Abstract
Background:
Therapeutic drug monitoring measures antiretroviral adherence more accurately than self-report but has not been available at the point-of-care (POC) until now. We compare a novel POC test for urine tenofovir to laboratory-based enzyme-linked immunosorbent assay (ELISA) testing in diverse patient populations urine pre-exposure prophylaxis (PrEP).
Setting:
Urine samples were analyzed using ELISA and the POC lateral flow immunoassay (LFA) test from two cohorts of PrEP users taking tenofovir disoproxil fumarate/emtricitabine: the Partners PrEP Study, which recruited Kenyan and Ugandan heterosexual men and women, and the IBrEATHe Study, which recruited U.S. transgender women and men using gender-affirming hormone therapy.
Methods:
We calculated the sensitivity, specificity, and accuracy of the POC test compared to ELISA at a cut-off of 1,500 ng/mL.
Results:
Overall, 684 urine samples were tested from 324 participants in the two cohorts. In Partners PrEP, 454 samples from 278 participants (41% women) were tested with a median age of 33 years. In IBrEATHe, 231 samples from 46 individuals (50% transwomen) were tested with a median age of 31 years. Comparison of the LFA read-out to ELISA yielded 100% sensitivity (97.5% one-sided confidence interval (CI)=99.3%), 98.3% specificity (95% CI=95.2%-99.7%), and 99.6% accuracy (95% CI=98.7%-99.9%).
Conclusion:
The sensitivity, specificity, and accuracy of a novel POC test for urine tenofovir all exceeded 98% when compared to a laboratory-based ELISA method when tested in diverse patient populations. Given the LFA’s high accuracy and expected low cost, this POC test is a promising tool to support antiretroviral adherence that could be widely scalable to real-world clinical settings.
Keywords: Pre-exposure prophylaxis, point-of-care, adherence, real-time, antiretroviral therapy
Introduction:
Adherence is the primary determinant of pre-exposure prophylaxis’ (PrEP) efficacy.1,2 Since self-reported PrEP adherence can be subject to bias,3 therapeutic drug monitoring (TDM), where drug levels are measured in a biomatrix such as dried blood spots,4 hair,5 plasma,6 or urine,7 is increasingly used to interpret and assess outcomes in PrEP studies. However, laboratory-based methods for TDM require expensive spectrometry-based technology and laboratory personnel, limiting their scalability and use outside of research settings.8 A point-of-care (POC) test to objectively assess adherence to PrEP using antibody-based detection could have substantial utility in clinical settings.
Availability of TDM in real-time could permit targeting of PrEP adherence counseling to PrEP users who can benefit most.9-11 In addition to use for PrEP, a POC test for adherence monitoring could also benefit patients on antiretroviral treatment (ART). During treatment monitoring, HIV viral loads serve both as surrogates of adherence and indicators of efficacy, although in some contexts they are used infrequently due to cost and logistical challenges. Availability of adherence information in real-time could support clinical decision-making and enhanced adherence counseling while the patient is still in clinic, increasing the efficiency of ART delivery.9 Tenofovir-based drugs are the backbone of PrEP and most ART regimens worldwide. A POC, real-time, metric of adherence for tenofovir would therefore likely have broad applications, including outside of research settings, to measure and support adherence to therapy for both HIV prevention and treatment.
We previously reported on the development of a highly-selective antibody against tenofovir and used it to develop an enzyme-linked immunosorbent assay (ELISA) to measure tenofovir levels in urine, validating it against spectrometry-based methods with high accuracy.7,8 Urine tenofovir testing examines recent adherence over the last 4–7 days, analogous to plasma tenofovir testing.12 Using data from a directly-observed therapy (DOT) study administering different dosing patterns of tenofovir disoproxil fumarate (TDF), we established that a cut-off of 1500 nanograms (ng)/milliliter (mL) of tenofovir in urine accurately classified recent TDF-based PrEP dosing.7 We then developed a lateral flow immunoassay (LFA), which permits testing at the POC, using the tenofovir-specific antibody designed to signal urine tenofovir levels below this cut-off.13 Finally, we validated the LFA against liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based methods.13
Prior to widespread use of this novel adherence metric, it is now necessary to: 1) establish that the LFA provides accurate read-outs when compared to laboratory-based testing; and 2) test the LFA in diverse patient populations. To accomplish these two objectives, in this analysis we validate the novel LFA POC test against ELISA drug-level testing using urine samples from two large previously completed PrEP studies.
Methods:
Study Populations:
The Partners PrEP Study was the first study that established the efficacy of TDF/emtricitabine (FTC)-based PrEP in heterosexual men and women ( NCT00557245).14 The study enrolled heterosexual serodiscordant couples in Kenya and Uganda, randomizing the HIV-uninfected partner to receive TDF/FTC, TDF, or placebo (n=4747).14 Our analysis leverages urine specimens available from participants in the pharmacokinetic substudy of Partners PrEP (n=292).15
The TRIUMPH study is a U.S. demonstration project of PrEP in transgender women and men. The I-BrEATHe study is an ongoing pharmacokinetic substudy of this demonstration project which collected biospecimens, including urine, from 48 participants also using gender-affirming hormone therapy ( NCT04050371).
Laboratory Methods:
Urine samples collected in Partners PrEP Study and I-BrEATHe were aliquoted for analysis by both ELISA and the LFA. For the ELISA-based immunoassay, working solutions of tenofovir of known concentrations were prepared. Calibrators or different concentrations of tenofovir were incubated on a microtiter plate with the targeted analyte-enzyme conjugate to generate a dose–response curve. An ELISA plate reader extrapolated the concentration of tenofovir in the unknown specimen based on the calibration curve. The lower limit of quantification (LLOQ) for the ELISA-based immunoassay is 1000 ng/mL, which corresponds to no dosing in the prior 4–7 days.7,8 For the LFA, 2–3 drops of urine are applied from the urine sample on to the LFA and, after five minutes, the lines on the LFA window are visually read (Figure 1). The cut-off for the LFA is 1,500 ng/ml, which was selected based on a prior DOT study because it optimized specificity for dosing in the prior 24 hours, while maintaining high sensitivity.7
Figure 1:
Photograph of the Point-of-Care Test showing Control and Test Lines and the Sample Window
Statistical Analysis:
We calculated the sensitivity, specificity and accuracy of the LFA compared to ELISA using urine samples in the two different studies by cross-tabulating values above/below the 1500ng/mL threshold by the two different assays.7 Because misclassification was very rare, we present confidence intervals based on exact calculations using the binomial distribution.
Results:
A photograph of the LFA showing test and control lines, as well as the sample window, is shown in Figure 1. Overall, 684 urine samples were tested from 324 participants in the two cohorts. In the Partners PrEP Study, 454 samples from 278 participants (41% women and 59% men) were tested; the median age was 33 years (interquartile range (IQR) of 28–39). In IBrEATHe, 231 samples from 46 individuals (50% transgender women on estrogen and 50% transgender men on testosterone) were tested; the median age was 31 years (IQR 25–40).
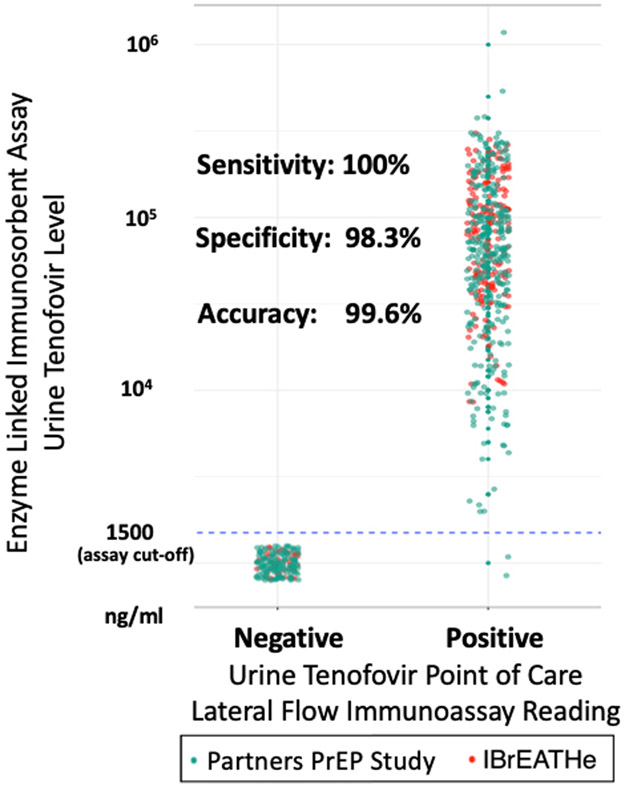
Overall, of the 505 samples with tenofovir levels greater than or equal to the cut-off of 1500ng/mL using laboratory-based ELISA, 505 of the POC test results were also positive, yielding 100% sensitivity (one-sided 97.5% confidence interval (CI)=99.3%). Of the 179 samples with TFV levels below the cut-off, 176 were negative with the POC test, yielding 98.3% specificity (95% CI=95.2%-99.7%). The accuracy of the POC LFA across the two studies was 99.6% compared to ELISA (95% CI=98.7%-99.9%) (Figure 2).
Figure 2:
Comparison of a Novel Point of Care Urine Tenofovir Test to a Laboratory-Based Enzyme-Linked Immunosorbent Assay
The three individuals who tested falsely positive with the LFA were all heterosexual men who were part of the Partners PrEP study; two were not on medications at the time, one was taking ampicillin, nitrofurantoin, and furosemide at the time of sample collection.
Discussion:
In a large, diverse sample of men and women (both cisgender and transgender) taking TDF/FTC-based PrEP, the sensitivity, specificity, and accuracy of a novel POC test for urine tenofovir were all over 98% when compared to a laboratory-based ELISA method. These results build on previous results showing high accuracy of the LFA compared to LC-MS/MS among 30 men and women in the TARGET study.13 An LFA, like a urine pregnancy test, can be performed easily by personnel without specialized training at the point-of-care. Given its excellent performance characteristics and estimated low-cost (<$2.00 per test), this POC test could be implemented widely to support both PrEP and HIV ART delivery.
Tenofovir-based regimens are the backbone of most ART regimens worldwide, and tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC), and recently tenofovir alafenamide (TAF)/FTC, are the only approved regimens for PrEP.16-18 As TAF is also metabolized into tenofovir and secreted in the urine at levels approximately eight-fold below TDF, the urine-based tenofovir assay can be readily adapted for monitoring TAF-based therapy.19 We are currently developing a version of the LFA to measure adherence to TAF-based ART. A POC urine tenofovir test could therefore provide real-time actionable recent adherence information in many contexts.
For PrEP, we have previously shown in a completed demonstration project that low versus high urine tenofovir concentrations confers a 14-fold higher odds of HIV seroconversion at subsequent visits.11 A negative urine tenofovir POC test during a PrEP visit, therefore, could trigger a discussion about the client’s adherence and potentially enhanced adherence counseling, closer follow-up intervals, and additional services (mental health, substance use, social work) as indicated. TDM has been previously used to support PrEP adherence counseling with promising results. The Los Angeles-based PATH-PrEP study utilized plasma tenofovir TDM to target motivational interviewing-informed adherence counseling to those with low recent adherence, finding that nearly 2/3 of participants with low adherence improved their subsequent adherence with counseling enhanced by drug-level testing.10 Future randomized controlled trials should examine the unique potential of POC adherence testing to both measure and support adherence counseling for PrEP.
In the context of HIV treatment, there is growing interest in using TDM to guide provision of costly resistance testing in resource-limited settings.20 An elevated HIV viral load with a high urine tenofovir level would suggest a higher likelihood of viral resistance, triggering a viral genotype and switch to second-line therapy. Conversely, a low urine tenofovir level in the setting of a high HIV viral load could spur enhanced adherence counseling. As a drop off in adherence may precede a rise in the HIV viral load by weeks to months for some individuals,21 the POC urine test could permit adherence intervention prior to the loss of virologic control. In contexts where viral load testing occurs annually or less often, urine tenofovir could be used in-between viral load tests to enhance adherence counseling and prioritize those who need more frequent viral load testing.20
This assay will have several limitations inherent to short-term adherence metrics. Urine tenofovir is highly correlated with plasma tenofovir,12 and similarly measures adherence over short periods of time (~1 week). It is possible that individuals could increase their adherence immediately prior to a clinical visit (“white-coat adherence”) and thereby have a positive urine tenofovir test, without this reflecting typical adherence patterns. Plasma-based tenofovir metrics supported the interpretation of all the major clinical trials and many of the demonstration projects of PrEP. In these studies, a pattern of increasing adherence prior to study visits was not seen, even when participants were informed drug-level testing would be performed.22 However, PrEP provision in the context of a clinical trial or demonstration project may differ from that occurring in real-world contexts so close observation of adherence patterns with widespread use of the POC test will be needed. Moreover, combining the urine POC test with a cumulative metric of adherence such as measurement of tenofovir or its metabolites in hair or dried blood spots (DBS), respectively, can reveal patterns of adherence.23 For these reasons, a positive urine tenofovir test should not imply perfect adherence. However, the interpretation of a low urine POC test is clear,11 with a negative test meriting discussion and potentially intervention to support the PrEP user’s adherence. An ongoing clinical trial of the feasibility, acceptability, and impact of enhanced adherence counseling with the POC test will shed additional light on implementation of the tool ( NCT03935464).
In conclusion, given the excellent performance characteristics of the tenofovir-based LFA in diverse populations on PrEP, this point-of-care test is emerging as a promising tool to support PrEP adherence that could be widely scalable to real-world clinical settings. Enhanced adherence support using this test should be evaluated in randomized clinical trials across a variety of populations.
Acknowledgements:
Sources of support: Funding for this work and the development of the antibody at Abbott Rapid Diagnostics was provided by the National Institute of Allergy and Infectious Diseases/National Institutes of Health (NIAID/NIH) R01AI143340 (P.I.: M.G.). Three authors (W.C.R., G.W., and M.V.) are from Abbott Rapid Diagnostics. M.A.S. was supported by National Institutes of Mental Health/NIH K23122286 and NIAID/NIH T32AI060530 (P.I.: Havlir). M.D., P.D., and R.G. were funded by California HIV/AIDS Research Program PR15-SF-007. P.D. and R.M.G. and P.D. were supported by NIAID/NIH R01AI118575. D.V.G. serves on advisory boards for Gilead and Merck. The other authors have no other conflicts of interest to declare. This data has not been presented previously.
Appendix
Partners PrEP Study Team
University of Washington Coordinating Center and Central Laboratories: Connie Celum (principal investigator, protocol co-chair), Jared M. Baeten (medical director, protocol co-chair), Deborah Donnell (protocol statistician), Robert W. Coombs, Lisa Frenkel, Craig W. Hendrix, Jairam Lingappa, M. Juliana McElrath.
Study sites and site principal investigators: Eldoret, Kenya (Moi University, Indiana University): Kenneth Fife, Edwin Were; Kabwohe, Uganda (Kabwohe Clinical Research Center): Elioda Tumwesigye; Jinja, Uganda (Makerere University, University of Washington): Patrick Ndase, Elly Katabira; Kampala, Uganda (Makerere University): Elly Katabira, Allan Ronald; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig Cohen; Mbale, Uganda (The AIDS Support Organization, CDC-Uganda): Jonathan Wangisi, James Campbell, Jordan Tappero; Nairobi, Kenya (University of Nairobi, University of Washington): James Kiarie, Carey Farquhar, Grace John-Stewart; Kenya Medical Research Institute, Nairobi, Kenya: Nelly Rwamba Mugo; Tororo, Uganda (CDC-Uganda, The AIDS Support Organization): James Campbell, Jordan Tappero, Jonathan Wangisi.
Data management was provided by DF/Net Research, Inc. (Seattle, USA) and site laboratory oversight was provided by Contract Laboratory Services (University of the Witwatersrand, Johannesburg, South Africa).
References:
- 1.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glidden DV, Amico KR, Liu AY, et al. Symptoms, Side Effects and Adherence in the iPrEx Open-Label Extension. Clin Infect Dis. 2016;62(9):1172–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koss CA, Hosek SG, Bacchetti P, et al. Comparison of Measures of Adherence to Human Immunodeficiency Virus Preexposure Prophylaxis Among Adolescent and Young Men Who Have Sex With Men in the United States. Clin Infect Dis. 2018;66(2):213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson PL, Liu AY, Castillo-Mancilla JR, et al. Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots following Directly Observed Therapy. Antimicrob Agents Chemother. 2018;62(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu AY, Yang Q, Huang Y, et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP). PLoS One. 2014;9(1):e83736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendrix CW, Andrade A, Bumpus NN, et al. Dose Frequency Ranging Pharmacokinetic Study of Tenofovir-Emtricitabine After Directly Observed Dosing in Healthy Volunteers to Establish Adherence Benchmarks (HPTN 066). AIDS Res Hum Retroviruses. 2016;32(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi M, Bacchetti P, Spinelli MA, et al. Brief Report: Validation of a Urine Tenofovir Immunoassay for Adherence Monitoring to PrEP and ART and Establishing the Cutoff for a Point-of-Care Test. J Acquir Immune Defic Syndr. 2019;81(1):72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi M, Bacchetti P, Rodrigues WC, et al. Development and Validation of an Immunoassay for Tenofovir in Urine as a Real-Time Metric of Antiretroviral Adherence. EClinicalMedicine. 2018;2–3:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson PL. What Can Urine Tell Us About Medication Adherence? EClinicalMedicine. 2018;2–3:5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landovitz RJ, Beymer M, Kofron R, et al. Plasma Tenofovir Levels to Support Adherence to TDF/FTC Preexposure Prophylaxis for HIV Prevention in MSM in Los Angeles, California. J Acquir Immune Defic Syndr. 2017;76(5):501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spinelli MA, Glidden DV, Rodrigues WC, et al. Low tenfovir level in urine by a novel immunoassay is associated with seroconversion in a PrEP demonstration project. AIDS. 2019;33(5):867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drain PK, Kubiak RW, Siriprakaisil O, et al. Urine Tenofovir Concentrations Correlate with Plasma and Relates to TDF Adherence: A Randomized Directly-observed Pharmacokinetic Trial (TARGET Study). Clin Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gandhi M, Wang G, King R, et al. Development and Validation of the First Point-of-Care Assay to Objectively Monitor Adherence to HIV Treatment and Prevention in Real-Time in Routine Settings. AIDS. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnell D, Ramos E, Celum C, et al. The effect of oral preexposure prophylaxis on the progression of HIV-1 seroconversion. AIDS. 2017;31(14):2007–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2018 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320(4):379–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hare CB, Coll J, Ruane P, et al. The Phase 3 Discover Study: DAILY F/TAF OR F/TDF FOR HIV PREEXPOSURE PROPHYLAXIS . Conference on Retroviruses and Opportunistic Infections 2019. March 3–7 [#104]. [Google Scholar]
- 18.Food and Drug Administration. FDA approves second drug to prevent HIV infection as part of ongoing efforts to end the HIV epidemic. Accessed October 3, 2019 https://www.prnewswire.com/news-releases/fda-approves-second-drug-to-prevent-hiv-infection-as-part-of-ongoing-efforts-to-end-the-hiv-epidemic-300930895.html. 2019.
- 19.Haaland RE, Martin A, Livermont T, et al. Urine emtricitabine and tenofovir concentrations provide markers of recent antiretroviral drug exposure among HIV-negative men who have sex with men. J Acquir Immune Defic Syndr. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermans LE, Steegen K, ter Heine R, Schuurman R, Tempelman H, Moraba R. PI DRUG-LEVEL TESTING AS A SCREENING TOOL FOR DRUG RESISTANCE IN 2ND-LINE ART FAILURE [Abstract 461] CROI 2019. March 4-7 Seattle, Washington. [Google Scholar]
- 21.Martin GE, Frater J. Post-treatment and spontaneous HIV control. Curr Opin HIV AIDS. 2018;13(5):402–407. [DOI] [PubMed] [Google Scholar]
- 22.Koss CA, Bacchetti P, Hillier SL, et al. Differences in Cumulative Exposure and Adherence to Tenofovir in the VOICE, iPrEx OLE, and PrEP Demo Studies as Determined via Hair Concentrations. AIDS Res Hum Retroviruses. 2017;33(8):778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen SE, Sachdev D, Lee SA, et al. Acquisition of tenofovir-susceptible, emtricitabine-resistant HIV despite high adherence to daily pre-exposure prophylaxis: a case report. Lancet HIV. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]