Abstract
Purpose of Review
To present the latest evidence related to the impact of ureteroscopy (URS) and percutaneous nephrolithotomy (PCNL) on the renal function.
Recent Findings
Our review suggests that the overall renal function is not detrimentally affected by endourological interventions (URS, PCNL). This is however influenced by the preoperative renal function, presence of comorbidities such as diabetes and hypertension. For PCNL procedures, tract multiplicity, preoperative UTI, and postoperative bleeding also contribute to a decline in renal function.
Summary
This review suggests that endourological interventions do not adversely affect renal function and tend to improve it in patients who do not have a poor renal function prior to the procedure. Several factors including poor preoperative renal function, diabetes, hypertension, and multiple percutaneous tracts appear to predispose patients to declining renal function after procedure, and these patients should be counseled for and followed up appropriately.
Keywords: Renal function, eGFR, Ureteroscopy, PCNL, Creatinine, Chronic kidney disease
Introduction
Kidney stone disease (KSD) is rising with a lifetime prevalence of 14% [1–3]. Surgical options such as shockwave lithotripsy (SWL), percutaneous nephrolithotomy (PCNL), and ureteroscopy (URS) are all used as treatment modalities [4–6]. The chosen treatment often depends on stone characteristics, patient fitness, comorbidities, surgical expertise, and underlying renal function. Preoperative assessment of these patients involves up-to-date imaging, urine culture, renal function, and fitness for a general anesthetic. The overall incidence of KSD has been rising, and hence more patients are subjected to surgical intervention [7, 8].
Kidney function can be impaired as a result of the disease, urinary infections, or ureteric obstruction related to the stone or surgical intervention related to the KSD. While it is generally believed that treatment of KSD would lead to an improvement of renal function, it is unclear if the surgical procedure required to remove the stone will have an adverse effect. There is a theoretical risk of deterioration of renal function with both PCNL and URS. The physical puncturing of the kidney during PCNL causes direct damage to the renal parenchyma, and this is amplified as PCNL is increasingly used to treat complex or staghorn calculi requiring multiple puncture tracts [9]. During endoscopic approach to the urinary tract, high pressure irrigation is often required to maintain a visual field, causing dilatation of the renal calyces that could potentially harm the function of the kidney. In addition, while the renal parenchyma is not breached as with PCNL, application of the holmium:yttrium-aluminum-garnet (Ho:YAG) laser may cause heat related tissue damage [10, 11].
Given the theoretical risk of renal function decline with both PCNL and URS, our aim was to conduct systematic review to clarify the effect of endourological interventions on renal function.
Method
Search Strategy
Our systematic review was performed as per the Cochrane guidelines and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) checklist [12]. The databases searched included MEDLINE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Excerpta Medica Database (EMBASE), Scopus, Clinicaltrials.gov, Google Scholar, Cochrane library and Web of Science with references cross-checked, and individual urology journals also hand-searched. The search terms included “stones,” “calculi,” “urolithiasis,” “nephrolithiasis,” “kidney,” “renal,” “ureteroscopy,” “URS,” “laser,” “fragmentation,” “percutaneous,” “PCNL,” “mini,” “miniaturized,” “percutaneous nephrolithotomy,” “lithotripsy,” “renal function,” “kidney function,” “chronic kidney disease,” “CKD,” “creatinine,” “eGFR,” “MAG3,”and “DMSA.” The references of identified studies were examined to find any further potential studies for inclusion. Boolean operators (AND, OR) were employed. The research was limited to English language articles from 1990 to June 2019.
A cut off of ten patients was set to include studies from centers with minimum relevant endourological experience in managing stones. All original studies were included, and where more than one article was available, the study with the longest follow-up was included. Experienced reviewers (TR, AP) not involved in the original work independently identified all the studies that appeared to fit the inclusion criteria, which were then included for a full review. All discrepancies were resolved with mutual agreement and consensus with the senior author (BKS).
Inclusion Criteria
Studies reporting on renal function of patients following endourological intervention (PCNL and URS)
Studies reporting on a minimum of 10 patients
Studies available in English
Exclusion Criteria
Laboratory, animal data, or review articles
Studies published before 2000
Data Extraction and Analysis
The following variables were extracted from included studies: author, year of publications, journal, country of study, treatment modality, patient characteristics, stone characteristics, method of monitoring renal function, follow up, and pre- and postoperative renal function. Data were collected using Microsoft Excel 2019 (version 16.28). Due to the heterogeneity of the included studies, the authors decided that meta-analysis of effect sizes was not suitable, and hence either pooled analysis was performed to calculate mean values or outcomes were summarized in a narrative fashion.
Quality of Studies Assessment
The Centre for Evidence-Based Medicine criteria were used to evaluate the levels of evidence of the included studies [13]. The quality of reporting outcomes was performed according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [14].
Results
Study Selection and Characteristics
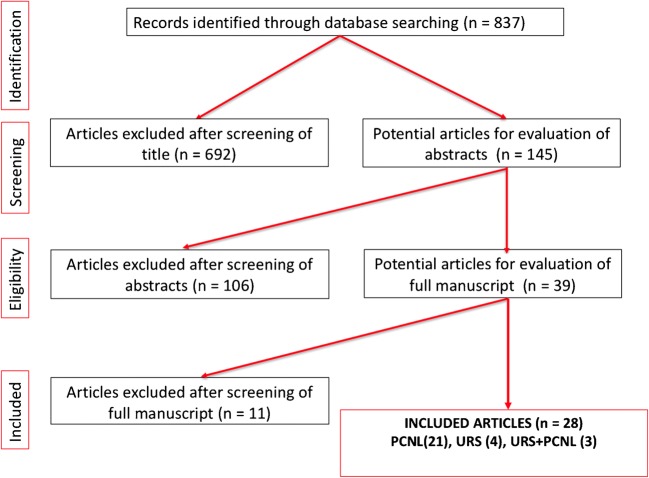
The literature search yielded 837 publications (Fig. 1). After excluding reports that were out of the scope of our systematic review, 145 abstracts were reviewed of which 39 full articles were reviewed for inclusion. Twenty-eight studies were included in the final review (5 were excluded as they were published before 1990, 4 did not mention the effect on renal function, 1 was an animal study and 1 was not in English language). Included studies were published between 2001 and 2019. Three papers compared the effect of PCNL and URS on renal function (Fig. 1).
Fig. 1.
PRISMA flow chart of the included studies
PCNL
The effect of PCNL on renal function was assessed in 21 studies published between 1999 and 2019. This included 1994 patients, and the mean age of patients was 49.3 years (Tables 1 and 2). The follow-up in these studies ranged from 1 day to 51 months [15, 16]. While 11 studies [15–26] used blood test to measure renal function, 1 study [27] used radionucleotide scans and 8 studies used combination of both [28–35].
Table 1.
Study details
| Author | Year | Journal | Country | Level of evidence | N | Age (years) | male/female | SFR | Mean operation time (min) | Average stone size (mm) | Cumulative stone diameter (mm2) | Diameter of the largest stone(mm) | Number of stones | Location | Composition | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCNL | Chatham | 2001 | Adult urology | USA | II | 45 | 49 (11–75) | 6//13 | 68% | 2.57 h (1.17–5.08) | 38 | 1432 (156–5220) | NS | NS | NS | 26% calc ox/calc phos, 21% calc ox, 16% struvite, 11% calc phos, 11% UA, 5% cystine, 5% matrix, 5% not-analyzed |
| Singh | 2001 | International urology and nephrology | India | III | 70 | 41.43 (9–70) | 68//22 | 75% | NS | NS | 928.6 | NS | NS | NS | Mixed, calc ox mono, calc ox di, and struvite stones were encountered in 48%, 14%, 17% and 21% respectively | |
| Kukreja | 2003 | Journal endourology | India | III | 84 | 47 ± 14 | 64//20 | 85.70% | NS | 1564 ± 1568 | NS | Staghorn 35 | 35 staghorn, 22 pelvic, 7 caliceal, 32 complex | NS | ||
| Al-Kohlany | 2005 | Journal of urology | Egypt | I | 43 | NS | 17//26 | 74% | 127 ± 30 | 187 ± 69 | Complete staghorn | NS | NS | NS | UA stones in 14 (28%), calc ox mono in 12 (24%), mixed calc ox and UA in 6 (12%), struvite in 5 (10%), cystine in 2 (4%), and mixed stones In 11 (22%) | |
| Handa | 2006 | Journal endourology | USA | III | 196 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Hegarty | 2006 | Journal endourology | USA | III | 20 | 54.4 ± 12.4 | 15//5 | 100% | NS | NS | 2157 ± 1441 (single tract); 423 ± 299 (multi tract) | NS | staghorn | NS | NS | |
| Moskovitz | 2006 | Journal endourology | Israel | II | 88 | 47 ± 16 | 47//41 | 90.00% | NS | > 150 | NS | NS | staghorn | NS | NS | |
| Yaycioglu | 2007 | Urological research | Turkey | III | 38 | 59 ± 16 | 13//6 | 72.7% IRF;84.2% NRF | 175 ± 69 | 54 ± 20 (IRF); 42 ± 24 (NRF) | NS | NS | staghorn | NS | IRF; calc ox 10, UA 4, Mg Am Phos 2, UA & calc ox 2, calc ox/phos 1. NRF calc ox 12, UA 2, UA/calc ox 2, and, Mg Am Phos 1 | |
| Kilic | 2008 | Journal endourology | Turkey | II | 24 | 36.67 ± 14.68 | 12//12 | NS | 122 ± 43 | 68.5 ± 32.7 | NS | NS | NS | NS | NS | |
| Kuzgunbay | 2010 | Journal endourology | Turkey | II | 16 | NS | NS | 75.00% | NS | NS | Complete staghorn | NS | NS | NS | calc ox 9, UA 2, UA and calc 2, Mg Am 2, calc phos and oxalate 1 | |
| Unsal | 2010 | Journal endourology | Turkey | II | 50 | 43.6 | 37//13 | 92%% | 80.5 (40–170) | NS | NS | NS | NS | NS | NS | |
| Akman | 2012 | Journal of urology | Turkey | III | 265 | 44.4 ± 15.3 | NS | 76.50% | 80 ± 35 | NS | 1191 ± 732 | NS | staghorn | NS | calc ox in 44, MG Am phos 13, UA 13, cystine 3, calc ox and phos 5, UA& calc phos in 2, Mg Am phos & calc ox 2 | |
| Chen | 2012 | Urology | China | I | 273 | 49.2 (22–73) | 165//108 | 84.70% | 129.2 ± 27 | 55 ± 7 | NS | NS | staghorn | NS | NS | |
| Ozden | 2012 | Urology | Turkey | III | 67 | 57 ± 14.1 | 47//20 | 76.80% | 88.5 ± 5.7 | NS | 670 ± 65 | NS | staghorn 11 | Pelvis 13, Pelvis plus polar 28, Polar 14, Staghorn 11, Upper ureter 3, | 3 UA, 16 infection, 24 calc ox, 4 calc phos | |
| Fayad | 2014 | Journal endourology | Egypt | II | 102 | 39.9 (35–76) | 70//32 | 70.70% | NS | NS | NS | NS | NS | NS | NS | |
| El Tabey SK | 2014 | Urology | Egypt | III | 200 | 52.3 ± 11.7 | 133/67 | 89.50% | NS | NS | NS | NS | Multiple 39, staghorn 66, single 95 | NS | NS | |
| Pérez-Fentes | 2014 | Urolithiasis | Spain | II | 30 | 60.1 ± 13.1 | 9//21 | 73% | 94 (69,134) | NS | 356 (220–820) | NS | NS | NS | NS | |
| EL-Nahas | 2016 | BJUI | Egypt | I | 42 | 50 ± 11 | 16//54 | 60% | US-130 ± 34; L-148 ± 35 | NS | L–15985 ± 8320; US–16656 ± 8211 | NS | staghorn | NS | NS | |
| Gorbachinsky | 2016 | Journal of urology | USA | II | 110 | 53 ± 15.4 | 52//58 | NS | NS | NS | NS | NS | NS | NS | NS | |
| Piao* | 2016 | World journal urology | South Korea | II | 31 | 58.0 ± 13.2 | 20//11 | 85.10% | 88.6 (±66.9) | NS | 3754 ± 6757 | 19.1 ± 13.2 | 2.9 ± 2.7 | NS |
calc ox mono 81 (54.7%) calc ox di 29 (19.6%) UA29 (19.6%) Carbonate apatite 9 (6.1%) |
|
| Zhou | 2017 | Journal endourology | China | III | 178 | 53.7 ± 15.0 | 78//100 | NS | NS | 783 ± 605 | NS | NS | NS | NS | NS | |
| Shi | 2018 | BJUI | China | III | 53 | 47.34 ± 12.24 | 26//27 | 92.45% | 117.55 ± 49 | 50 ± 24.77 | NS | NS | Staghorn 17, Multiple 32 | MP 2, LP1, pelvis 1, staghorn 17, multiple 32 | NS | |
| Jiao* | 2019 | Medicine | China | III | 58 | 51.67 ± 12.29 | 39//19 | 87.93% | 90.40 ± 31.29 | 12.27 ± 4.39 | NS | NS | NS | Renal pelvis or prox ureter 7; UC/MC 25; LC 26 | NS | |
| Cho* | 2019 | Scientific reports | Korea | II | 20 | 57.3 ± 13.5 | 14//6 | 85.50% | 72.1 ± 57.0 | 17.2 ± 13.5 | 3345 ± 7115 | 17.2 ± 13.6 | 3.1 ± 3.2 | NS | calc ox mono 64 (54.7); calc ox dehydrate 20 (17.1); UA 25 (21.4); Carbonate apatite 8 (6.8) | |
| URS | Ingimarsson | 2012 | Urology | USA | II | 113 | 56 | 55//58 | 91.40% | NS | 7 ± 4 | 905 | NS | NS |
Bilateral renal or ureteropelvic junction stones without ureteral stones 71 (60.7%), renal and ureteral 21.4% Bilateral renal stones with unilateral ureteral stones 25 (21.4%) Bilateral renal stones with bilateral ureteral stones 2 (1.7%) |
calc ox 83 (71%); apatite 21 (18%); brushite 6 (5%); UAin 5 (4%); AmU in 2 (2%) |
| Sninsky | 2014 | Journal of Endourology | USA | III | 26 | 54.3 ± 14.5 | 12//14 | NS | NS | 25 ± 12 | NS | NS | NS | 45% kidney; 55% kidney & ureter | NS | |
| Hoarau | 2015 | IBJU | France | III | 163 | 52.8 ± 17 | 86//77 | 74.40% | 96.4 ± 40.78 | 15 ± 9 | NS | 12.9 ± 5.7 | NS | NS | calc ox mono 34%, calc ox dehydrate 5%, UA 8%, carbapatite 16.6%, and unknown 53% | |
| Yang B | 2016 | Urologia Internationalis | China | III | 44 | 46.3 ± 10.1 | 29//15 | 86.40% | 94.8 ± 29.0 | 26 ± 6 | NS | NS | 3.0 ± 0.8 | Pelvis (14.6%) UC (11.5%) MC (1.69%) LC 47.7%) Ureter (9.2%) | NS | |
| Piao* | 2016 | World Journal Urology | South Korea | II | 117 | 58.0 ± 13.2 | 74//43 | 85.10% | 88.6 ± 66.9 | NS | 3754 ± 6757 | 19.1 ± 13.2 | 2.9 ± 2.7 | NS | calc ox mono 81 (54.7%) calc ox di 29 (19.6%) UA29 (19.6%) Carbonate apatite 9 (6.1%) | |
| Jiao* | 2019 | Medicine | China | III | 48 | 55.69 ± 12.70 | 33//15 | 81.25% | 105.56 ± 45.76 | 12 ± 4 | NS | NS | NS | Pelvis or proximal ureter 8; UC/MC 11: LC 29 | NS | |
| Choo* | 2019 | Scientific reports | Korea | II | 97 | 57.3 ± 13.5 | 66//31 | 85.50% | 72.1 ± 57.0 | NS | 3345 ± 7115 | 17.2 ± 13.6 | 3.1 ± 3.2 | NS | calc ox mono 64 (54.7); calc ox di (17.1); UA 25 (21.4); Carbonate apatite 8 (6.8) |
*Studies listed twice as include both URS and PCNL; IRF impaired renal function; NRF normal renal function; US ultrasound group; L laser group; Calc calcium; ox oxalate; UA uric acid; phos phosphate; Mg magnesium; Am ammonium; mono monohydrate; di dihydrate; NS not specified
Table 2.
Effect of PCNL and URS on the renal function of patients
| Author | Year | Measure | Mean follow up time | Mean preoperative renal function | Postoperative change | Study summary | |
|---|---|---|---|---|---|---|---|
| PCNL | Chatham | 2001 | creatinine, radionucleotide scan | 15 months | 0.9 mg/dL | No significant change. Trend to improvement | PCNL does not result in loss of renal function |
| Singh | 2001 | eGFR, radionucleotide scan | 9 months | NS | No significant change. Trend to improvement | The average fall in serum creatinine values was 1.53 mg/dl (32%) and the average functional improvement by renal dynamic scans stood at 20.7% | |
| Kukreja | 2003 | Creatinine | 2.2 ± 1.34 years | 2.87 ± 1.19 mg/dL | No significant change | 33 patients (39.3%) showed improvement, 24 (28.6%) showed stabilization, and 27 (32.1%) showed deterioration in renal function. Poor pre-operative function linked to deterioration. | |
| Al-Kohlany | 2005 | eGFR, creatinine, radionucleotide scan | 4.8 ± 2.5 months | 1 ± 0.3 mg/dL | No significant change. Trend to improvement | Split GFR of the treated kidneys improved or remained stable in 91% patients | |
| Handa | 2006 | Creatinine | 1 day | 0.97 ± 0.02 mg/dL | Significantly worse | Significant increase in serum creatinine of 0.14 mg/dL at 1 day | |
| Hegarty | 2006 | Creatinine | NS | 1.67 ± 1.33 mg/dL | No significant change in single-tract group. Significantly worse in multiple-tract group | Significant rise in serum creatinine (1.67 mg/dL to 1.91 mg/dL; P ± 0.05) and drop in creatinine clearance (76.9 mL/min to 67.2 mL/min; P ± 0.05) in the multiple-tract group; this was more pronounced in patients with existing renal insufficiency. No significant change in renal function was seen in the single-tract group | |
| Moskovitz | 2006 | Radionucleotide scan | 15–24 months | NS | No significant change | No significant change | |
| Yaycioglu | 2007 | Creatinine | 15.6 months | 2.8 mg/dL | No significant change. Trend to improvement | IRF group mean serum creatinine value was 2.8 mg/dl before surgery and 2.6 mg/dl after. NRF Group mean serum creatinine levels before and after were 0.93 ± 0.16 and 0.94 ± 0.17 mg/dl. | |
| Kilic | 2008 | Creatinine | 12 months | 0.83 ± 0.42 mg/dL | No significant change | PCNL does not cause obvious renal dysfunction and significant parenchymal scarring | |
| Kuzgunbay | 2010 | Creatinine | 51.1. ±10/1 | 2.30 ± 0.56 mg/dL | No significant change. Trend to improvement | Creatinine values decreased to normal range in six patients (37.5%), six patients (37.5%) had stable renal function and values increased in four patients (25%) | |
| Unsal | 2010 | Creatinine, radionucleotide scan | 6 months | 1.19 ± 0.46 mg/dL | No significant change | QSPECT of 99mTc-DMSA confirms that renal function is preserved or often improved after percutaneous stone removal | |
| Akman | 2012 | eGFR, creatinine | 36. ±24. months | 1.08 ± 0.51 mg/dL | No significant change. Trend to improvement | Renal function was improved or maintained in 80% patients | |
| Chen | 2012 | eGFR, creatinine, radionucleotide scan | 6 months | 0.94 ± 25.4 mg/dL | Significant improvement | A combination of multitract MPCNL and high-power Ho:YAG laser does not delay postoperative renal function recovery | |
| Ozden | 2012 | eGFR | 45.7 ± 17.08 months | 37.9 ± 14.05 ml/min | No significant change. Trend to improvement | Mean eGFR was preoperatively 37.9 (±14) and postoperatively 45.1 (±16). Diabetes mellitus and urinary infection were predictive of renal function deterioration at 1 year on multivariate analysis | |
| El Tabey | 2014 | eGFR | 3 ± 1.4 years | 2 ± 0.8 mg/dL | Significant improvement | Significant improvement in renal function at long term follow-up | |
| Fayad | 2014 | creatinine, radionucleotide scan | 12 months | 1.52 ± 0.56 mg/dL | Poor preoperative function significantly worsened. Normal function no significant change | Patients with normal preoperative renal function showed no significant change, those with baseline renal impairment showed significant worsening. Risk factors for this were elevated (1.4 mg/dL) preoperative serum creatinine level, diabetes, and hypertension | |
| Pérez-Fentes | 2014 | eGFR, creatinine, radionucleotide scan | 3 months | 74.73 ± 24.5 ml/min; 1.0 ± 0.4 mg/dL | No significant change | PCNL has a minimal impact on global kidney function. Perioperative complications increased PCNL functional damage | |
| EL-Nahas | 2016 | eGFR | 3 months | 0.9 ± 0.04 mg/dL | No significant change | 4 patients improved, 2 decreased, 36 stationary renal function | |
| Gorbachinsky | 2016 | Creatinine, radionucleotide scan | 4.1 months | 1.08 ± 0.49 mg/dL | No significant change. Trend to worse in multi tract group | 2.28% decrease in function in multi tract group | |
| Piao* | 2016 | eGFR, creatinine, radionucleotide scan | 3 months | 78.3 ± 26.2 ml/min, 1.1 ± 0.4 mg/dL | No significant change. Trend to improvement | Abnormal separate renal function showed postoperative recovery in 31 patients (58.5%), three cases (5.7%) showed deterioration | |
| Zhou | 2017 | eGFR, radionucleotide scan | 7.6 (6–12) months | 29.8 ± 21.2 ml/min | Significant improvement. No significant difference in single/multiple tracts | Significant improvement. No significant difference in single/multiple tracts | |
| Shi | 2018 | eGFR | 6 months | 1.15 ± 0.48 mg/dL | No significant change. Trend to improvement | Mean eGFR was 78.58 post op compared withn75.51 (27.08) pre op. Diabetes and high pre-operative creatinine predictive of postoperative decline | |
| Cho* | 2019 | eGFR, creatinine | 60–90 days | 0.99 ± 0.31 mg/dL | Poor preoperative function significantly worsened. Normal function no significant change | Preoperative severe deterioration of separate renal function was a significant predictor for the postoperative deterioration of renal function. Low preoperative deterioration showed high probability of recovery | |
| Jiao* | 2019 | eGFR | 1 month | 87.45 ± 49.73 ml/min | No significant change | Mean change in eGFR 87.45- > 89.21 difference not statistically significant | |
| URS | Ingimarsson | 2012 | Creatinine | 2.8 years | 0.99 mg/dL | No significant change. Trend to improvement | Mean creatinine was 0.99 (SD 0.28) preoperatively and 1.00 (SD 0.29) postoperatively |
| Sninsky | 2014 | eGFR | 28.1 months (5–75) | 68 ± 13.3 ml/min | No significant change. Trend to improvement | The mean eGFR improved from 68.0 - > 75.4, not statistically significant | |
| Hoarau | 2015 | eGFR | 15.5 ± 11.5 months | 84.30 ± 26.2 ml/min | No significant change. Trend to improvement | Significant renal function deterioration occurred in 8 cases (4.9%) and significant renal function amelioration occurred in 23 cases. Median GFR was not significantly changed from 84.3 ± 26.2 to 84.9 ± 24.5 | |
| Piao* | 2016 | eGFR, creatinine, radionucleotide scan | 3 months | 78.3 ± 26.2 ml/min; 1.1 ± 0.4 mg/dL | No significant change. Trend to improvement | Abnormal separate renal function showed postoperative recovery in 31 patients (58.5%), 3 cases (5.7%) showed deterioration | |
| Yang B | 2016 | Creatinine | 4 weeks | 1.40 ± 1.68 mg/dL | Significant improvement | Mean serum creatinine improved from 81– > 75 | |
| Choo* | 2019 | eGFR, radionucleotide scan | 60–90 days | NS | Poor preoperative function significantly worsened. Normal function no significant change | Preoperative severe deterioration of separate renal function was a significant predictor for the postoperative deterioration of renal function. Low preoperative deterioration showed high probability of recovery | |
| Jiao* | 2019 | eGFR | 1 month | 74.46 ± 17.50 ml/min | No significant change. Trend to improvement | Mean eGFR 74.46- > 77.83 not statistically significant |
NS not specified
Three studies showed significantly improved renal function following PCNL [19, 30, 35]. Eight studies showed no significant improvement but a trend toward improved renal function [16, 17, 23–25, 28, 29, 34]. Eight studies showed no significant change in renal function [18, 21–23, 26, 27, 32, 34]. Handa et al. showed that on day 1 post-procedure the renal function was significantly worse [15].
Hegarty et al. showed significantly worse renal function in patients who underwent multiple tracts PCNLs, but no significant change in those with single tract approach [20]. Fayad et al. showed that those with poor preoperative renal function had significantly worsened renal function post-procedure, but those with normal preoperative function had a stable renal function [31]. Fayad, Ozden, and Chi et al. showed that diabetes was associated with poor postoperative renal function [17, 23, 31]. In addition, Fayad et al. and Ozden et al. showed that postoperative UTI was associated with poor postoperative renal function [17, 31]. Perez-Fentes et al. suggested that postoperative complications were associated with more parenchymal damage following PCNL [33].
Ureteroscopy
The effect of ureteroscopy on renal function was assessed in four studies published between 2014 and 2019 [36–39] (Tables 1 and 2). This included 608 patients, 355 males and 253 females, and the mean age of patients was 54.9 years (Table 1). The follow-up ranged from 4 weeks to 28.1 months [36, 40]. All 4 studies used blood tests (creatinine, eGFR) for renal function monitoring [36–39].
Yang et al. [36] showed that URS significantly improve postoperative renal function. The other three studies showed no statistically significant change but trend to improvement in postoperative renal function [37–39].
Comparative Studies between PCNL and URS
Three studies included both PCNL and URS published between 2016 and 2019 (Tables 1 and 2). This included 262 patients with a mean age of 57.3 (Table 1) [40–42]. The follow-up ranged from 60 days to 90 days. Jiao et al. and Cho et al. used blood tests (creatinine, eGFR) to measure renal function while Piao et al. used combination of blood test and radionucleotide scans [40–42].
Both Piao et al. and Jiao et al. showed no significant change in renal function but a trend towards improvement [40, 42]. Cho et al. showed that if preoperative renal function was normal and then postoperative renal function was statistically normal, but if the renal function was abnormal, then it had a tendency to deteriorate significantly postoperatively [41].
Quality Assessment
The Centre for Evidence-Based Medicine criteria were used to evaluate the levels of evidence of the included studies and found that 3 studies were level one [18, 28, 30], 11 were level two [16, 21, 26, 27, 29, 31–33, 38, 41, 42], and 14 were level three evidence ( [15, 17, 19, 20, 22–25, 34–37, 39, 40])(Table 1). In addition, the quality of all studies was assessed for inclusion against the STROBE criteria [14].
Discussion
Meaning of the Study
Here we present the only systematic review on the effect of PCNL and URS on renal function. Our study suggests that overall renal function is not detrimentally affected by endourological intervention, but there are potentially some important predictive factors including preoperative renal function, diabetes, and hypertension, hence patients should be appropriately counseled and followed up.
For patients undergoing PCNL, the results were varied. Handa et al. showed a significantly worse postoperative renal function but their follow up time frame was only 1 day, and this may not have been replicated at subsequent follow up [15]. Gorbachinsky et al., Hegarty et al., and El-Tabey et al. showed that multiple tracts were predictive of significant deterioration in renal function [19, 20, 32]. This is perhaps a reflection of the theoretical risk of parenchymal damage causing a decline in renal function, but this wasn’t replicated across all studies using multiple tracts. Several studies showed that a poor preoperative renal function was predictive of the postoperative function [23, 31, 41]. Additionally, Fayad et al. showed that diabetes and hypertension were independent risk factors for poor outcome [31], El-Tabey et al. showed that postoperative bleeding was a factor [19], and Ozden et al. showed that diabetes and urinary tract infection were independent factors [23]. This suggests that declining renal function maybe attributable to patient comorbidities and other underlying disease as opposed to the effect of the endourological procedure alone. Especially as three studies showed significant improvement in function, and the majority of others showed a trend toward improvement [19, 30, 35].
With patients undergoing ureteroscopy only, Yang et al. showed a significant improvement in postoperative renal function [36]. Cho et al. demonstrated that poor preoperative renal function predicated deterioration, but the renal function was protected for those with good pre-operative renal function [41]. All the other studies showed a trend towards improvement of renal function. Interestingly Sninksy et al. concluded that there was no association between poor preoperative function or multiple procedures on the post-procedural function [37].
Strengths, Limitations, and Areas for Future Research
This study gives and overview of the effect of endourological techniques effect on renal function. Due to the heterogeneity of the studies and methods for monitoring renal function meta-analysis was not possible; this also made it difficult to compare the studies directly. The patient population inherently contains a number of confounders in terms of comorbidities. In addition, many of papers were retrospective case series and prone to bias. It is prudent that future studies look at the procedural cost differences and quality of life in these patients [43–45]. Similarly, the laser settings and the heat generated by them need to be addressed especially in the context of patients with poor-preoperative renal function [46].
The review highlights that although the renal function is unaffected in most endourological interventions, yet there is a lack of prospective real-life data addressing this issue. Similarly, perhaps there is a need for a randomized control trial addressing both PCNL and URS, with an emphasis on pre- and postoperative renal function, taking into consideration the comorbidities such as diabetes, hypertension, obesity, and chronic kidney disease [47]. This is especially important as previous studies have shown a direct link of these factors on the renal function [48]. Identification of high-risk patients and periodic monitoring of renal function would help in early intervention and is likely to protect further deterioration [49]. PCNL does not seem to result in loss of renal function [29]. However, increasing multiplicity of tracts seems to negatively impact the renal function [50]. Minimally invasive PCNL however does not seem to effect renal function even when there are multiple tracts [35]. In patients with pre-existing CKD or diabetes/hypertension and non-obstructed pelvicalyceal system multi-tract PCNL may result in a kidney function deterioration and thus endoscopic combined intrarenal surgery (ECIRS) should be contemplated [51].
Conclusion
This review suggests that endourological interventions do not adversely affect renal function and tend to improve it in patients who do not have a poor renal function prior to the procedure. Several factors including poor preoperative renal function, diabetes, hypertension, and multiple percutaneous tracts appear to predispose patients to declining renal function after procedure, and these patients should be counseled for and followed up appropriately.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Endourology
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thomas Reeves, Email: reevesth@gmail.com.
Amelia Pietropaolo, Email: ameliapietr@gmail.com.
Nariman Gadzhiev, Email: nariman.gadjiev@gmail.com.
Christian Seitz, Email: drseitz@gmx.at.
Bhaskar K. Somani, Email: bhaskarsomani@yahoo.com
References
- 1.Raheem OA, Khandwala YS, Sur RL, Ghani KR, Denstedt JD. Burden of urolithiasis: trends in prevalence, treatments, and costs. Eur Urol Focus. 2017;3(1):18–26. doi: 10.1016/j.euf.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Rukin N, Siddiqui Z, Chedgy E, Somani BK. Trends in upper tract stone disease in England: evidence from the hospital episodes statistics (HES) database. Urol Int. 2017;98(4):391–396. doi: 10.1159/000449510. [DOI] [PubMed] [Google Scholar]
- 3.Pietropaolo A, Proietti S, Geraghty R, et al. Trends of ‘urolithiasis: interventions, simulation and laser technology’ over the last 16 years (2000-2015) as published in the literature (PubMed): a systematic review. World J Urol. 2017;35(11):1651–1658. doi: 10.1007/s00345-017-2055-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh A, Somani BK. Safety and feasibility of day case ureteroscopy and laser lithotripsy (URSL) in patients with a solitary kidney. Cent European J Urol. 2016;69(1):91–95. doi: 10.5173/ceju.2016.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones P, Elmussareh M, Aboumarzouk OM, Mucksavage P, Somani BK. Role of minimally invasive (micro and ultra-mini) PCNL for adult urinary stone disease in the modern era: evidence from a systematic review. Curr Urol Rep. 2018;19(4):27. doi: 10.1007/s11934-018-0764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Türk C, Petřík A, Sarica K, Seitz C, Skolarikos A, Straub M, Knoll T. EAU guidelines on interventional treatment for urolithiasis. Eur Urol. 2016;69(3):475–482. doi: 10.1016/j.eururo.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 7.Geraghty R, Proietti S, Traxer O, Archer M, Somani BK. Worldwide impact of warmer seasons on the incidence of renal colic and kidney stone disease (KSD): evidence from a systematic review of literature. J Endourol. 2017;31(8):729–735. doi: 10.1089/end.2017.0123. [DOI] [PubMed] [Google Scholar]
- 8.Rob S, Bryant T, Wilson I, Somani BK. Ultra low dose, low dose and standard dose CTKUB: is there a difference?' results from a systematic review of literature. Clin Radiol. 2017;72(1):11–15. doi: 10.1016/j.crad.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Emiliani E, Talso M, Baghdadi M, et al. Renal parenchyma injury after percutaneous nephrolithotomy tract dilatations in pig and cadaveric kidney models. Cent European J Urol. 2017;70(1):69–75. doi: 10.5173/ceju.2017.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winship B, Wollin D, Carlos E, Peters C, Li J, Terry R, Boydston K, Preminger GM, Lipkin ME. The rise and fall of high temperatures during ureteroscopic holmium laser lithotripsy. J Endourol. 2019;33(10):794–799. doi: 10.1089/end.2019.0084. [DOI] [PubMed] [Google Scholar]
- 11.Tokas T, Herrmann TRW, Skolarikos A, et al. Pressure matters: intrarenal pressures during normal and pathological conditions, and impact of increased values to renal physiology. World J Urol. 2019;37(1):125–131. doi: 10.1007/s00345-018-2378-4. [DOI] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 13.Oxford centre for evidence-based medicine - Levels of evidence. https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ (accessed Nov 2019).
- 14.Masse J. Strengthening the reporting of observational studies in epidemiology-molecular epidemiology (STROBE-ME): an extension of the STROBE statement. J Clin Epidemiol. 2013;66(1):113. doi: 10.1016/j.jclinepi.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Handa RK, Matlaga BR, Connors BA, Ying J, Paterson RF, Kuo RL, Kim SC, Lingeman JE, Evan AP, Willis LR. Acute effects of percutaneous tract dilation on renal function and structure. J Endourol. 2006;20(12):1030–1040. doi: 10.1089/end.2006.20.1030. [DOI] [PubMed] [Google Scholar]
- 16.Kuzgunbay B, Gul U, Turunc T, Egilmez T, Ozkardes H, Yaycioglu O. Long-term renal function and stone recurrence after percutaneous nephrolithotomy in patients with renal insufficiency. J Endourol. 2010;24(2):305–308. doi: 10.1089/end.2009.0362. [DOI] [PubMed] [Google Scholar]
- 17.Akman T, Binbay M, Aslan R, Yuruk E, Ozgor F, Tekinarslan E, Yazici O, Berberoglu Y, Muslumanoglu AY. Long-term outcomes of percutaneous nephrolithotomy in 177 patients with chronic kidney disease: a single center experience. J Urol. 2012;187(1):173–177. doi: 10.1016/j.juro.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 18.El-Nahas AR, Elshal AM, El-Tabey NA, et al. Percutaneous nephrolithotomy for staghorn stones: a randomised trial comparing high-power holmium laser versus ultrasonic lithotripsy. [Article] BJU Int. 2016;118(2):307–312. doi: 10.1111/bju.13418. [DOI] [PubMed] [Google Scholar]
- 19.El-Tabey NA, El-Nahas AR, Eraky I, et al. Long-term functional outcome of percutaneous nephrolithotomy in solitary kidney. Urology. 2014;83(5):1011–1015. doi: 10.1016/j.urology.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Hegarty NJ, Desai MM. Percutaneous nephrolithotomy requiring multiple tracts: comparison of morbidity with single-tract procedures. J Endourol. 2006;20(10):753–760. doi: 10.1089/end.2006.20.753. [DOI] [PubMed] [Google Scholar]
- 21.Kilic S, Altinok T, Altunoluk B, et al. Long-term effects of percutaneous nephrolithotomy on renal morphology and arterial vascular resistance as evaluated by color Doppler ultrasonography: preliminary report. Urol Res. 2006;34(3):178–183. doi: 10.1007/s00240-006-0038-4. [DOI] [PubMed] [Google Scholar]
- 22.Kukreja R, Desai M, Patel SH, Desai MR. Nephrolithiasis associated with renal insufficiency: factors predicting outcome. J Endourol. 2003;17(10):875–879. doi: 10.1089/089277903772036181. [DOI] [PubMed] [Google Scholar]
- 23.Ozden E, Mercimek MN, Bostanci Y, et al. Long-term outcomes of percutaneous nephrolithotomy in patients with chronic kidney disease: a single-center experience. Urology. 2012;79(5):990–994. doi: 10.1016/j.urology.2011.10.066. [DOI] [PubMed] [Google Scholar]
- 24.Shi X, Peng Y, Li L, Li X, Wang Q, Zhang W, Dong H, Shen R, Lu C, Liu M, Gao X, Sun Y. Renal function changes after percutaneous nephrolithotomy in patients with renal calculi with a solitary kidney compared to bilateral kidneys. BJU Int. 2018;122(4):633–638. doi: 10.1111/bju.14413. [DOI] [PubMed] [Google Scholar]
- 25.Yaycioglu O, Egilmez T, Gul U, Turunc T, Ozkardes H. Percutaneous nephrolithotomy in patients with normal versus impaired renal function. Urol Res. 2007;35(2):101–105. doi: 10.1007/s00240-007-0081-9. [DOI] [PubMed] [Google Scholar]
- 26.Moskovitz B, Halachmi S, Sopov V, Burbara J, Horev N, Groshar D, Nativ O. Effect of percutaneous nephrolithotripsy on renal function: assessment with quantitative SPECT of (99m)Tc-DMSA renal scintigraphy. J Endourol. 2006;20(2):102–106. doi: 10.1089/end.2006.20.102. [DOI] [PubMed] [Google Scholar]
- 27.Unsal A, Koca G, Resorlu B, et al. Effect of percutaneous nephrolithotomy and tract dilatation methods on renal function: assessment by quantitative single-photon emission computed tomography of technetium-99m-dimercaptosuccinic acid uptake by the kidneys. J Endourol. 2010;24(9):1497–1502. doi: 10.1089/end.2010.0008. [DOI] [PubMed] [Google Scholar]
- 28.Al-Kohlany KM, Shokeir AA, Mosbah A, et al. Treatment of complete staghorn stones: a prospective randomized comparison of open surgery versus percutaneous nephrolithotomy. J Urol. 2005;173(2):469–473. doi: 10.1097/01.ju.0000150519.49495.88. [DOI] [PubMed] [Google Scholar]
- 29.Chatham JR, Dykes TE, Kennon WG, et al. Effect of percutaneous nephrolithotomy on differential renal function as measured by mercaptoacetyl triglycine nuclear renography. Urology. 2002;59(4):522–525. doi: 10.1016/s0090-4295(02)01519-4. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Zhu L, Yang S, Wu W, Liao L, Tan J. High- vs low-power holmium laser lithotripsy: a prospective, randomized study in patients undergoing multitract minipercutaneous nephrolithotomy. Urology. 2012;79(2):293–297. doi: 10.1016/j.urology.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 31.Fayad AS, Elsheikh MG, Mosharafa A, el-Sergany R, Abdel-Rassoul MA, Elshenofy A, Ghamrawy H, el Bary AA, Fayad T. Effect of multiple access tracts during percutaneous nephrolithotomy on renal function: evaluation of risk factors for renal function deterioration. J Endourol. 2014;28(7):775–779. doi: 10.1089/end.2013.0771. [DOI] [PubMed] [Google Scholar]
- 32.Gorbachinsky I, Wood K, Colaco M, Hemal S, Mettu J, Mirzazadeh M, Assimos DG, Gutierrez-Aćeves J. Evaluation of renal function after percutaneous nephrolithotomy-does the number of percutaneous access tracts matter? J Urol. 2016;196(1):131–136. doi: 10.1016/j.juro.2016.01.121. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Fentes D, Cortes J, Gude F, et al. Does percutaneous nephrolithotomy and its outcomes have an impact on renal function? Quantitative analysis using SPECT-CT DMSA. Urolithiasis. 2014;42(5):461–467. doi: 10.1007/s00240-014-0693-9. [DOI] [PubMed] [Google Scholar]
- 34.Singh I, Gupta NP, Hemal AK, Aron M, Dogra PN, Seth A. Efficacy and outcome of surgical intervention in patients with nephrolithiasis and chronic renal failure. Int Urol Nephrol. 2001;33(2):293–298. doi: 10.1023/a:1015230510071. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y, Gurioli A, Luo J, Li Z, Zhu J, Li J, Liu Y. Comparison of effect of minimally invasive percutaneous nephrolithotomy on split renal function: single tract vs multiple tracts. J Endourol. 2017;31(4):361–365. doi: 10.1089/end.2016.0822. [DOI] [PubMed] [Google Scholar]
- 36.Yang B, Ning H, Liu Z, Zhang Y, Yu C, Zhang X, Pan D, Ding K. Safety and efficacy of flexible ureteroscopy in combination with holmium laser lithotripsy for the treatment of bilateral upper urinary tract calculi. Urol Int. 2017;98(4):418–424. doi: 10.1159/000464141. [DOI] [PubMed] [Google Scholar]
- 37.Sninsky BC, Jhagroo RA, Astor BC, Nakada SY. Do multiple ureteroscopies alter long-term renal function? A study using estimated glomerular filtration rate. J Endourol. 2014;28(11):1295–1298. doi: 10.1089/end.2014.0322. [DOI] [PubMed] [Google Scholar]
- 38.Ingimarsson J, Knoedler J, Amy K. Same-session bilateral ureteroscopy: safety and outcomes: MP51–08. [Miscellaneous] J Urol. 2016;195(Supplement 4):e683–e684. doi: 10.1016/j.urology.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 39.Hoarau N, Martin F, Lebdai S, Chautard D, Culty T, Azzouzi AR, Bigot P. Impact of retrograde flexible ureteroscopy and intracorporeal lithotripsy on kidney functional outcomes. Int Braz J Urol. 2015;41(5):920–926. doi: 10.1590/S1677-5538.IBJU.2014.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiao B, Lai S, Xu X, et al. The efficacy of flexible ureteroscopy lithotripsy and miniaturized percutaneous nephrolithotomy for the treatment of renal and proximal ureteral calculi of <=2 cm: A retrospective study. [Article] Medicine. 2019;98(11):e14535. doi: 10.1097/MD.0000000000014535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho S, Choo M, Lee D, et al. A prospective, observational study to investigate change of separate renal function in patients who underwent minimally invasive renal stone surgery according to the preoperative functional deterioration. J Endourol. 2016;30(Supplement 2):A86–AA7. [Google Scholar]
- 42.Piao S, Park J, Son H, et al. Evaluation of renal function in patients with a main renal stone larger than 1 cm and perioperative renal functional change in minimally invasive renal stone surgery: a prospective, observational study. World J Urol. 2016;34(5):725–732. doi: 10.1007/s00345-015-1653-x. [DOI] [PubMed] [Google Scholar]
- 43.Somani BK, Robertson A, Kata SG. Decreasing cost of flexible ureterorenoscopic procedures: cost volume relationship. Urology. 2011;78(3):528–530. doi: 10.1016/j.urology.2010.12.073. [DOI] [PubMed] [Google Scholar]
- 44.Geraghty R, Jones P, Herrmann T, et al. Ureteroscopy seems to be clinically and financially more cost effective than shock wave lithotripsy for stone treatment: systematic review and meta-analysis. World J Urol. 2018;36(11):1783–1793. doi: 10.1007/s00345-018-2320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.New F, Somani BK. A complete world literature review of quality of life in patients with kidney stone disease. Curr Urol Rep. 2016;17(12):88. doi: 10.1007/s11934-016-0647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kronenberg P, Somani BK. Advances in lasers for the treatment of stones. Curr Urol Rep. 2018;19(6):45. doi: 10.1007/s11934-018-0807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishii H, Couzins M, Obumarzouk O, et al. Outcomes of systematic review of ureteroscopy for stone disease in the obese and morbidly obese population. J Endourol. 2016;30(2):135–145. doi: 10.1089/end.2015.0547. [DOI] [PubMed] [Google Scholar]
- 48.Kawamoto R, Akase T, Ninomiya D, Kumagi T, Kikuchi A. Metabolic syndrome is a predictor of decreased renal function among community-dwelling middle-aged and elderly Japanese. Int Urol Nephrol. 2019;51:2285–2294. doi: 10.1007/s11255-019-02320-0. [DOI] [PubMed] [Google Scholar]
- 49.Moore SL, Cook P, de Conick V, et al. Outcomes and long-term follow-up of patients with cystine stones: a systematic review. Curr Urol Rep. 2019;20(6):27. doi: 10.1007/s11934-019-0891-7. [DOI] [PubMed] [Google Scholar]
- 50.Yadav R, Agarwal S, Sankhwr S, et al. A prospective study evaluating impact on renal function following percutaneous nephrolithotomy using Tc99m ethylenedicysteine renal scan: does multiplicity of access tracts play a role? Investig Clin Urol. 2019;60(1):21–28. doi: 10.4111/icu.2019.60.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scoffone CM, Cracco CM, Cossu M, Grande S, Poggio M, Scarpa RM. Endoscopic combined intrarenal surgery in Galdakao-modified supine Valdivia position: a new standard for percutaneous nephrolithotomy? Eur Urol. 2008;54(6):1393–1403. doi: 10.1016/j.eururo.2008.07.073. [DOI] [PubMed] [Google Scholar]