurement period and the participant’s baseline value. Similarly, we calculated the deviation between the binary classification of these variables at a follow-up measurement period from their baseline value. Thus, the binary deviation score is a 3-level variable such that if the participant’s classification as depressed or non-adherent was unchanged from baseline to the follow-up time period a code of “0” was assigned, whereas “1” indicated their classification improved from depressed or non-adherent at baseline to not depressed or adherent at follow-up, and “−1” indicated their status worsened from not depressed or adherent at baseline to depressed or non-adherent at follow-up. Such a deviation score estimates the effect of change (in either direction captured as +1 or –1) from no change (value of 0).
We used two different linear model specifications to estimate the simultaneous, longitudinal relationship between change in depression and change in non-adherence measured continuously (33). First, in two separate analyses, we modeled the longitudinal trends in change (from baseline) in continuous measures of non-adherence (depression) as the outcome with a longitudinal linear regression model including linear time (in months), baseline depression (non-adherence) and change in depression (change in non-adherence) from baseline measured continuously, as the independent variables. The coefficient of the baseline predictor indicates the degree to which between-individual differences in baseline level is related to the change in the outcome. The coefficient of the deviation score reflects the degree to which within-person change in the predictor is associated with change in the outcome, providing an estimate of the longitudinal relationship between the two.
In the second specification, we used a longitudinal logistic regression model to examine trends in binary non-adherence and depression classification. Again, change in the predictor variable was coded as the difference between binary classifications at the follow-up period and baseline. Each model included linear time (coded as months), baseline value of the binary predictor, and time-varying change from baseline value. The interpretation of these coefficients is similar to what was described above in the first model specification, with results presented as odds ratios.
In both model specifications described above, we also estimated an additional model for the continuous and binary measure of depression or non-adherence with an interaction term between the change score and month. A significant interaction term indicates that the longitudinal relationship between the predictor and the outcome varies across the months enrolled in the study. In the binary outcomes modeling, interaction terms were non-significant. Therefore, we only present the simplified model without interaction terms.
The regression models were covariate-adjusted with the inclusion of socio-demographic characteristics (age, sex, income, education, marital status), baseline CD4 count and study arm (intervention vs. usual care control) assignment. All analyses accounted for clustering of repeated assessments on each participant. The number of individuals with initial baseline assessments (PHQ-9 and adherence) is 143; of those, 132 (92.3%) completed an assessment at month 20. There was also a small amount (<1%) of item nonresponse. To maximize sample size and avoid bias stemming from omission of observations with any variables missing a value, we also imputed missing values using the fully conditional specification in PROC MI in SAS. Regression models were estimated using PROC GENMOD in SAS vs. 9.4 (37).
RESULTS
Sample Description
A sample of 153 participants enrolled in the study, of whom 143 had electronically monitored adherence data at month 2 (which served as the adherence baseline data) and composed the analytic sample. The longitudinal modeling was conducted with four waves of change scores, or 572 observations on 143 study participants. Just over a third (n=51; 35.7%) of these 143 participants were male, mean age was 39.1 years (SD=10.2), half (n=72; 50.3%) were married, 16 (11.2%) had any secondary education, mean monthly disposable income was ~ $67 USD (SD= 67), and mean baseline CD4 count was 442 cells/mm3 (SD=227). Two-thirds were assigned to one of the two intervention arms (n=96; 67.1%) with the remaining assigned to the usual care arm, and 132 (92.3%) completed the month-20 follow-up assessment. The 11 participants without month-20 follow up data did not significantly differ from the others on any of the above described characteristics.
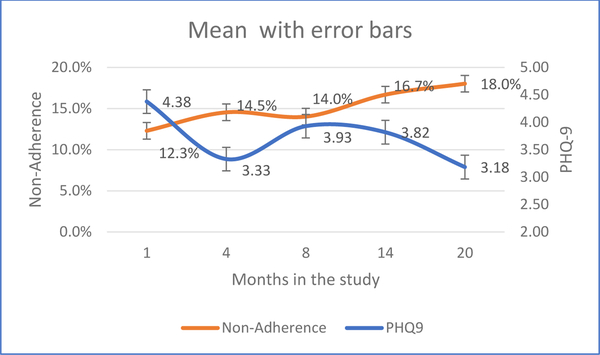
Mean PHQ-9 score at baseline was 4.38 (SD = 3.86), with 44 (30.8%) having scores of 5–9 (minor depression) and another 14 (9.8%) having scores greater than 9 (clinical depression). Therefore, a total of 58 individuals (40.6%) were classified as having at least minor depression at baseline. Mean of the continuous non-adherence variable at baseline was 12.3% (SD = 19.6%), with just over one-third (n=53; 37.1%) classified as non-adherent (using a binary classification of non-adherence > 10%). Figure 1 displays time trends in sample means for continuous PHQ-9 and non-adherence between baseline and month 20. At month 20, we see that average PHQ-9 score is 3.18 (SD = 4.17) while mean non-adherence is 18.0% (SD = 20.4%). While average PHQ-9 decreased over time, average non-adherence increased over time in this sample.
Figure 1:
Mean trends for depression and non-adherence with 95% confidence intervals
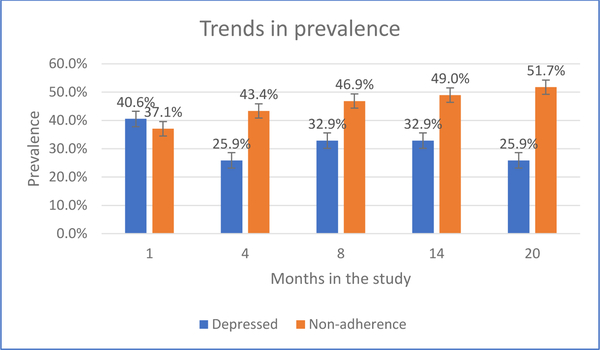
Figure 2 shows the time trend in the prevalence rates of the binary classifications of depression and non-adherence in this sample. At month 20, 25.9% had at least minor depression, while just over half (51.7%) were non-adherent. The slopes are consistent with that seen for the mean trends of the continuous measures of depression and non-adherence (figure 1). The average (within-person) change in PHQ-9 between baseline and month 20 was −1.19 (SD = 4.90). The average (within-person) change in non-adherence between baseline and month 20 was 7.8% (SD = 20.1%).
Figure 2:
Trends in prevalence of binary classification of depression and non-adherence
Longitudinal Relationship Between Change in Continuous Measures of Depression and Non-adherence
Table-I presents the longitudinal relationship between continuous measures of depression and ART non-adherence. The first column provides results for change in non-adherence (percentage of prescribed doses not taken). In the main effects model (top panel), non-adherence increased over time (2.4% over 12 months) among those with zero change in PHQ-9, as reflected by the significant coefficient of time. Also, an increase in PHQ-9 was associated with increase in non-adherence. For example, if a hypothetical person had a 5-point increase in PHQ-9, then this would contribute to an additional 2.5% increase in non-adherence (effect size = .135). Combining the coefficients of the main effects and interaction terms, this would result in a net increase of 4.9% in non-adherence over 12 months.
Table-I:
Longitudinal association between change in continuous measures of depression (PHQ-9) and non-adherence (proportion of prescribed doses not taken)
| Outcome | ||||
|---|---|---|---|---|
| Predictor | Change in nonadherence a | Change in depression a | ||
| Main effects Model | Beta (SE) | p-value | Beta (SE) | p-value |
| Baseline depression | .002 (.003) | .596 | ||
| Month | .002 (.001) | .025 | ||
| Change in depression a | .005 (.002) | .027 | ||
| Baseline non-adherence | 3.04 (1.54) | .049 | ||
| Month | -.03 (.02) | .237 | ||
| Change in non-adherence a | 3.98 (1.45) | .006 | ||
| Interaction Model | ||||
| Baseline depression | .002 (.003) | .652 | ||
| Month | .003 (.001) | .005 | ||
| Change in depression a | -.004 (.003) | .194 | ||
| Change in depression * month | .001 (.0002) | .002 | ||
| Baseline non-adherence | 3.02 (1.54) | .049 | ||
| Month | -.03 (.02) | .149 | ||
| Change in non-adherence a | 1.53 (2.92) | .600 | ||
| Change in non-adherence * month | .18 (.19) | .354 | ||
Change is computed as follow-up minus baseline; values in bold indicate significant findings with p-value < .05. All mixed-effects regression models are covariate-adjusted with the inclusion of socio-demographic characteristics (age, gender, income, education level, marital status), baseline CD4 count and study arm (intervention vs. usual care control) assignment.
In the interaction model (bottom panel), the coefficient of time was higher than before (0.3% per month). Also, a significant interaction term suggested that the effect of PHQ-9 depends on time (0.1% per month). For example, a 5-point change in PHQ-9 would contribute to an additional 4% increase in non-adherence over 12 months, and an 8% increase in non-adherence over 20 months because of this interaction term, and not accounting for the constant slope of the time trend. Now combining the coefficients of the main effects and interaction terms, a hypothetical 5-point increase in PHQ-9 would result in an increase in non-adherence of 7.6% at 12 months and 14% at 20 months. Baseline depression score did not predict change in non-adherence in either model.
The models in the second column predict change in the continuous measure of depression (PHQ-9). In the first model (top panel), baseline non-adherence was an independent predictor. An increase of 10% in non-adherence between two study participants contributed to a difference of 0.3 units in PHQ-9, at baseline. Change in non-adherence was also significantly associated with depression – for example, a hypothetical 10% change in non-adherence corresponded to 0.4 unit increase in depression (effect size = .148). Looking at the coefficient of time, those with perfect adherence (or, zero value of non-adherence) saw a slight, albeit non-significant reduction in depression over time. When we tested for an interaction between change in non-adherence and time (bottom panel), we found that the interaction term was non-significant. Therefore, we decided to drop the interaction term in favor of the simpler main effects model in the top panel.
Longitudinal Relationship Between Change in Binary Depression and Non-adherence
Table-II presents the results of longitudinal logistic regression models examining the relationship between binary classifications of depression and non-adherence. In the model predicting non-adherence classification, rates of non-adherence were stable over time (that is, coefficient of linear time was not significant), and significantly associated with both baseline classification of having at least minor depression and change in classification of depression, suggesting that becoming depressed corresponded to 1.65 times higher odds of non-adherent status. The second model predicting change in classification of depression showed that classification of depression increased over time, overall, and was significantly associated with both baseline classification of non-adherence and change in non-adherence status. We estimated that becoming non-adherent was associated with a 2.35 times higher odds of having at least minor depression. We do not present the additional models that include the interaction of time and the predictor, because the interaction terms in the models for both dependent variables were not significant.
DISCUSSION
While most studies have focused on how depression is related to and may influence ART adherence, this is one of the few studies to examine the potential bidirectional nature of this relationship. This was accomplished by assessing how change in each of these constructs relates to the baseline level and change in the other, estimated at the same time point, using longitudinal assessments over 20 months of follow-up. Our data from ART experienced patients in Uganda provide empirical support for the bidirectional nature of the relationship between depression and ART non-adherence. A temporal trend of increased non-adherence was accelerated by greater change in depression, and measures of change in non-adherence and depression were predictive of each other.
When looking at our data from a within-person change perspective, our data did not reveal a temporal trend for measures of change in depression over time when controlling for measures of change in non-adherence. However, we did observe a temporal trend towards increased change in the continuous measure of non-adherence over time when controlling for change in the continuous measure of depression, which reflected a trend towards worsening adherence. This trend was accelerated with greater change (or increase) in depression; our data indicated that the effect of change in depression on non-adherence depends on time, as the effect is minimal at first, but increases with time.
Furthermore, like other studies that have shown increased depression over time to be associated with increased ART non-adherence (38–42), our data revealed that time varying change in the classification of having at least minor depression (e.g., becoming depressed) was an independent predictor of change in the classification of non-adherence (e.g., becoming non-adherent), and this association remained when examining continuous measures of the constructs as well (when the interaction with time was not included in the model). Similarly, looking at the flip side of this association, time varying measures of change in non-adherence were independently predictive of change in depression, such that increased non-adherence was predictive of increased depression. This finding was evident regardless of whether continuous or binary classification measures of both depression and non-adherence were used.
We also examined between-person variation with the inclusion of the baseline measure of our predictors. Baseline classification of depression was significantly associated with average change in classification of non-adherence across the follow-up time points, indicating that those who had at least minor depression at baseline were more likely to be classified as non-adherent. However, higher non-adherence levels at baseline was associated with higher levels of depression, averaged across follow-up time points, only when the interaction with time was included in the model; non-adherent status at baseline was not predictive of depression classification at follow-up.
These findings suggest that not only may depression treatment have indirect benefits on ART adherence, but efforts to support adherence and address barriers to adherence and retention may have mental health benefits as well. There is some empirical evidence that antidepressant treatment (43–45) and cognitive behavioral therapy for depression (22) improve adherence among depressed patients. Similarly, studies that evaluate the effects of interventions to improve adherence have the opportunity to examine whether the effects extend to mental health and psychological well-being among depressed individuals.
A key limitation of this analysis is that while our data is longitudinal, the inferences from our findings are confined to associations, and cannot infer causality or temporal precedence due to the observational design of this study. Our data support the bidirectional nature of the relationship between depression and non-adherence, and that change in one construct is associated with change in the other, but our data cannot evaluate whether change in one construct causes changes in the other. Another limitation is with regard to our measurement of depression. Although the self-report PHQ-9 has established scoring cutoffs that have been found to correspond with diagnostic levels of depression, our data would be strengthened if depression diagnoses had been derived from diagnostic interviews (46). Furthermore, the relatively low number of participants with clinical depression resulted in us including manifestations of minor depression in the analytic classification of depression. Therefore, our findings related to this classification cannot be considered reflective of patients diagnosed with clinical depression. Lastly, although our use of an objective measure of adherence is a strength, electronic monitoring caps nonetheless can either underestimate or overestimate actual adherence; we tried to limit measurement error with instructions on how to use the cap so that is provides accurate adherence data, as well as the collection of self-reported instances of incorrect use of the cap, which was used to adjust the electronic monitoring data.
CONCLUSIONS
While other research has examined and supported the influence of depression and changes in depression on ART adherence, our data support the reciprocal relationship—that non-adherence and changes in adherence are associated with levels and changes in depression. The findings support the potential benefits of depression care and adherence support for improving adherence and mental health, respectively, and argue for the value of integrating such services into HIV care programs.
Table-II:
Longitudinal association between change in depression and non-adherence status and binary classifications of these constructs at follow-up
| Non-adherence classification b |
Depression classification c |
|
|---|---|---|
| O.R. (295% C.I.) | O.R. (95% C.I.) | |
| Depression classification at baseline | 1.02 (1.003, 1.05) | |
| Month | 1.90 (1.08, 3.36) | |
| Change in depression classificationa | 1.65 (1.19, 2.31) | |
| Nonadherence classification at baseline | 0.99 (0.97, 1.02) | |
| Month | 2.80 (1.56, 5.04) | |
| Change in nonadherence classificationa | 2.35 (1.51, 3.67) |
Change is represented by the 3-level categorical variable (−1, 0 or 1);
Non-adherence > .10 is classified as non-adherent;
PHQ9 > 4 is classified as depressed. All mixed-effects regression models are covariate-adjusted with the inclusion of socio-demographic characteristics (age, gender, income, education level, marital status), baseline CD4 count and study arm (intervention vs. usual care control) assignment.
Acknowledgments
This project was funded by the National Institute of Mental Health (NIMH), grant number R34MH096609 (P.I., Sebastian Linnemayr).
Footnotes
Conflicts of interest: The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
All procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
REFERENCES
- 1.Brandt R The mental health of people living with HIV/AIDS in Africa: a systematic review. Afr J AIDS Res. 2009; 8:123–133. [DOI] [PubMed] [Google Scholar]
- 2.Collins PY, Homan AR, Freeman MC, Patel V. What is the relevance of mental health to HIV/AIDS care and treatment programs in developing countries? a systematic review. AIDS 2006; 20:1571–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myer L, Smit J, Le Roux L, Parker S, Stein DJ, Seedat S. Common mental disorders among HIV-infected individuals in South Africa: Prevalence, predictors, and validation of brief psychiatric rating scales. AIDS Patient Care STDs. 2008; 22:147–157. [DOI] [PubMed] [Google Scholar]
- 4.Abas M, Ali G-C, Nakimuli-Mpungu E, Chibanda D. Depression in people living with HIV in sub-Saharan Africa: time to act. Trop Med Int Health. 2014;19: 1392–1396. [DOI] [PubMed] [Google Scholar]
- 5.Ickovics JR, Hamburger ME, Vlhaov, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV sero-positive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001; 285:1466–74. [DOI] [PubMed] [Google Scholar]
- 6.Evans DL, Ten Have TR, Douglas SD, et al. Association of depression with viral load, CD8 T lymphocytes, and natural killer cells in women with HIV infection. Am J Psychiatry. 2002; 159:1752–9. [DOI] [PubMed] [Google Scholar]
- 7.Lima VD, Geller J, Bangsberg DR, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS. 2007; 21:1175–1183. [DOI] [PubMed] [Google Scholar]
- 8.Antelman G, Kaaya S, Ruilan W, et al. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. J Acquir Immune Defic Syndr. 2007; 44:470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pence BW, Miller WC, Gaynes BN, Eron JJ. Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007; 44:159–166. [DOI] [PubMed] [Google Scholar]
- 10.Nakimuli-Mpungu E, Bass JK, Alexandre P, Mills EJ, Musisi S, Ram M, et al. Depression, Alcohol Use and Adherence to Antiretroviral Therapy in Sub-Saharan Africa: A Systematic Review. AIDS Behav. 2012;16: 2101–2118. [DOI] [PubMed] [Google Scholar]
- 11.Mayston R, Kinyanda E, Chishinga N, Prince M, Patel V. Mental disorder and the outcome of HIV/AIDS in low-income and middle-income countries: a systematic review. AIDS Lond Engl. 2012;26 Suppl 2: S117–135. [DOI] [PubMed] [Google Scholar]
- 12.Gordillo V, Del Amo J, Soriano V, González-Lahoz J. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999, 13:1763–1769. [DOI] [PubMed] [Google Scholar]
- 13.Cichowitz C, Maraba N, Hamilton R, Charalambous S, Hoffmann CJ. Depression and alcohol use disorder at antiretroviral therapy initiation led to disengagement from care in South Africa. PLOS One. 2017; 12(12): doi.org/10.1371/journal.pone.0189820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandura A Self-efficacy: Toward a unifying theory of behavioral change. Psychol Rev. 1977; 84:191–215. [DOI] [PubMed] [Google Scholar]
- 15.Fisher JD, Fisher WA, Misovich SJ, Kimble DL, Malloy TE. Changing AIDS risk behavior: effects of an intervention emphasizing AIDS risk reduction information, motivation, and behavioral skills in a college student population. Health Psychol. 1996; 15(2):114–123. [DOI] [PubMed] [Google Scholar]
- 16.Olatunji BO, Mimisga MJ, O’Clerigh C, Safren SA. A review of treatment studies of depression in HIV. Top HIV Med. 2006; 14:116–128. [PubMed] [Google Scholar]
- 17.Wagner GJ, Ghosh-Dastidar B, Robinson E, Ngo VK, Glick P, Mukasa B, Musisi S, Akena D. Effects of depression alleviation on ART adherence and HIV clinic attendance in Uganda, and the mediating roles of self-efficacy and motivation. AIDS & Behavior, 2017; 21(6): 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yun LW, Maravi MBS, Kobayashi JS, Barton PL, Davidosn AJ. Antidepressant treatment improves adherence to antiretroviral therapy among depressed HIV-infected patients. J Acquir Immune Defic Syndr. 2005; 38:432–8.57. [DOI] [PubMed] [Google Scholar]
- 19.Wagner GJ, Goggin K, Remien RH, et al. A closer look at depression and its relationship to HIV antiretroviral adherence. Ann Behav Med 2011; 42:352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wing RR, Phelan S, Tate D. The role of adherence in mediating the relationship between depression and health outcomes. J Psychosom Res. 2002; 53(4): 877–81. [DOI] [PubMed] [Google Scholar]
- 21.Wagner GJ, Ghosh-Dastidar B, Garnett J, Kityo C, Mugyenyi P. Impact of HIV antiretroviral therapy on depression and mental health among clients with HIV in Uganda. Psychosom Med 2012; 74:883–90. [DOI] [PubMed] [Google Scholar]
- 22.Safren SA, O’Cleirigh CO, Tan JY, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009; 28: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antoni MH, Carrico AW, Duran RE, et al. Randomized clinical trial of cognitive behavioral stress management on human immunodeficiency virus viral load in gay men treated with highly active antiretroviral therapy. Psychosom Med. 2006; 68(1): 143–51. [DOI] [PubMed] [Google Scholar]
- 24.Linnemayr S, Stecher C. Behavioral economics matters for HIV research: the impact of behavioral biases on adherence to antiretrovirals (ARVs). AIDS Behav. 2015; 19:2069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001; 16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monahan PO, Shacham E, Reece M, et al. Validity/reliability of PHQ-9 and PHQ-2 depression scales among adults living with HIV/AIDS in western Kenya. J Gen Intern Med. 2009; 24:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic Statistical Manual of Mental Disorders, 4th Edition. American Psychiatric Association, Washington, D.C., 1994. [Google Scholar]
- 28.Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14(4):357–66. [DOI] [PubMed] [Google Scholar]
- 29.Wagner GJ, Kanouse DE, Golinelli D, et al. Cognitive-behavioral intervention to enhance adherence to ART: a randomized controlled trial (CCTG 578). AIDS. 2006; 20:1295–1302. [DOI] [PubMed] [Google Scholar]
- 30.Finocchario-Kessler S, Catley D, Berkley-Patton J, et al. Baseline predictors of ninety percent or higher antiretroviral therapy adherence in a diverse urban sample: the role of patient autonomy and fatalistic religious beliefs. AIDS Patient Care STDs. 2011;25:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deschamps AE, Graeve VDE, van Wijngaerden E, et al. Prevalence and correlates of nonadherence to antiretroviral therapy in a population of HIV patients using Medication Event Monitoring System. AIDS Patient Care STDS. 2004; 18:644–657. [DOI] [PubMed] [Google Scholar]
- 32.Wilson IB, Bangsberg DR, Shen J, et al. Heterogeneity among studies in rates of decline of ART adherence over time: Results from the MACH14 study. J Acquir Immune Defic Syndr. 2013; 64:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hedeker D,Gibbons RD. Longitudinal Data Analysis Wiley Series in Probability and Statistics. Longitudinal data analysis. Hoboken, NJ, US: Wiley-Interscience; 2006 [Google Scholar]
- 34.Bryk AS, audenbusch SW. Hierarchical Linear Models: Application and Data Analysis Methods. Newbury Park, CA: Sage; 1992 [Google Scholar]
- 35.Goldstein H Multilevel Models in Educational and Social Research. London: Griffin; 1987 [Google Scholar]
- 36.Duckworth AL, Tsukayama E, May H. Establishing causality using longitudinal hierarchical linear modeling: an illustration predicting achievement from self-control. Soc Psychol Personal Sci. 2010; 1(4): 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.SAS/STAT software version 9.4 Copyright (c) 2002–2012 by SAS Institute Inc., Cary, NC, USA. [Google Scholar]
- 38.Starace F, Ammassari A, Trotta MP, et al. Depression is a risk factor for suboptimal adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002; 31: S136–9. [DOI] [PubMed] [Google Scholar]
- 39.Holzemer WL, Corless IB, Nokes KM, et al. Predictors of self-reported adherence in persons living with HIV disease. AIDS Patient Care and STDs. 1999; 13(3): 185–97. [DOI] [PubMed] [Google Scholar]
- 40.Ammassari A, Antinori A, Aloisi MS, et al. Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosomatics. 2004; 45(5): 394–402. [DOI] [PubMed] [Google Scholar]
- 41.Vranceanu AM, Safren SA, Lu M, et al. The relationship of post-traumatic stress disorder and depression to antiretroviral medication adherence in persons with HIV. AIDS Patient Care and STDs. 2007; 22: 313–21. [DOI] [PubMed] [Google Scholar]
- 42.Wagner GJ, Kanouse DE, Koegel P, Sullivan G. Correlates of HIV antiretroviral adherence in persons with serious mental illness. AIDS Care. 2004; 16(4): 501–6. [DOI] [PubMed] [Google Scholar]
- 43.Yun LW, Maravi M, Kobayashi JS, Barton PL, Davidson AJ. Antidepressant treatment improves adherence to antiretroviral therapy among depressed HIV-infected patients. J Acquir Immune Defic Syndr. 2005; 38(4): 432–8. [DOI] [PubMed] [Google Scholar]
- 44.Horberg MA, Silverberg MJ, Hurley LB, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr. 2008; 47(3): 384–90. [DOI] [PubMed] [Google Scholar]
- 45.Tsai AC, Weiser SD, Petersen ML, Ragland K, Kushel MB, Bangsberg DR. A marginal structural model to estimate the causal effect of antidepressant medication treatment on viral suppression among homeless and marginally housed persons with HIV. Arch Gen Psychiatry. 2010; 67: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simoni JM, Safren SA, Manhart LE, et al. Challenges in Addressing Depression in HIV Research: Assessment, Cultural Context, and Methods. AIDS & Behavior. 2011; 15: 376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]