Abstract
Staphylococcus aureus relies on quorum sensing to exert virulence to establish and maintain infection. Prior research demonstrated the potent quorum sensing inhibition effects of “430D-F5”, a refined extract derived from the fruits of Schinus terebinthifolia, a medicinal plant used for the traditional treatment of skin and soft tissue infections. We report the isolation and identification of three compounds from 430D-F5 that reduce virulence and abate dermonecrosis: 3-oxo-olean-12-en-28-oic acid (1), 3-oxotirucalla-7,24Z-dien-26-oic acid (2) and 3α-hydroxytirucalla-7,24 Z-dien-27-oic acid (3). Each compound inhibits all S. aureus accessory gene regulator (agr) alleles (IC50 2–70 μM). Dose-dependent responses were also observed in agr-regulated reporters for leucocidin A (lukA, IC50 0.4-25 μM) and glycerol ester hydrolase or lipase (gehB, IC50 1.5–25 μM). Surprisingly, dose-dependent activity against the nuclease reporter (nuc), which is under the control of the sae two-component system, was also observed (IC50 0.4–12.5 μM). Compounds 1-3 exhibited little to no effect on the agr-independent mgrA P2 reporter (a constitutive promoter from the mgrA two-component system) and the esxA reporter (under control of mgrA). Compounds 1-3 inhibited δ-toxin production in vitro and reduced dermonecrosis in a murine in vivo model. This is the first report of triterpenoid acids with potent anti-virulence effects against S. aureus.
Subject terms: Drug discovery, Chemical biology, Bacteriology
Introduction
Staphylococcus aureus has long been recognized as a significant cause of both community-acquired (CA) and healthcare associated (HA) infections, such as endocarditis, septic arthritis, osteomyelitis, and necrotizing pneumonia1. In the past decade, an increasing number of infections by methicillin-resistant S. aureus (MRSA) have been documented and estimates in the United States suggest that MRSA causes between 11,000–18,000 deaths and 80,000 invasive infections annually2,3. Furthermore, a single clone of CA-MRSA (USA300) has emerged as the most common cause of all skin and soft tissue infections in the United States and continues to pose a serious public health threat in community and healthcare settings4,5. With the last novel class of antibiotics to be brought to market being discovered in the 1980s, new strategies are necessary to respond to the widespread development of antibiotic resistant infections6. Some promising future approaches include promoting infection prevention to reduce the need for antibiotics, encouraging investment in antimicrobial agents with new regulatory policies and economic models, slowing the spread of resistance in order to preserve the useful lives of available antibiotics, and developing novel therapeutics that modulate host-microbe interactions without placing direct selective pressures known to drive resistance resistance7.
S. aureus antibiotic resistance can be due to various extrinsic, or acquired, mechanisms such as enzymatic drug modification, mutated drug targets, enhanced efflux pump expression, and altered membrane permeability8. Additionally, intrinsic mechanisms of resistance such as biofilm formation and production of virulence factors that subvert the host immune response, play a key role in prolonging and increasing the pathogenicity of S. aureus infections. The production of a suite of superantigens, toxins and exo-enzymes heavily contribute to the invasive nature of staphylococcal infections, and this virulence mechanism is largely controlled by quorum sensing (QS).
S. aureus uses multiple two-component systems to sense and respond to changes in cell density and environmental cues. One of these two-component systems is the Accessory Gene Regulator (agr) system, which has been extensively characterized for its complex regulatory role in global MRSA virulence and its requirement for MRSA skin infection pathogenesis1,2,9. Agr senses and responds to its cognate auto-inducing peptide (AIP) signal in a cell-density dependent manner. All described AIP signals are between 7 and 12 amino acids long, with the C-terminal amino acids constrained in a thiolactone or lactone ring and an N-terminal “tail” extension10,11. S. aureus has four allelic variants of the agr system (agr-I, II, III, IV), and each recognizes and responds to its cognate AIP signal (AIP-I, II, III, IV). The dominant CA-MRSA USA300 is agr Type-I, while health-care associated MRSA strains are predominantly agr Type-II12,13.
The agrBDCA operon encodes the AIP precursor (AgrD) and integral membrane protease (AgrB) necessary for final AIP processing, as well as the membrane-localized histidine kinase sensor (AgrC) and response regulator (AgrA). A hypervariable region spanning agrBDC determines the agr type. At sufficient local concentration, cognate AIPs bind AgrC, which dimerizes and phosphorylates the response regulator AgrA. Downstream, activated AgrA binds chromosomal promoters P2 and P3 to induce the transcription of the agrBDCA operon and the small RNA regulator RNAIII, respectively. As the primary effector of the agr system, RNAIII post-transcriptionally regulates the expression of more than 200 virulence-associated genes including toxins (α, β, δ, and γ), proteases, lipases, superantigens (toxic shock syndrome toxin-1, enterotoxins B, C, and D), and leukocidins10,14–16. Additionally, the RNAIII transcript includes the hld gene for δ-toxin, an amphipathic 26 amino acid peptide with cytolytic activity.
Given such extensive agr-dependent virulence factor production, inhibition of agr signaling (i.e. quorum sensing) has been proposed as an alternative strategy to prevent or treat MRSA skin infections. Several promising molecules have been reported, including the AgrA inhibitor savarin3, AgrB inhibitor ambuic acid4, and the pan-agr inhibitor apicidin5. Previously, we reported on the quorum sensing inhibitory activity of a refined extract from the fruit of the Brazilian peppertree (Schinus terebinthifolia Raddi, Anacardiaceae) against several MRSA strains6. S. terebinthifolia is an evergreen shrub native to South and Central America and grows as a noxious weed in the southern United States. It was introduced to the USA just over 100 years ago as an ornamental plant, and has since spread via a process of stratified dispersal around established populations and by long-distance jumps due to human activities17. In Florida, where we collected it for the present study, S. terebinthifolia is listed as a Category I invasive exotic species throughout the state18. Eradication efforts are underway in Florida, and have included largescale removal in the Everglades, application of herbicides, and most recently, deployment of parasitizing insects as a biocontrol measure19.
However, in Brazil where the plant is valued as a medicinal species and popularly known as “pimenta-rosa”, it is used in folk medicine for the treatment of an array of illnesses, including several associated with infection and inflammation. Several parts of the plant have been found to contain chemicals with antimicrobial20,21, anti-inflammatory22, antioxidant22,23 and anti-tumor24 bioactivities. The current study characterizes the anti-virulence activity of three triterpenoid acids isolated from the fruit of S. terebinthifolia against clinically-relevant MRSA strains.
Results
Bioassay-guided isolation of three bioactive triterpenoid acids
Bioassay-guided fractionation of an organic extract of S. terebinthifolia fruits (named extract 430) was directed by a set of reporter strain assays of MRSA agr::P3 activation for agr types I-IV25. Inhibition of YFP fluorescence in these strains was used as a preliminary indication for the inhibition of quorum sensing. An aqueous suspension of 430 was sequentially partitioned against hexanes (430B), then ethyl acetate (430 C). The final remaining water layer (430 F) was determined to be the most bioactive under these testing parameters and was selected for further fractionation with a flash chromatography system using a gradient of hexane, ethyl acetate and methanol. The most active fraction, 430F-F5, was chosen for compound isolation via reverse phase HPLC using a gradient system of water and methanol. A first round of HPLC yielded 12 fractions (430F-F5-PF1 through PF12). The most bioactive fractions, 430F-F5-PF11 and 430F-F5-PF12, were selected for a second round of HPLC fractionation, which led to the isolation of three active triterpenoid acids. An overview of the fractionation strategy, with percent yields of the active fractions and compounds is provided (Supplementary Fig. 1).
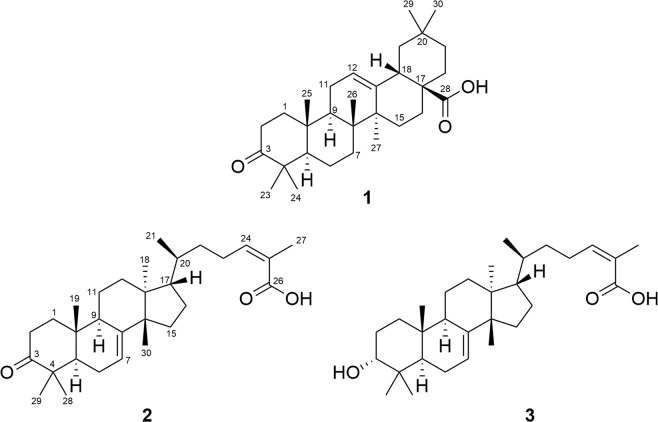
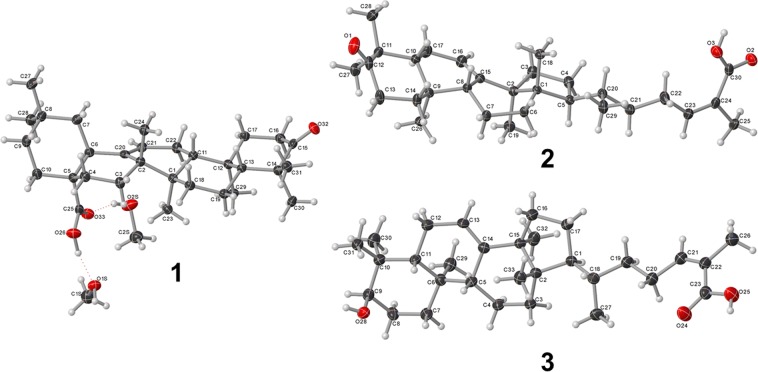
Structure determination was performed using Nuclear Magnetic Resonance (NMR) (Supplementary Figs. 2–4) and Mass Spectrometry (MS) (Supplementary Figs. 5–7). The compounds were identified as 3-oxo-olean-12-en-28-oic acid (1)26, 3-oxotirucalla-7,24Z-dien-26-oic acid (2)27 and 3α-hydroxytirucalla-7,24Z-dien-26-oic acid (3)28 (Fig. 1).The structures of 1-3 were further confirmed by single-crystal, X-ray diffractometry (Supplementary.cif files provided) with the assignment of the absolute configuration at the chiral centers (Fig. 2).
Figure 1.
Structures of isolated bioactive compounds: 3-oxo-olean-12-en-28-oic acid (1), 3-oxotirucalla-7,24Z-dien-26-oic acid (2) and 3α-hydroxytirucalla-7,24Z-dien-26-oic acid (3).
Figure 2.
OLEX2 projections of compounds 1-3 (crystallographic numbering).
Spectroscopic data for Compounds 1–3
3-oxo-olean-12-en-28-oic acid (1). Crystal solid. 1H NMR (600 MHz, CD3OD): δ 5.27 (1 H, t, J = 3.7 Hz, H-12), 2.87 (1 H, dd, J = 4.6, 14.0 Hz, H-18), 2.56 (1 H, dt, J = 8.0, 10.7 Hz, H-2β), 2.38 (1 H, m, H-2α), 1.18 (3 H, s, H-27), 1.08 (3 H, s, H-23), 1.07 (3 H, s, H-25), 1.05 (3 H, s, H-24), 0.95 (3 H, s, H-30), 0.91 (3 H, s, H-29), 0.88 (3 H, s, H-26); HRMS-APCI m/z 453.3370 [M-H]- (calcd for C30H44O3, 453.3374).
3-oxotirucalla-7,24Z-dien-26-oic acid (2). Crystal solid. 1H NMR (600 MHz, CD3OD): δ 5.92 (1 H, m, H-24), 5.34 (1 H, m, H-7), 2.84 (1 H, d, J = 5.5 Hz, H-2), 1.87 (3 H, s, H-27), 1.13 (3 H, s, H-19), 1.05 (3 H, s, H-29), 1.03 (6 H, s, H-28 and H-30), 0.92 (3 H, d, J = 6.5 Hz, H-21), 0.85 (3 H, s, H-18), HRMS-APCI m/z 453.3369 [M-H]- (calcd for C30H45O3, 455.3374).
3α-hydroxytirucalla-7,24Z-dien-27-oic acid (3). Amorphous solid. 1H NMR (600 MHz, CDCl3): δ 6.08 (1 H, t, J = 7.5 Hz, H-24), 5.25 (1 H, m, H-7), 3.46 (1 H, t, J = 2.9 Hz, H-3), 1.91 (3 H, s, H-27), 0.97 (3 H, s, H-28), 0.94 (3 H, s, H-30), 0.91 (3 H, s, H-29), 0.89 (3 H, s, H-21), 0.82 (3 H, s, H-18), 0.77 (3 H, s, H-19); HRMS-APCI m/z 455.3525 [M-H]− (calcd for C30H47O3, 455.3531).
X-ray Crystallographic Analysis of Compounds 1-3
Crystallographic data for compounds 1-3 have been deposited with the Cambridge Crystallographic Data Centre, 12 Union Road, CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk) and are available on request quoting the deposition number CCDC: 1949041 (compound 1), 1949042 (compound 2) and 1949043 (compound 3).
Crystallographic data of 1. (C30H46O3). 2 (CH4O), Mr = 518.75, monoclinic, I2 (No. 5), a = 11.8169 (3) Å, b = 7.3667(2) Å, c = 34.0413(12) Å, β = 95.459(3), α = γ = 90°, V = 2949.88(16) Å3, T = 106 (6) K, Z = 4, Z’ = 1, μ(CuKα) = 0.601 mm-1, 14060 reflections measured, 5225 unique (Rint = 0.0516) which were used in all calculations. The final wR2 was 0.1073 (all data) and R1 was 0.0403 (I > 2σ(I)).
Crystallographic data of 2. C30H46O3, Mr = 454.67, monoclinic, P21 (No. 4), a = 6.81969(10) Å, b = 19.7846(3) Å, c = 19.4400(3) Å, β = 94.4735(13)°, α = γ = 90°, V = 2614.95(7) Å3, T = 107(8) K, Z = 4, Z’ = 2, μ(CuKα) = 0.557 mm-1, 29953 reflections measured, 10230 unique (Rint = 0.0408) which were used in all calculations. The final wR2 was 0.0939 (all data) and R1 was 0.0350 (I > 2σ(I)).
Crystallographic data of 3. (C30H48O3). (CH4O), Mr = 488.72, orthorhombic, P212121 (No. 19), a = 7.43165(12) Å, b = 12.8741(2) Å, c = 59.8281(12) Å, α = β = γ = 90°, V = 5724.12(18) Å3, T = 102(2) K, Z = 8, Z’ = 2, μ(CuKα) = 0.564, 61328 reflections measured, 10360 unique (Rint = 0.1169) which were used in all calculations. The final wR2 was 0.1301 (all data) and R1 was 0.0503 (I > 2σ(I)).
Triterpenoid acids 1-3 exhibit limited growth inhibitory activity in S. aureus strains
Compounds 1-3 from S. terebinthifolia were screened against eight S. aureus strains at the test range of 4-563 µM in broth microdilution assays. Growth inhibition by dose response of the agr reporter strains is reported in Fig. 3, with a summary of MICs on all tested strains reported in Table 1. None of the three compounds exhibited an IC50 (≥50% growth inhibition in comparison to vehicle control) and MIC (≥90% inhibition) values for MRSA agr group I (AH1677), II and IV reporter strains. With regards to MRSA agr group III, Compound 1 exhibited weak growth inhibition (~15% inhibition) at up to 80 µM, but reached an IC50 by 141 µM and MIC at 281 µM, leaving a small gap between growth and quorum sensing inhibitory activity (QS IC50: 141 µM; IC00: 281 µM). Compound 3 exhibited stronger growth inhibitory activity against this agr group III reporter strain, reaching an MIC at 18 µM. Compounds 2 and 3 exhibited similar growth inhibitory activity against UAMS-929 and UAMS-1, IC50 and MIC of 35 and 70 µM, respectively. Also, 2 and 3 inhibited the growth of MRSA agr group I (AH1263, NRS249) with MICs of 563 and 18 µM, respectively for 2 and MICs of 141 and 281 µM for 3. Importantly, MICs for AH1263 (LAC, the agr group I USA300 strain used in the murine model of dermonecrosis) were not detected at concentrations of up to 563 µM for compound 1, and were noted at the higher concentrations of 563 and 141 µM for compounds 2 and 3, respectively.
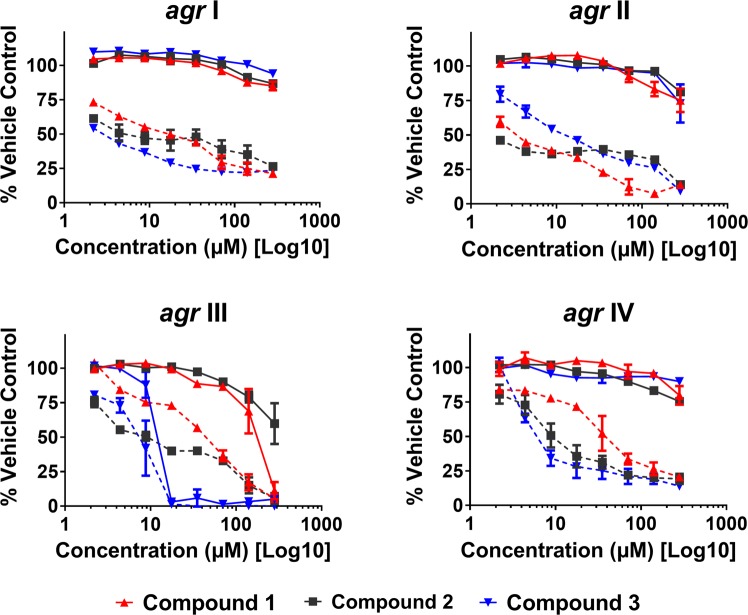
Figure 3.
Schinus compounds 1-3 inhibit agr activity for all four agr alleles in a dose-dependent manner. Data are represented as percent agr activity or growth of the vehicle (DMSO) control at 24 hours against the following strains: AH1677 (agr I), AH430 (agr II), AH1747 (agr III), and AH1872 (agr IV). The dashed lines represent agr activity, measured by fluorescence, and the solid lines represent growth, measured using OD600. Significant differences (P < 0.05) in quorum sensing activity between the vehicle control and compounds 1-3 were observed at all doses tested in agr groups I, II and IV, with exception of the lowest concentration tested (2 µM) of 1 in agr III and 3 in agr IV.
Table 1.
MICs (μM) of S. terebinthifolia compounds 1-3 on S. aureus. Extract 430F-F5 is reported as μg/mL.
| Strain | 430F-F5 | Compound 1 | Compound 2 | Compound 3 | ||||
|---|---|---|---|---|---|---|---|---|
| IC50 | MIC | IC50 | MIC | IC50 | MIC | IC50 | MIC | |
| AH1263; LAC (agr I) | ND | ND | ND | ND | 141 | 563 | 35 | 141 |
| NRS249 (agr I) | ND | ND | ND | ND | 9 | 18 | 18 | 281 |
| AH1677 (agr I) | ND | ND | ND | ND | ND | ND | ND | ND |
| AH430 (agr II) | ND | ND | ND | ND | ND | ND | ND | ND |
| AH1747 (agr III) | ND | ND | 141 | 281 | ND | ND | 9 | 18 |
| AH1872 (agr IV) | 4 | 32 | ND | ND | ND | ND | ND | ND |
| UAMS-929 | − | − | ND | ND | 35 | 70 | 35 | 70 |
| UAMS-1 | 128 | ND | ND | ND | 35 | 70 | 35 | 70 |
Note: “ND“: MIC not detected at the test range (4 – 563 μM).
Triterpenoid acids (1-3) inhibit quorum sensing and toxin production
To identify small molecule inhibitors of S. aureus quorum sensing from the 430F-F5 fraction, we employed a bioassay-guided fractionation strategy using agr-fluorescent reporter stains that represent each of the four known agr allelic groups (Fig. 3, Supplementary Table 1). Strains were grown in the presence of the compounds and monitored for growth inhibition by optical density and agr activity by fluorescence.
Compounds 1-3 significantly inhibited quorum sensing in all four agr types, exhibiting IC50 values ranging from 4–70 µM for 1, 2–9 µM for 2, and 4–9 µM for 3 (Fig. 3, Table 2). Compound 3 showed the most potent quorum sensing inhibition activity in this model (IC90 of 18 µM), but this was influenced by high growth inhibition of the agr III reporter by this compound. With the exception of this case (compound 3 growth inhibition of the agr III reporter strain), 1-3 exhibited limited growth inhibitory activity against the reporter strains.
Table 2.
IC50 and IC90 (μM) for quorum sensing inhibitory activity of S. terebinthifolia compounds 1-3 on S. aureus.
| Strain | Compound 1 | Compound 2 | Compound 3 | |||
|---|---|---|---|---|---|---|
| IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | |
| AH1677 (agr I) | 18 | ND | 4 | ND | 4 | ND |
| AH430 (agr II) | 4 | 70 | 2 | 281 | 9 | 281 |
| AH1747 (agr III) | 70 | 281 | 9 | 281 | 9 | 18 |
| AH1872 (agr IV) | 35 | ND | 9 | ND | 9 | 281 |
Note: “ND“: Value not detected at the test range (2 – 281 μM).
To verify the observed quorum-inhibition effects of 1-3, we assessed their capacity to inhibit δ-toxin production at concentrations ranging from 9 to 141 µM. High level δ-toxin producing of strains of S. aureus were treated with the triterpenoid acids and the supernatants harvested for RP-HPLC and human keratinocyte cell (HaCaT) toxicity analysis. Each of compounds 1-3 were effective in significantly reducing δ-toxin production, with weak or no growth inhibition in the S. aureus strains examined (Fig. 4A).
Figure 4.
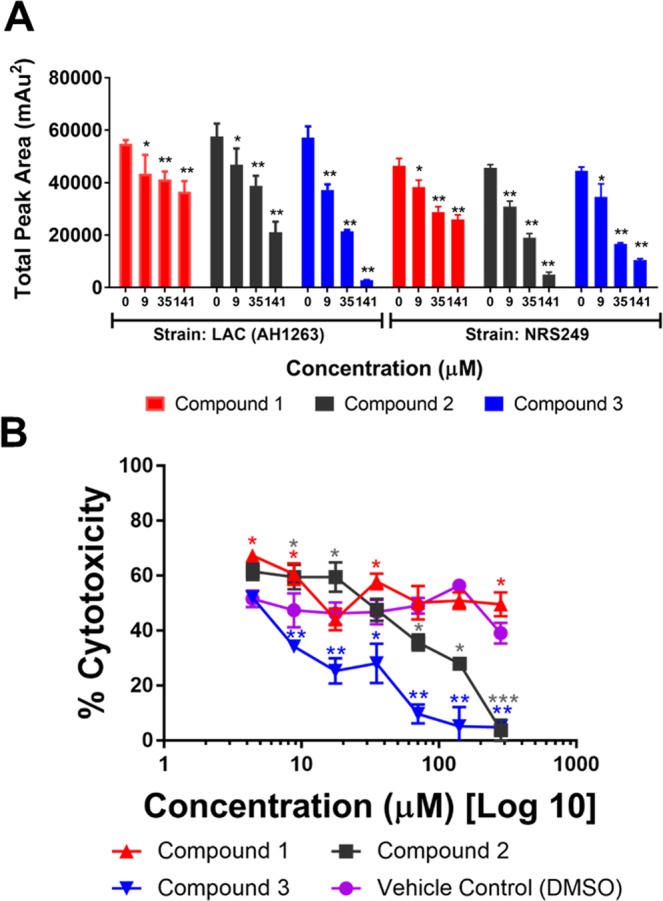
Compounds 1-3 inhibit δ-toxin production in a dose-dependent manner. (A) Levels of δ-toxin were quantified by HPLC analysis of culture supernatant following treatment with sub-MIC50 concentrations of compounds 1-3. (B) General toxicity of S. aureus supernatants to HaCaTs. An immortalized line of human keratinocytes was treated with supernatants of S. aureus (NRS249) that were grown + /− compounds 1-3 or vehicle (DMSO). Statistical significance by ANOVA in comparison to the vehicle treated wild type control is denoted as *P < 0.05, **P < 0.01, ***P < 0.001. Error bars represent mean and SEM.
To assess any remaining virulence factors linked to cytotoxicity (e.g., phenol soluble modulins, PSMs), sterile filtered supernatants of NRS249 cultures grown with compounds 1-3, were exposed to an immortalized line of human keratinocytes (HaCaTs) and assessed for damage by lactate dehydrogenase (LDH) assay. The toxicity of S. aureus supernatants to HaCaTs cells was inhibited in a dose dependent manner (Fig. 4B).
To examine the specificity of 1-3 against agr, we also chose reporter strains (promoter-GFP fusions in the Staphylococcus aureus AH1263 background) that are known to be either agr-regulated or independent of agr regulation.
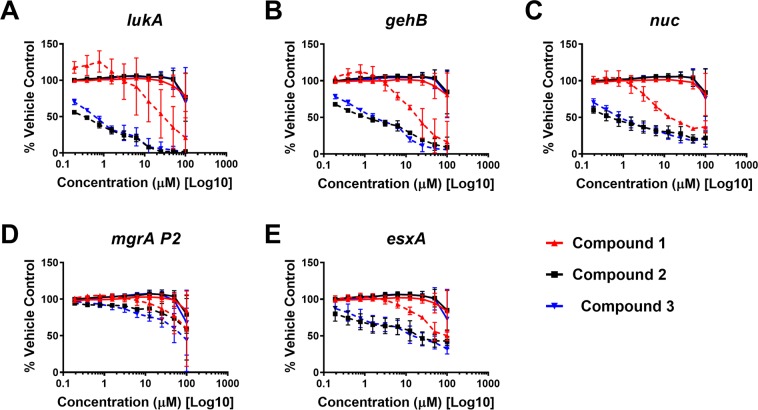
The agr-regulated reporters (lukA: leukocidin A, gehB: glycerol ester hydrolase or lipase) showed dose-dependent inhibition by 1-3 as expected since we have shown that these compounds target the agr system (Fig. 5A,B).
Figure 5.
Schinus compounds 1-3 inhibit other classes of MRSA virulence factors in a dose dependent manner. Select MRSA virulence factor reporter strains were treated with increasing doses of Schinus compounds 1-3 and growth (OD600, solid lines) and reporter activity (GFP, dashed lines) were measured over 24 hours. The 24-hour time point of treated groups normalized to the DMSO-only control is shown. Results are pooled from 3 independent experiments and the mean + /− SD is shown.
To our surprise, the nuc reporter (nuclease), which is primarily under the control of the sae two-component system, is also inhibited in a dose-dependent manner by all of the Schinus compounds (1-3), suggesting broader inhibitory effects against multiple MRSA virulence factors (Fig. 5C). Additionally, we tested the agr-independent mgrA P2 reporter (a constitutive promoter from the mgrA two-component system) and saw little effect of any Schinus compound (1-3), suggesting that these two components do not primarily target this system (Fig. 5D). We found for the agr-independent esxA reporter, which is a component of the MRSA type VII secretion system and generally thought to be under the control of the mgrA two-component system (but may be regulated by other systems as well), that there was modest inhibition by 1-3 (Fig. 5E).
Taken together, these results confirm that 1-3are potent agr inhibitors but are also broader MRSA virulence inhibitors that may also target the sae two-component system.
Triterpenoid acids 1-3 impact biofilm formation
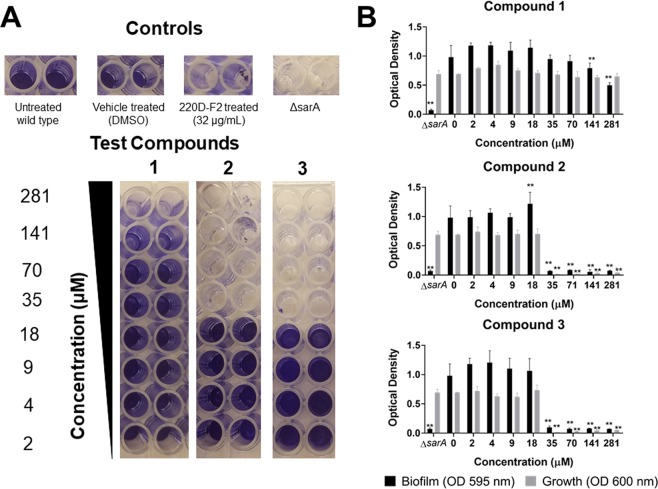
In microtiter plate biofilm assays, compound 1 increased biofilm formation at low dosages (2–70 µM), and reduced biofilm at higher concentrations (141– 281 µM) without growth inhibition. This is noteworthy as the neither the growth inhibitory IC50 or MIC for 1 in the biofilm strain (UAMS-1) was detected at a maximum test concentration of 563 µM (Table 1). Compounds 2 and 3 caused significant, dose-dependent increases in biofilm biomass at concentrations ranging from 2–16 μM. In contrast, both biofilm formation and bacterial growth were strongly reduced at concentrations of 35 μM and above (Fig. 6). For compounds 2 and 3, these biofilm inhibitory concentrations are roughly 4-18 times higher than the IC50 for quorum sensing inhibition in the four reporter strains (Table 2).
Figure 6.
Impact of compounds 1-3 on S. aureus biofilm formation and planktonic growth in a biofilm model. USA 200 isolate UAMS-1 and its isogenic sarA mutant (UAMS-929) were used in the biofilm assay. (A) Images of crystal violet stained biofilm in 96-well plates. (B) The optical density of the crystal violet eluent from the adherent biofilm (OD 595 nm) is plotted along with the optical density for the planktonic cells (OD 600 nm), measured by transfer of the well supernatants to a new 96-well plate. Statistical significance by ANOVA in comparison to the vehicle treated wild type control is denoted as **P < 0.05, **P < 0.01, ***P < 0.001. Error bars represent mean and SEM.
Impact of compounds 1-3 on human keratinocytes
To determine the potential toxicity to human cells, compounds 1-3 were tested on human keratinocyte cells (HaCaTs) using a lactate dehydrogenase (LDH) assay for cytotoxicity. The IC50 values for cytotoxicity to HaCaTs and therapeutic indices for each reporter strain are reported in Fig. 7. The therapeutic index ranged from 2 to 35, depending on the reporter strain and compound combination.
Figure 7.
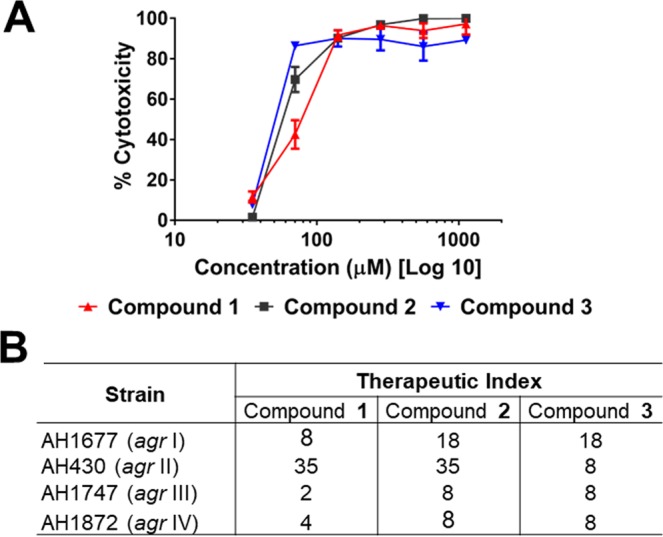
Impact of compounds on quorum sensing inhibition in comparison to cytotoxicity in a human keratinocyte cell line for calculation of the therapeutic index. (A) Cytotoxicity of compounds 1-3 on human keratinocyte (HaCaT) cell line by LDH assay for cell viability, with IC50 of 140 µM for 1 and 70 µM for 2 and 3. (B) The therapeutic index for each compound is calculated as IC50 for HaCaT cytotoxicity divided by the IC50 for agr activity (see Table 2) in each reporter strain.
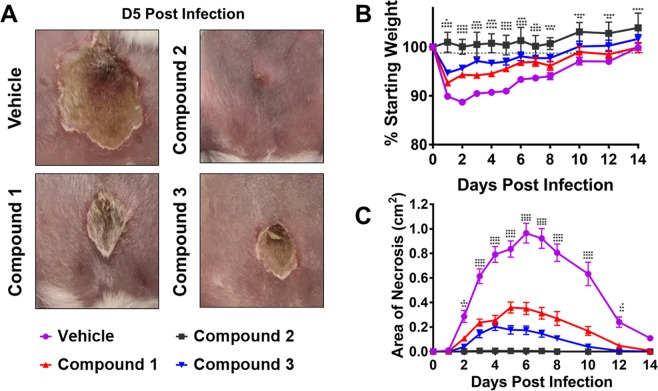
Compounds 1-3 abate quorum sensing and dermonecrosis in vivo
The antivirulence activity of compounds 1-3 observed in vitro led us to assess their efficacy as an in vivo anti-infective. Using a previously established murine model of dermo-necrosis15,25,29, BALB/c mice were intradermally challenged with MRSA (USA300) and 50 µg of each Schinus compound or DMSO vehicle control at the time of infection. Over the two-week course of infection, compounds 1 and 3 exhibited similar potency in reducing MRSA-mediated skin damage (Fig. 8). Compound 2 effectively reduced the dermonecrotic lesion area to nearly undetectable levels (Fig. 8A). All compounds significantly minimized animal weight loss, indicating decreased morbidity compared to wild-type infection (Fig. 8B). Additionally, each compound significantly reduced dermonecrotic lesion size throughout the course of MRSA infection (Fig. 8C). Taken together, these results demonstrate that all of the compounds protect mice from MRSA infection-associated dermonecrotic injury and morbidity.
Figure 8.
Compounds 1-3 inhibit MRSA quorum sensing and protect skin from dermonecrosis in a murine model of skin and soft tissue infection. (A) BALB/c mice were intradermally challenged with 1×108 CFUs of MRSA (LAC) and 50 µg of respective compound or the vehicle control (DMSO). A representative image of the dermo-necrotic lesion is shown for Day 5 post-infection. (B) Compounds 1-3 significantly reduce morbidity as measured by animal weight loss over time compared to vehicle control. (C) A single 50 µg dose of each Schinus compound significantly attenuates MRSA-mediated dermatopathology as measured by lesion size. Significant differences between vehicle and compounds 1-3 were determined by Ordinary 2-way ANOVA with Dunnett’s correction for multiple comparisons. *P < 0.05, **P < 0.005, ***P < 0.0005, ****P < 0.0001. Error bars represent mean and SEM.
Discussion
The emergence of antibiotic resistance, together with the lack of antibiotics based on novel molecular scaffolds, marks the entry to the ‘post antibiotic era’. Interference with bacterial virulence has emerged as an attractive approach among the current strategies for developing new anti-infective drugs. Prior studies have reported the capacity of plant extracts and phytochemicals to interfere in intra and inter-species quorum sensing communication systems30,31. The ability of plants to interrupt quorum sensing systems may serve as a defense mechanism to fight against bacterial invasion. One of the keys factors of success of phytochemicals could be their similitude to what is considered the ideal quorum sensing inhibitor, which includes being chemically stable, highly effective low-molecular-mass molecules and harmless for human health32.
In a previous study, we confirmed the quorum sensing activity of a refined S. terebinthia fruit extract (430D-F5) against multiple MRSA strains25. Here, we successfully isolated three triterpenoid acids using a bioassay-guided fractionation approach. The structure of these compounds (1-3) was determined via NMR, MS, and X-ray crystallography, and matches with substances previously isolated from S. terebinthifolia and other plants33–35. The crystal structures of compounds 2 and 3 were previously reported36,37.
We found that the three triterpenoid acids (1-3) inhibited expression of all S. aureus agr types in a dose dependent manner with limited inhibition of bacterial growth in agr groups I, II, and IV, but moderate growth inhibition in the agr group III reporter. Compound 3 showed the most potent anti-virulence activity against S. aureus in vitro (Table 2, Fig. 3), while compound 2 exhibited the greatest level of protection from dermonecrosis in vivo. To confirm the inhibitory activity of isolated compounds with a downstream protein level readout, we assessed their ability to inhibit the production of agr-regulated δ-toxin. These triterpenoid acids (1-3) notably decreased δ-toxin production in two S. aureus strains, and the general toxicity of pre-treated S. aureus supernatants to HaCaTs also confirmed these effects.
The impact on other agr-regulated reporters were also assessed, revealing dose-dependent inhibition of leucocidin A (lukA) and glycerol ester hydrolase or lipase (gehB). Little to no effect of any compound was noted for the agr-independent mgrA P2 or esxA reporters. Interestingly, a dose-dependent response was noted in tests with the nuclease reporter, which is under the control of the sae two-component system, suggesting a dual role of 1-3 in broader virulence inhibition across both agr and sae systems.
The agr operon is essential for virulence-factor production during MRSA skin-infection and reducing agr functionality could potentially mitigate acute tissue damage. This is supported by the results of our in vivo experiments, where 2 decreased MRSA-associated tissue damage to the limit of detection. Notably, 1 and 3 also exhibited significant anti-virulence effects, and mice were protected from severe dermonecrosis and morbidity. However, it is well-established that the absence of agr signaling drives MRSA biofilm formation under in vitro conditions38. Indeed, low concentrations of all compounds resulted in significant biomass accumulation while higher concentrations mitigated biomass accumulation in our in vitro assays. These results suggest that careful titration of quorum sensing inhibitory compound doses will certainly be necessary if administered in a human infection scenario. Additionally, this study employed inhibitory compounds at the onset of infection in a prophylactic model, effectively reducing MRSA virulence during infection initiation and allowing for rapid clearance of bacteria by the immune system. The use of quorum sensing inhibitors at the onset of human infection is far less likely if disease progression is slow or access to healthcare providers is limited. Thus, we postulate that natural product quorum sensing inhibitors, such as those proposed in this study, could be paired with a secondary bactericidal or biofilm dispersal agents to fully abrogate MRSA infection. However, further research is needed to identify antibiotics that synergize with virulence inhibitory compounds while mitigating the risk of acquired MRSA resistance39.
Our results also suggest that the biological activity of these compounds may be particularly sensitive to chemical modifications of their terpenoid skeleton. In particular, this is the case for compounds 2 and 3, where the substituent at carbon 3 could play a key role in the inhibition of virulence factors. A medicinal chemistry approach to modification of the terpenoid skeleton could also be explored to improve the therapeutic indices of these compounds, with an optimal difference between activity and toxicity being several hundredfold. This will require study of a large number of related compounds in order to approach the minimum requirements necessary for reaching a more definitive conclusion with respect to structure-activity relationships (SAR).
Previous studies have reported related pentacyclic triterpenoids with anti-virulence and biofilm formation/eradication activities against S. aureus and other strains, such as betulinic acid40, asiatic and corosolic acids41, ursolic acid and derivatives42,43, glycyrrhetinic acid44 and oleanolic acid40. However, to the best of our knowledge, this is the first time that the anti-quorum sensing activity of tirucallane-type triterpenoids (2 and 3) is described.
Materials and Methods
Plant material
Schinus terebinthifolia Raddi, Anacardiaceae fruits were collected in bulk from private lands in DeSoto County, Florida in November of 2013 and 2014 after obtaining permission from the land owner. Vouchers were deposited at the Emory University Herbarium (GEO) (Voucher CQ-400, GEO Accession No. 020063) and were identified using the standard Flora for Florida. Plant material was then dried in a desiccating cabinet at low heat. Once dry, plant material was sealed in paper bags and stored at room temperature until further processing.
Extraction and bioassay guided fractionation
Fruits of S. terebinthifolia were dried in a dehumidifying chamber, ground into a powder with a Wiley Mill at 2 mm mesh and extracted using an ultrasonic bath at room temperature with methanol (ratio of 1 g dry material: 10 mL MeOH) for a total of three times. Filtered extracts were combined, concentrated at reduced pressure with rotary evaporators (<40 °C), and lyophilized. The crude extract (15.0 g) was suspended in 400 mL methanol-water (1:4) solution and underwent sequential liquid-liquid partitioning with hexane (3×500 mL), ethyl acetate (3×500 mL), and n-butanol (3×500 mL). The combined partitions were evaporated to dryness to yield 1.16 g (3.92%) of hexane residue, 1.15 g (2.98%) of ethyl acetate residue and 12.61 g (32.71%) of aqueous residue.
Following initial quorum sensing assays, the most active partition, the aqueous residue (430 F) was subjected to fractionation through flash chromatography. Fractionation was performed using a CombiFlash® Rf+ Lumen (Teledyne ISCO) flash chromatography system with a RediSep Rf Gold silica column. The dry load column was prepared by binding 430 F to Celite 545 at a ratio of 1:4. Flash chromatography was performed using a three-solvent system of (A) hexane, (B) dichloromethane, and (C) methanol. The gradient began with 100% A for 6 column volumes (CV), then went to 100% B over 12 CV and was held for 18.2 CV. The gradient was then changed to 74.5:25.5 B:C over the course of 3.1 CV. These conditions were held for 6.8 CV, following which the gradient changed to 68.8:31.2 B:C over 0.7 CV and was held at these conditions for 7.5 CV. Finally, the gradient was adjusted to 100% C over 2.2 CV and held for 14.6 CV. The chromatography was monitored at 254 and 280 nm, as well as via evaporative light scanning detector (ELSD). Tube volumes (25 mL each) were combined to create eight fractions 430F-F1 (tubes 1–10), 430F-F2 (tubes 11-17), 430F-F3 (tubes 18-27), 430F-F4 (tubes 28-42), 430F-F5 (tubes 44-45), 430F-F6 (tubes 46-62), 430F-F7 (tubes 63-71), 430F-F8 (tubes 72-82). 430F-F5 was the most active fraction after testing for quorum sensing activity.
430F-F5 was further fractionated by preparative HPLC (Prep-HPLC) [Agilent 1260 infinity II LC system (CA, USA) equipped with a UV-vis detector, auto collector, Agilent XDB-C18 (30 × 250 mm, 5 μm) column, eluted with A: H2O, B: MeOH at 42.5 mL/min using the following gradient: 0 min 50% B, 0-11.5 min 50% B, 11.5-36.5 min 100% B, 36.5-46.5 min 100% B, detection at 254 and 320 nm)] to afford 12 sub-fractions. 430F-F5-PF11 and 430F-F5-PF12 showed the highest activity for the inhibition of quorum sensing.
430F-F5-PF11 and 430F-F5-PF12 were fractionated/purified using the same prep-HPLC system described above and eluted with A: H2O, B: Acetonitrile at 42.5 mL/min using the following gradient: 0 min 90% B, 12 min 95% B, 30 min 95% B. From 430F-F5-PF11 were obtained compound 1 (100.0 mg). From 430F-F5-PF12 were isolated compound 2 (178.1 mg) and 3 (52.0 mg).
Analytical chemistry procedures
Nuclear magnetic resonance (NMR) data, 1H NMR (600 MHz) were measured on a Bruker AVANCE III HD 600 (600 MHz for 1H-NMR, 5 mm CryoProbe) spectrometer in CD3OD or CDCl3 solvents. Chemical shifts (δ) are reported in ppm with the solvent peaks used as reference.
High resolution/accurate mass-Atmospheric pressure chemical ionization (HRMS-APCI) experiments were performed on a Thermo Exactive Plus using the Ion Max Source with APCI probe. The sample was placed on a melting point capillary and then placed in an Ion Sense ASAP (Atmospheric Solid Analysis Probe) probe, which was inserted into the source to enable gas from the APCI probe to blow on the capillary tube. The APCI probe uses nitrogen gas at arbitrary units of 50 and was heated to a temperature of 450 °C. A discharge on the needle with a discharge current of 5 µA was used to ionize the sample, with a capillary temperature of 320 °C, S-lens RF level 70 and an AGC setting of 1 E-6. The maximum injection time was 50 ms. Spectra were collected with 140,000 resolution at m/z 200 using Tune software and analyzed with Thermo’s Freestyle software.
Single crystals of compounds 1-3 were recrystallized from methanol by placing them into a −20 °C freezer overnight. A suitable crystal was selected and the crystal was mounted on a Rigaku XtaLAB Synergy-S diffractometer. The crystal was kept at 107(8) K during data collection. Using Olex2, the structure was solved with the ShelXT structure solution program using Intrinsic Phasing and refined with the ShelXL refinement package using Least Squares minimization.
Bacterial strains
Quorum sensing reporter strains of S. aureus representing the four agr groups included AH1677, AH430, AH1747 and AH1872, were used. AH1677 is an agr I reporter from strain AH845 (CA-MRSA USA 300 LAC), AH430 is an agr II reporter from SA502A, AH1747 is an agr III reporter from strain CA- MRSA MW2, and AH1872 is an agr IV reporter from MN EV. All strains contained plasmid pDB59 that served as an agr fluorescence reporter and was maintained through culture in media containing chloramphenicol at a concentration of 10 μg mL−1. Chloramphenicol (Sigma-Aldrich) was dissolved in 95% EtOH to a stock concentration of 10 mg mL−1 and stored at −20 °C until being added to the media. Cultures were grown on Tryptic Soy Agar (TSA) supplemented with 10 μg mL−1 chloramphenicol. Two S. aureus strains (UAMS-1, UAMS-929) served as biofilm test strains, with the latter being an isogenic Δsar mutant that has a limited capacity to produce a biofilm. One S. aureus strain (NRS385) was used for δ-toxin quantification experiments. UAMS-1, UAMS-929 and NRS385 were grown in Tryptic Soy Agar (TSA) and Tryptic Soy Broth (TSB). A summary of strain characteristics is provided in Supplementary Table 1.
Minimum inhibitory concentration
Fraction 430F-F5 and isolated compounds of S. terebinthifolia 1-3, were evaluated for minimum inhibitory concentrations (MICs) against the four agr groups, the biofilm test strain (UAMS-1) and NRS249 (for δ-toxin quantification experiments) following the Clinical Laboratory Standards Institute (CLSI) M100-S23 guidelines for microtiter broth dilution testing45. Fraction, compounds 1-3 and vehicle were tested at a concentration range of 4 to 563 μM, using 2-fold serial dilution. The optical density (OD600nm) was measured with a BioTek Cytation 3 multimode plate reader, to calculate percent inhibition of growth. The IC50 for growth was defined as the lowest concentration at which an extract displayed ≥ 50% inhibition and MIC (IC90) at ≥ 90% inhibition. Control included the vehicle (DMSO) and antibiotic (Ampicillin, MP Biomedicals Inc). All tests were performed in triplicate and repeated using a new stock of bacteria on a separate day.
Agr reporter assay for quorum sensing inhibition
S. aureus agr reporter strains AH1677 (agr I), AH430 (agr II), AH1747 (agr III) and AH1872 (agr IV) were grown overnight in TSB supplemented with chloramphenicol at 37 °C while shaking at 200 rpm. Cultures of S. aureus were standardized by optical density (OD600nm) matching to a 0.5 McFarland standard to reach a final inoculum density in the wells of 5 × 105 CFU mL−1 in TSB, supplemented with 10 μg/mL chloramphenicol. Compounds 1-3 were tested to escalating concentrations (2-281 μM) of each compound. Black sided, 96-well, clear bottom, tissue-culture treated plates (Costar 3603) with final well volume of 200 μL were used for all agr inhibition assays. Plates were incubated in a humidified chamber at 37 °C while shaking at 260 rpm. After 24 hours, plates were removed and OD600 and fluorescence were measured by plate reader at an excitation of 493 nm and emission of 535 nm. All tests were performed in triplicate and repeated using a new stock of bacteria on a separate day.
MRSA virulence factor reporter assays
For reporter assays, overnight (ON) cultures of MRSA reporter strains (AH511646, pCM29-PlukA, CmR; AH538246, pCM29-PgehB, CmR; AH361347, mgrA P2-sGFP fusion, ErmR; AH510148, Pnuc-sGFP, CmR; AH509546, pCM29-PesxA, CmR) were prepared by sub-culturing 1:500 in fresh TSB (5% DMSO) with chloramphenicol (10 µg/mL) or erythromycin (5 µg/mL). Compounds 1-3 were resuspended in DMSO, and then diluted fresh in TSB for each assay replicate. 200 µL of compound was added to a black 96-well, clear bottom, tissue-culture treated plates (Costar) and diluted 2-fold in TSB (100 µM - 0.2 µM final compound concentrations). 100 µL of reporter culture was added to each well for 200 µL final volume. A vehicle control (TSB + DMSO only) was included for each reporter for each experimental replicate. Cultures were grown in a Stuart humidified incubator at 37 °C with shaking at 1000 RPM. At hourly time points, the plates were measured on a TECAN Group Ltd. Infinite 200 Pro plate reader to quantify growth (OD600 nm) and sGFP signal (Excitation: 480 nm, Emission: 515 nm).
HaCaT cytotoxicity assay
The HaCaT cell line was maintained and cytotoxicity of compounds (1-3) were assessed using the LDH cytotoxicity assay as previously described49. The Therapeutic Index (TI) was calculated as a ratio of the TD50 (Toxic dose for cytotoxicity at IC50) and ED50 (effective dose for quorum sensing inhibiton IC50): = . Cytotoxicity of supernatants was further evaluated using a viability/cytotoxicity assay and imaged with fluorescent microscopy as previously described49. All tests were performed in triplicate and the full experiment was repeated on a separate day using fresh cell stock.
Quantification of δ-toxin
Levels of δ-toxin present in the culture supernatant of treated and untreated samples was quantified using NRS249 and AH1263 strains of S. aureus and following a previously described protocol50. Experiments were conducted in quadruplicate in 14 mL snap-cap tubes with a final volume of 1.5 mL, and repeated in full with fresh bacterial stock on a separate day. All compounds were tested at concentrations from 9-141 µM. Tubes were incubated at a 45° angle at 37 °C while shaking (275 rpm) for 15 hours. After incubation, cultures were placed on ice, an aliquot was taken to determine OD, then centrifuged at 13,000 rcf for 5 min at a temperature of 4 °C. Supernatants were removed and 750 μL of each supernatant was placed in a vial for HPLC quantification of δ -toxin. The remaining volume of supernatant was sterile filtered with a 0.22 μm nylon syringe filter and stored at −20 °C until needed for later treatment of cells in the HaCaT cytotoxicity assay. Level of δ-toxin in the bacterial supernatant treated and untreated with the compounds was quantified by RP-HPLC (Reversed-Phase High Performance Liquid Chromatography) as previously described50.
Biofilm formation
All compounds were examined (2–281 μM) for impact on S. aureus biofilm formation using a human plasma protein-coated assay as previously described49,51,52. Additionally, to monitor for any growth impact in this assay, the optical density of planktonic cells in the biofilm wells was calculated by transferring the supernatant to a new 96-well plate and reading the OD600nm in a BioTek plate reader. S. aureus isolate UAMS-1 was used for the biofilm assay, and its isogenic sarA mutant (UAMS-929), which has a biofilm deficient phenotype, served as a positive control. All tests were performed in triplicate and repeated using a new stock of bacteria on a separate day.
Animal Studies
A previously described murine model of MRSA skin infection was used to determine the efficacy of each Schinus compound as an anti-infective25. All animal experiments described herein were approved by and conducted in accordance with the recommendations of the Animal Care and Use Committee at the University of Colorado Anschutz Medical Campus (IACUC protocol number 117217). One day prior to inoculation, the abdominal hair of 8 week old female BALB/c mice was shaved and chemically removed with topical application of Nair for 1 minute. LAC (USA300, AH1263) was grown in TSB media overnight at 37 °C in a shaking incubator (200 rpm). Overnight LAC culture was diluted 1:100 in fresh TSB media and allowed to grow to early log phase (~2 hr to OD600nm 0.5). Cells were washed in sterile PBS and resuspended to achieve an inoculum of 1×108 CFU. 50 μL inoculum suspensions containing 1×108 CFU LAC and 50 μg of each compound 1-3 (diluted in DMSO) or DMSO vehicle control were injected intradermally into the abdominal skin of each BALB/c mouse. Baseline body weights for the mice were taken prior to injection and each day following. For determination of lesion size, digital photos of skin lesions were taken with an iPhone camera and analyzed with ImageJ software for Mac. Inoculum CFU was verified by serial dilution, plating, and colony counting after overnight incubation of the plate.
Statistical Analyses
All data were analyzed using a two-tailed Student’s t-test as calculated by GraphPad Prism 7 software (GraphPad Software, La Jolla, CA). DMSO or dH2O treated (vehicle control) cultures were used as a vehicle control and were compared to those treated with extract for all statistical analyses. P < 0.05 were considered statistically significant. All assays and other experiments were performed in triplicate or quadruplicate to ensure sufficient technical replicates and repeated on two separate days to ensure sufficient biological replicates.
Supplementary information
Acknowledgements
We acknowledge the use of the Rigaku Synergy-S diffractometer, supported by the National Science Foundation under grant CHE-1626172. ARH was supported by NIH grant AI133089 from the National Institute of Allergy and Infectious Diseases. CLQ was supported by Emory University development funds. The authors thank the Emory College Mass Spectrometry Center for performing the HRMS-APCI analysis. Thanks to the Emory University NMR Research Center for maintaining and providing access to the NMR instrumentation.
Author contributions
G.P., H.T., A.R.H. and C.L.Q. wrote the manuscript. C.Q. and A.H. designed the experimental plan. H.T. undertook chemical isolation and microbiological studies on biofilms, agr and growth inhibition. F.C. undertook the human cell culture studies. J.T.L. assisted with compound isolation and contributed to analysis of chemistry data. G.P. undertook M.S. and N.M.R. work. J.B. performed the X-ray crystallography experiments and data analysis. M.B. performed the animal experiments and experiments on reporters other than agr. All authors contributed to the revision and final review of the manuscript.
Competing interests
C.L.Q., H.T., G.P. and J.T.L. are named inventors on a provisional patent application concerning the technology presented in this paper. The authors confirm that any competing interests do not alter their adherence to all the Nature Publishing Group policies on sharing data and materials.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Huaqiao Tang and Gina Porras.
Supplementary information
is available for this paper at 10.1038/s41598-020-65080-3.
References
- 1.Lee AS, et al. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers. 2018;4:18033. doi: 10.1038/nrdp.2018.33. [DOI] [PubMed] [Google Scholar]
- 2.Wilcox, M. et al. Reporting elevated vancomycin minimum inhibitory concentration in methicillin-resistant Staphylococcus aureus: consensus by an International Working Group. Future Microbiol., 10.2217/fmb-2018-0346 (2019). [DOI] [PMC free article] [PubMed]
- 3.Challagundla, L. et al. Range expansion and the origin of USA300 North American epidemic Methicillin-Resistant Staphylococcus aureus. mBio9, 10.1128/mBio.02016-17 (2018). [DOI] [PMC free article] [PubMed]
- 4.King MD, et al. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann. Intern. Med. 2006;144:309–317. doi: 10.7326/0003-4819-144-5-200603070-00005. [DOI] [PubMed] [Google Scholar]
- 5.Stryjewski ME, Chambers HF. Skin and soft-tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2008;46:S368–S377. doi: 10.1086/533593. [DOI] [PubMed] [Google Scholar]
- 6.Silver LL. Challenges of antibacterial discovery. Clin Microbiol Rev. 2011;24:71–109. doi: 10.1128/CMR.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N. Engl. J. Med. 2013;368:299–302. doi: 10.1056/NEJMp1215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Duijkeren, E., Schink, A. K., Roberts, M. C., Wang, Y. & Schwarz, S. Mechanisms of bacterial resistance to antimicrobial agents. Microbiol. Spectr. 6, 10.1128/microbiolspec.ARBA-0019-2017 (2018). [DOI] [PMC free article] [PubMed]
- 9.Cheung GY, Wang R, Khan BA, Sturdevant DE, Otto M. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect. Immun. 2011;79:1927–1935. doi: 10.1128/iai.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thoendel M, Kavanaugh JS, Flack CE, Horswill AR. Peptide signaling in the staphylococci. Chem. Rev. 2011;111:117–151. doi: 10.1021/cr100370n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson ME, et al. Staphylococcus epidermidis agr quorum-sensing system: signal identification, cross talk, and importance in colonization. J. Bacteriol. 2014;196:3482–3493. doi: 10.1128/JB.01882-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuji BT, Rybak MJ, Cheung CM, Amjad M, Kaatz GW. Community- and health care-associated methicillin-resistant Staphylococcus aureus: a comparison of molecular epidemiology and antimicrobial activities of various agents. Diagn. Microbiol. Infect. Dis. 2007;58:41–47. doi: 10.1016/j.diagmicrobio.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Grundstad ML, et al. Quorum sensing, virulence, and antibiotic resistance of USA100 methicillin-resistant Staphylococcus aureus isolates. mSphere. 2019;4:e00553–00519. doi: 10.1128/mSphere.00553-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu. Rev. Genet. 2008;42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 15.Parlet CP, et al. Apicidin attenuates MRSA virulence through quorum-sensing inhibition and enhanced host defense. Cell Rep. 2019;27:187–198.e186. doi: 10.1016/j.celrep.2019.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi SD, Malachowa N, DeLeo FR. Pathogenesis of Staphylococcus aureus abscesses. Am J Pathol. 2015;185:1518–1527. doi: 10.1016/j.ajpath.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams DA, Muchugu E, Overholt WA, Cuda JP. Colonization patterns of the invasive Brazilian peppertree, Schinus terebinthifolius, in Florida. Heredity. 2007;98:284–293. doi: 10.1038/sj.hdy.6800936. [DOI] [PubMed] [Google Scholar]
- 18.Pernas, T. et al. Florida Exotic Pest Plant Council’s 2019 List of Invasive Plant Species. (Florida Exotic Pest Plant Council, 2019).
- 19.Gioeli, K. T., Enloe, S. F., Minteer, C. R. & Langeland, K. A. Brazilian peppertree control. 1-5 (University of Florida Agronomy Department, UF/IFAS Extension. SS-AGR-17, 2018).
- 20.da Silva Dannenberg G, Funck GD, Mattei FJ, da Silva WP, Fiorentini ÂM. Antimicrobial and antioxidant activity of essential oil from pink pepper tree (Schinus terebinthifolius Raddi) in vitro and in cheese experimentally contaminated with Listeria monocytogenes. Innov. Food Sci. Emerg. Technol. 2016;36:120–127. doi: 10.1016/j.ifset.2016.06.009. [DOI] [Google Scholar]
- 21.Silva A, et al. Antibacterial activity, chemical composition, and cytotoxicity of leaf’s essential oil from Brazilian pepper tree (Schinus terebinthifolius, Raddi) Braz. J. Microbiol. 2010;41:158–163. doi: 10.1590/S1517-83822010000100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.da Silva MM, et al. Schinus terebinthifolius: phenolic constituents and in vitro antioxidant, antiproliferative and in vivo anti-inflammatory activities. Rev Bras Farmacogn. 2017;27:445–452. doi: 10.1016/j.bjp.2016.12.007. [DOI] [Google Scholar]
- 23.Jeribi C, Karoui IJ, Hassine DB, Abderrabba M. Comparative study of bioactive compounds and antioxidant activity of Schinus terebinthifolius Raddi fruits and leaves essential oils. Int. J. Sci. Res. 2014;3:453–458. [Google Scholar]
- 24.Ramos DMB, et al. Evaluation of antitumor activity and toxicity of Schinus terebinthifolia leaf extract and lectin (SteLL) in sarcoma 180-bearing mice. J. Ethnopharmacol. 2019;233:148–157. doi: 10.1016/j.jep.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Muhs, A. et al. Virulence inhibitors from Brazilian Peppertree block quorum sensing and abate dermonecrosis in skin infection models. Sci. Rep. 7, 42275, 10.1038/srep42275, https://www.nature.com/articles/srep42275#supplementary-information (2017). [DOI] [PMC free article] [PubMed]
- 26.Shirane N, Hashimoto Y, Ueda K, Takenaka H, Katoh K. Ring-A cleavage of 3-oxo-olean-12-en-28-oic acid by the fungus Chaetomium longirostre. Phytochemistry. 1996;43:99–104. doi: 10.1016/0031-9422(96)00266-X. [DOI] [Google Scholar]
- 27.Mulholland DA, Nair JJ. Triterpenoids from Dysoxylum pettigrewianum. Phytochemistry. 1994;37:1409–1411. doi: 10.1016/S0031-9422(00)90421-7. [DOI] [Google Scholar]
- 28.Morais TR, et al. Antiparasitic activity of natural and semi-synthetic tirucallane triterpenoids from Schinus terebinthifolius (Anacardiaceae): structure/activity relationships. Molecules. 2014;19:5761–5776. doi: 10.3390/molecules19055761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paharik AE, et al. Coagulase-negative staphylococcal strain prevents Staphylococcus aureus colonization and skin infection by blocking quorum sensing. Cell Host Microbe. 2017;22:746–756.e745. doi: 10.1016/j.chom.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandeputte OM, et al. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2010;76:243. doi: 10.1128/AEM.01059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vattem DA, Mihalik K, Crixell SH, McLean RJC. Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia. 2007;78:302–310. doi: 10.1016/j.fitote.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen TB, Givskov M. Quorum sensing inhibitors: a bargain of effects. Microbiology. 2006;152:895–904. doi: 10.1099/mic.0.28601-0. [DOI] [PubMed] [Google Scholar]
- 33.Xie P, et al. Enhanced extraction of hydroxytyrosol, maslinic acid and oleanolic acid from olive pomace: Process parameters, kinetics and thermodynamics, and greenness assessment. Food Chem. 2019;276:662–674. doi: 10.1016/j.foodchem.2018.10.079. [DOI] [PubMed] [Google Scholar]
- 34.Silva-Júnior E, et al. Phytochemical compounds and pharmacological properties from Schinus molle Linnaeus and Schinus terebinthifolius Raddi (Anacardiaceae). J. Chem. Pharm. Res. 2015;7:389–393. [Google Scholar]
- 35.Morais TR, et al. Application of an ionic liquid in the microwave assisted extraction of cytotoxic metabolites from fruits of Schinus terebinthifolius Raddi (Anacardiaceae) J. Braz. Chem. Soc. 2017;28:492–497. [Google Scholar]
- 36.Jain MK, et al. Specific competitive inhibitor of secreted phospholipase A2 from berries of Schinus terebinthifolius. Phytochemistry. 1995;39:537–547. doi: 10.1016/0031-9422(94)00960-2. [DOI] [PubMed] [Google Scholar]
- 37.Da Silva GF, et al. G. Lanost-7-en triterpenes from stem bark of Santiria trimera. Phytochemistry. 1990;29:1629–1632. doi: 10.1016/0031-9422(90)80135-4. [DOI] [Google Scholar]
- 38.Boles BR, Horswill AR. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008;4:e1000052–e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lister, J. L. & Horswill, A. R. Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Front Cell Infect Microbiol4, 10.3389/fcimb.2014.00178 (2014). [DOI] [PMC free article] [PubMed]
- 40.Gilabert M, et al. Sesqui- and triterpenoids from the liverwort Lepidozia chordulifera inhibitors of bacterial biofilm and elastase activity of human pathogenic bacteria. Phytomedicine. 2015;22:77–85. doi: 10.1016/j.phymed.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Garo E, et al. Asiatic acid and corosolic acid enhance the susceptibility of Pseudomonas aeruginosa biofilms to tobramycin. Antimicrob. Agents Chemother. 2007;51:1813–1817. doi: 10.1128/aac.01037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu JF, et al. Bacterial biofilm inhibitors from Diospyros dendo. J. Nat. Prod. 2006;69:118–120. doi: 10.1021/np049600s. [DOI] [PubMed] [Google Scholar]
- 43.Ren D, et al. Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl. Environ. Microbiol. 2005;71:4022–4034. doi: 10.1128/aem.71.7.4022-4034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li HE, et al. Glycyrrhetinic acid protects mice from Staphylococcus aureus pneumonia. Fitoterapia. 2012;83:241–248. doi: 10.1016/j.fitote.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 45.Tugume P, et al. Ethnobotanical survey of medicinal plant species used by communities around Mabira Central Forest Reserve, Uganda. J. Ethnobiol. Ethnomed. 2016;12:5. doi: 10.1186/s13002-015-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crosby HA, et al. The Staphylococcus aureus ArlRS two-component system regulates virulence factor expression through MgrA. Mol. Microbiol. 2020;113:103–122. doi: 10.1111/mmi.14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crosby HA, et al. The Staphylococcus aureus global regulator MgrA modulates clumping and virulence by controlling surface protein expression. PLoS Pathog. 2016;12:e1005604–e1005604. doi: 10.1371/journal.ppat.1005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan H, Wang Q, Teng M, Li X. The DNA-binding mechanism of the TCS response regulator ArlR from Staphylococcus aureus. J. Struct. Biol. 2019;208:107388. doi: 10.1016/j.jsb.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Quave CL, et al. Castanea sativa (European Chestnut) leaf extracts rich in ursene and oleanene derivatives block Staphylococcus aureus virulence and pathogenesis without detectable resistance. PLoS One. 2015;10:e0136486. doi: 10.1371/journal.pone.0136486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quave C, Horswill A. in Methods in Molecular Biology. 2018;1673:363–370. doi: 10.1007/978-1-4939-7309-5_27. [DOI] [PubMed] [Google Scholar]
- 51.Quave CL, et al. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS One. 2012;7:e28737. doi: 10.1371/journal.pone.0028737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beenken KE, Blevins JS, Smeltzer MS. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect. Immun. 2003;71:4206–4211. doi: 10.1128/iai.71.7.4206-4211.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.