Highlights
-
•
Severe COVID-19 infection is characterized by inflammatory dysregulation and cytokine storm.
-
•
We report a case of clinical improvement after treatment with anakinra in association with remdesivir.
-
•
The high tolerability, short half-life and immunomodulatory profile of anakinra may be useful in such a setting.
-
•
Further studies are needed to confirm the safety and efficacy of anakinra in this setting.
Keywords: Novel coronavirus disease 2019 (COVID-19), Cytokine-release syndrome, Interleukin-1 receptor antagonist, Anakinra, Remdesivir
Abstract
We report the first successful treatment with the IL-1 receptor antagonist anakinra, in association with the most promising and available antiviral therapy, of a severe case of novel coronavirus disease 2019 (COVID-19). We describe the diagnosis, clinical course, and management of the case, including the respiratory failure at presentation, the progression to a scenario characterized by profound inflammatory dysregulation similar to that observed during macrophage activation syndrome, and the clinical improvement after treatment with the IL-1 receptor antagonist anakinra. This case highlights the high tolerability and the interesting immunomodulatory profile of the IL-1 receptor antagonist anakinra in the setting of severe COVID-19 associated with remdesivir therapy. Further studies are needed to confirm the safety and efficacy of this combination strategy in the treatment of this emerging infection.
1. Introduction
Since the first reports of cases from the Hubei Province of China at the end of 2019, more than 80,000 cases of novel coronavirus disease 2019 (COVID-19) have been diagnosed in China and thousands of cases have been reported in all continents (Wu and McGoogan, 2020). Italy has been one of the most involved countries since the end of February 2020 (Livingston and Bucher, 2020).
The lung involvement and the clinical deterioration seen in severe cases have been associated with a substantial increase in the levels of pro-inflammatory cytokines and interleukins (IL-2, IL-6, IL-7, Il-10) and inflammatory markers (D-dimer, ferritin, and C-reactive protein), with pro-inflammatory cytokine levels proportional to the severity of lung disease (Conti et al., 2020b, Conti et al., 2020a, Lin et al., 2020, Wang et al., 2020). This clinical condition resembles the inflammatory derangement seen in other scenarios, such as sepsis-induced macrophage activation syndrome (MAS) (Huang et al., 2020) or immune dysregulation characterized by low expression of human leukocyte antigen D related (HLA-DR) on CD14 monocytes (Lukaszewicz et al., 2009).
Moreover, preliminary observations have shown favorable outcomes when the IL-6 receptor inhibitor tocilizumab was used to treat severe COVID-19 patients (Luo et al., 2020). However, the long half-life of tocilizumab and its limited availability in the clinical arena promote the investigation of other therapeutic options. Among these, the recombinant interleukin-1 receptor antagonist anakinra may be beneficial in reducing the inflammatory storm observed in severe COVID-19 cases. Anakinra has been commonly used in the treatment of rheumatologic conditions, but previous studies report its efficacy in reducing mortality in septic subjects with MAS (Lopalco et al., 2016, Shakoory et al., 2016).
Of note, no specific antiviral therapy has been approved by randomized clinical trials at the moment, even though some antiviral compounds may be associated with clinical improvement as reported by a recent preliminary report of individuals receiving remdesivir on a compassionate-use basis (Grein et al., 2020).
2. Case description
On March 10, 2020, a 57-year-old man presented to the emergency department of our hospital with a 5-day history of sore throat, cough, and fever (maximal body temperature of 39 °C). The patient did not disclose any contact with COVID-19 patients. Apart from a history of tobacco smoke and a body mass index of 30.8 kg/m2, the patient was otherwise healthy.
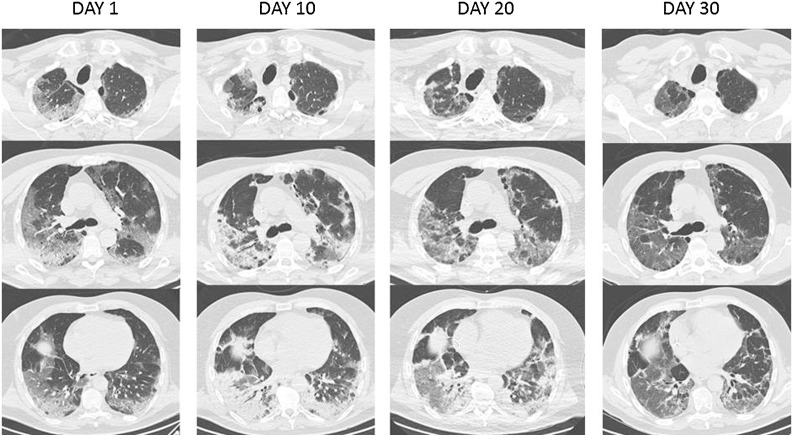
As shown in Table 1 , a body temperature of 38.5 °C and oxygen saturation of 92% while breathing on a Venturi mask were reported at presentation. Laboratory results revealed lymphopenia, a slight elevation of C-reactive protein (CRP) and troponin I, while the electrocardiogram and the echocardiogram performed did not show any abnormalities. A high-resolution computed tomography (HRCT) of the thorax revealed multiple patchy ground-glass opacities in subpleural regions bilaterally, with apical signs of emphysema (Fig. 1 ). A pharyngeal swab specimen was obtained and sent for detection of SARS-CoV-2 by real-time reverse-transcriptase polymerase chain reaction (rRT-PCR) assay: this was reported back within 6 hours as positive. The patient was tested for other respiratory viruses by nucleic acid amplification test and for Legionella pneumophila and Streptococcus pneumoniae by urinary antigens, without any evidence of concurrent infection. The patient was admitted at an infectious diseases unit with health care workers following airborne and droplets precautions.
Table 1.
Vital signs, laboratory tests and treatments during the hospital course.
 |
P/F: ratio of arterial oxygen partial pressure to fractional inspired oxygen; QD: every 24 h; BID: every 12 h; TID: every 8 h; QID: every 6 h; LD: loading dose.
Fig. 1.
(or Supplementary Material). High-resolution computed tomography scans of the thorax at hospital day 1, 10, 20 and 30. Day 1. Multiple patchy ground-glass opacities in bilateral subpleural areas, especially in the posterior segment of the upper lobe and in the lower right lobe. Some traction bronchiectasis. Emphysematous changes. Day 10. Diffuse bilateral increase of parenchymal consolidations, with substantial involvement of the lower lung fields. Day 20. Favorable radiographic evolution with reduction of parenchymal consolidation in the lower lobes. Day 30. Further reduction in extension of the areas of parenchymal consolidation. Ectasia of the vascular structures in interstitial fibrosis areas.
An on off-label treatment with lopinavir/ritonavir (400/100 mg every 12 h per os) and hydroxychloroquine (200 mg every 8 h per os) was started. Azithromycin (500 mg every 24 h per os) and ceftriaxone (2 g every 24 h intravenously) were empirically associated to the ongoing treatment. Despite this, the respiratory status of the patient further deteriorated, with oxygen saturation values dropping to 89% while on reservoir. During this time ventilatory support was offered to the patient, who repeatedly refused both non-invasive and invasive ventilation. A psychiatric evaluation did not disclose any conditions impairing the decision-making ability of the patient and the ethical committee of the hospital accepted the patient's decision to refuse ventilatory support.
On day 7 of hospitalization the patient's clinical condition appeared extremely critical: ratio of arterial oxygen partial pressure to fractional inspired oxygen (P/F) was 50. Fever, asthenia and anorexia worsened and an increase of inflammatory markers (CRP, D-dimer, and ferritin) was noted with a profound change in lipid distribution. Due to the evidence of inflammatory dysregulation and the unavailability of tocilizumab in our hospital, treatment with anakinra was introduced (100 mg every 6 h subcutaneously for seven days). The patient was screened for HBV and Mycobacterium tuberculosis infection: test results were negative. In addition, lopinavir/ritonavir was discontinued and the investigational antiviral remdesivir was started as compassionate use (loading dose 200 mg, followed by 100 mg every 24 h intravenously for seven days).
Progressive normalization of the body temperature was observed, with persistently critical respiratory function. Because of a concern of hospital-acquired pneumonia, on day 10 ceftriaxone and azithromycin were discontinued and treatment with linezolid (600 mg every 12 h intravenously) and piperacillin/tazobactam (4.5 g every 6 h intravenously) was started. Blood and urine cultures performed on day 10 yielded negative results.
In the following days, the patient became afebrile and inflammatory markers dropped. By day 16, a substantial improvement in the respiratory function of the patient was also noticed, with oxygen saturation levels of 92% while on Venturi mask. New HRCT scans showed a progressive reduction of the consolidative lesions previously noticed. Pharyngeal swab specimens for SARS-CoV-2 testing were collected on day 24 and were positive. On day 32, supplemental oxygen was discontinued, oxygen saturation was 93% while the patient was breathing ambient air and he was proposed for transition to subacute care.
3. Discussion
To the best of our knowledge, we report the first treatment with the IL-1 receptor antagonist anakinra and remdesivir of a severe COVID-19 case (Conti et al., 2020b, Conti et al., 2020a). IL-1 is the apical pro-inflammatory mediator, inducing both its own production and the synthesis of several secondary inflammatory mediators, such as IL-6 (Sönmez et al., 2018). Due to the possible role of IL-1/IL-6 axis blockade in conditions resembling MAS, the critical status of our patient, and the shortage of tocilizumab in our institution, we considered the administration of anakinra, in association with the most promising available antiviral agent and with adequate anti-thrombotic therapy (Shakoory et al., 2016, Tanaka et al., 2019). Indeed, another possible and even more frequent pathological mechanism seems to be immune dysregulation with a decrease in HLA-DR expression on CD14 monocytes and overproduction of tumor necrosis factor-α (TNF-α) and IL-6 (Giamarellos-Bourboulis et al., 2020). Although this process could not implicate the overexpression of IL-1, because it may not affect the expression of this interleukin (Giamarellos-Bourboulis et al., 2020), the prompt resolution of fever, the reduction of inflammatory markers, and the improvement in the respiratory function observed in our patient are suggestive of a potential positive effect of anakinra. Nevertheless, the long-lasting positive detection of SARS-CoV-2 on pharyngeal swab specimens did not preclude the clinical recovery of the patients. As described in previous reports, this may suggest a long viral shedding, despite clinical improvement or resolution of symptoms (Yang et al., 2020).
Moreover, even if no venous thromboembolism was detected on a chest CT angiogram with contrast performed at day 10 after admission, anti-thrombotic therapy was also added as shown in Table 1, as this strategy was suggested by progressively available evidence, particularly in consideration of the increase of D-dimer levels (Tang et al., 2020).
The main adverse effects associated with the use of anakinra are injection site reactions and severe infections (Cohen et al., 2004, Lopalco et al., 2016). In our experience no injection site reaction was identified and the elevation of procalcitonin reported on day 7 preceded the administration of anakinra. Moreover, the progression of the lung consolidation detected on day 10 could be expression of ongoing viral infection and inflammation. Nonetheless, the short half-life of anakinra allows a very rapid interruption of its immunomodulatory effect, different from what is observed for long-acting agents, such as tocilizumab (Campbell et al., 2011).
The present report has several limitations. First, IL-1 and IL-6 levels were not evaluated during the hospital stay of the patient due to logistic limitations in an already overwhelmed health system. Second, quantitation of SARS-CoV-2 viral loads were not performed, due to similar constrains. Finally, we cannot make any causal inference regarding the clinical improvement of our patient and the use of anakinra, because of the observational design of this report.
In conclusion, this report highlights the potential role of anakinra in the treatment of respiratory dysfunction in COVID-19 patients. Clinical trials are needed to assess safety and efficacy of this immunomodulatory agent.
Conflict of interest
None.
Funding
None.
Ethical approval
A written consent was obtained by the patient for all off-label treatments that have been provided. The contribution of the ethical committee of the hospital was required for difficult clinical choices as reported.
Acknowledgements
We want to thank nurses (A Basilio, G Bettiga, V Borrini, B Buffoni, E Colombo, C Corti, M Esposito, M Gatti, L Invernizzi, I Molteni, L Pascarelli, A Ravasio, S Salvo, ML Zuffi and the head nurse F Pinoli), nurse assistants (L Abderrazak, B Alessandrino, L Maffei), the personnel implied in pharmaceutical supply (G La Torre, D Commisso, V Colombo), our colleagues and all those facing with us terrible days, sharing every day the effort to take care of each patient in the best possible way.
References
- Campbell L., Chen C., Bhagat S.S., Parker R.A., Östör A.J. Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology. 2011;50(3):552–562. doi: 10.1093/rheumatology/keq343. [DOI] [PubMed] [Google Scholar]
- Cohen S.B., Moreland L.W., Cush J.J., Greenwald M.W., Block S., Shergy W.J. 990145 Study Group. A multicentre, double blind, randomised, placebo-controlled trial of anakinra (Kineret), a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Ann Rheum Dis. 2004;63:1062–1068. doi: 10.1136/ard.2003.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti P., Gallenga C.E., Tetè G., Caraffa A., Ronconi G., Younes A. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection and lung inflammation mediated by IL-1. J Biol Regul Homeost Agents. 2020;34(10):23812. doi: 10.23812/Editorial-Conti-2. [DOI] [PubMed] [Google Scholar]
- Conti P., Ronconi G., Caraffa A., Gallenga C., Ross R., Frydas I. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:1. doi: 10.23812/CONTI-E. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.04.009. S1931-3128(20)30236-5 [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007016. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection – a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9:727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston E., Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4344. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- Lopalco G., Rigante D., Giannini M., Galeazzi M., Lapadula G., Iannone F. Safety profile of anakinra in the management of rheumatologic, metabolic and autoinflammatory disorders. Clin Exp Rheumatol. 2016;34:531–538. [PubMed] [Google Scholar]
- Lukaszewicz A.C., Grienay M., Resche-Rigon M., Pirracchio R., Faivre V., Boval B. Monocytic HLA-DR expression in intensive care patients: interest for prognosis and secondary infection prediction. Crit Care Med. 2009;37:2746–2752. doi: 10.1097/CCM.0b013e3181ab858a. [DOI] [PubMed] [Google Scholar]
- Luo P., Liu Y., Qiu L., Liu X., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020 doi: 10.1002/jmv.25801. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoory B., Carcillo J.A., Chatham W.W., Amdur R.L., Zhao H., Dinarello C.A. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönmez H.E., Demir S., Bilginer Y., Özen S. Anakinra treatment in macrophage activation syndrome: a single center experience and systemic review of literature. Clin Rheumatol. 2018;37:3329–3335. doi: 10.1007/s10067-018-4095-1. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Diallo D., Delbosc S., Genève C., Zappella N., Yong-Sang J. High-density lipoprotein (HDL) particle size and concentration changes in septic shock patients. Ann Intensive Care. 2019;9(1):68. doi: 10.1186/s13613-019-0541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- Yang J.R., Deng D.T., Wu N., Yang B., Li H.J., Pan X.B. Persistent viral RNA positivity during recovery period of a patient with SARS-CoV-2 infection. J Med Virol. 2020 doi: 10.1002/jmv.25940. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]