Highlights
-
•
COVID-19 is a pandemic caused by the SARS-Cov-2 coronavirus.
-
•
The pneumonia caused by COVID-19 may cause high mortality.
-
•
Treatment and/or vaccination for virus is an ongoing proccess, they have not been discovered yet.
-
•
Interferons could be effective for the treatment of COVID-19.
-
•
Further studies of the experiences of people with MS who take type I interferons, such as beta interferons who get COVID-19, will help to inform.
Abstract
Background
The Coronavirus (COVID-19), (Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)) has been spreading worldwide since its first identification in China. It has been speculated that patients with comorbidities and elderly patients could be at high risk for the pandemic reasoned respiratory insufficiency and death. At first, it was thought that the patients who use immunmodulator therapy could be even at higher risks of disease complications. However, it has been also speculated about that using immunmodulators could be an advantage for the clinical prognosis. Therefore, several immunmodulators are currently being tested as potential treatment for COVID-19.
Methods
In this paper we report on a patient that has been treated with type 1 interferon for multiple sclerosis who developed COVID-19.
Results
Despite using immunmodulator, the symptoms of the patient at hospitalization were mild and he did not show elevated D-dimer, and there was no lymphopenia. He was discharged to home-quarantine with no symptoms.
Discussion
This report supports the idea of using type 1 interferon in the treatment could be effective in COVID-19 affected patients.
1. Main text
The Coronavirus (COVID-19), (Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)) has been spreading since its identification in cluster of cases of pneumonia in Wuhan, Hubei Province, China. The symptoms of the infected patients have wide range from asymptommatic or common cold symptoms to pneumonia symptoms. The prognosis may vary from full recovery to the severe acute respiratory distress syndrom and death. So far, many different treatment modalities have been tried and offered by different groups of physicians. Nowadays, many studies are on progress to develop vaccine for prevention, and to develop drugs for medical treatment. There are a few studies about the effect of interferon therapy in COVID-19 patients (Sallard et al., 2020; Sheahan et al., 2020; Lokugamage et al., 2020). Effect of type 1 interferon (such as IFN-α and IFN-β) therapy as a potential treatment against coronavirus (COVID-19, MERS and SARS) was shown in many studies and researches are maintaining, more comprehensive data will be available soon (Sallard et al., 2020; Sheahan et al., 2020; Lokugamage et al., 2020). A recently published study has supported that type 1 interferons can be used as a potential therapy against COVID-19 (Sallard et al., 2020). It is noted in this research that interferon therapy can be effective against SARS-CoV, and SARS-CoV-2 may be more sensitive to interferon than other coronaviruses and necessity for the use of interferons in early stage of treatment (Sallard et al., 2020). As to be parallel with this treatment suggestion and researches, we wanted to present our 31 years old male patient with Multiple Sclerosis (MS) who was under beta interferon treatment since he got the diagnosis approximately for 2 years. At the medical history of the patient there is only seasonal allergic rhinitis and MS. Before the onset of the symptoms he has a history of contact with COVID-19 positive patients who were his co-workers. After 1week of exposure he was admitted to the emergency room (ER) with dry cough and shortness of breath complaints.
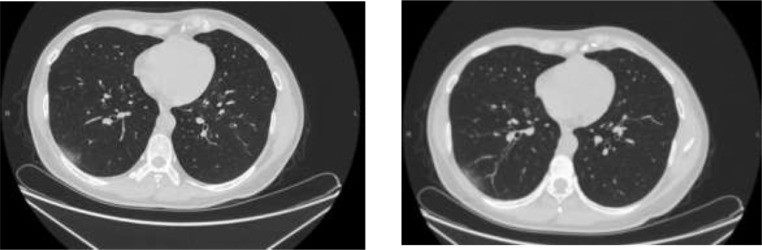
There was no drug use other than interferon which he had been using for 2 years. He had no fever. His respiratory rate and oxygen saturation were normal. In physical examination, respiratory sounds were normal, White blood cells (WBC), hemoglobin (HGB), platelet count (PLT), C-reactive protein level, liver function tests, kidney function tests, D-Dimer level were normal. COVID PCR test obtained from oropharyngeal and nasopharyngeal swab was positive. There were subpleural located ground glass opacities in lower regions at right lung in chest computed tomography (Fig. 1 ). Hydroxychloroquine, azithromycin and enoxaparin sodium treatment were initiated as an addition his interferon therapy. In his follow up, he didn't develop other symptoms except cough. Therapy maintained 5 days. All laboratory tests that performed in fifth day were normal. Our patient who did not have any other complaints has been using interferon therapy for 2 years. Not only length of hospital stays of our patient who was hospitalized with diagnosis of COVID-19 pneumonia was short but also his symptoms remained markedly faint. Hydroxychloroquine and azithromycin were given our patient combined with interferon during time of hospital stay. In his follow up, our patient's clinical features were better and our patient was discharged in the seventh day of his admission.
Fig. 1.
Subpleural located ground glass opacities in lower regions at right lung in chest computed tomography, comprehensive to the first chest CT ground glass opacity of the lesion is suitable with regression.
IFNs play a crucial role in the immune response to viral infections. Type 1 interferon binds to ubiquitously expressed co-receptors on the cell surface (O'Brien et al., 2020). Jak stat signaling pathway activation and also the upregulation of numerous genes stimulated by IFN begin after this binding (O'Brien et al., 2020). IFN stimulated genes encode many proteins that mediate antiviral activities. This case made us consider that interferon therapy might have favorable effect on severity of symptoms and length of hospital stay of patients with COVID-19. IFN-I treatment may be an efficient and safe against SARS-CoV-2 and this should be kept in mind. However, as this individual was treated with other anti-viral agents, it is not possible to definitively link the short hospital stay to treatment. However, that the patient was relatively young and exhibited good prognostic features such as lack of D-dimer, lymphopenia (Huang et al., 2020) could suggest that prophylactic interferon may aid recovery. Further studies of the experiences of people with MS who take type I interferons, such as beta interferons who get COVID 19 (Sormani, 2020), may clarify this further.
2. Scientific responsibility statement
The authors declare that they are responsible for the article's scientific content including study design, data collection, analysis and interpretation, writing, some of the main line, or all of the preparation and scientific review of the contents and approval of the final version of the article.
3. Animal and human rights statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. No animal or human studies were carried out by the authors for this article.
Declaration of Competing Interest
The authors have no conflict of interest about material presented in this manuscript.
References
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond. Engl. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. PMID: 31986264 DOI: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokugamage K.G., Schindewolf C., Menachery V.D. SARS-CoV-2 sensitive to type I interferon pretreatment. BioRxiv. 2020 03.07.982264. [Google Scholar]
- O’Brien T.R., Thomas D.L., Jackson S.S., Prokunina-Olsson L., Donnelly R.P., Hartmann R. Weak Induction of Interferon Expression by SARS-CoV-2 Supports Clinical Trials of Interferon Lambda to Treat Early COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa453. Apr 17pii: ciaa453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallard E., Lescure F.-.X., YazdanYazdanpanah F.M., Smadj N.P. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104791. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11(1):222. doi: 10.1038/s41467-019-13940-6. Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P. Italian Study Group on COVID-19 infection in multiple sclerosis. An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol. 2020 doi: 10.1016/S1474-4422(20)30147-2. Apr 30pii: S1474-4422(20)30147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]