Abstract
In this work, the concept of lipase cocktail has been proposed in the ultrasound-assisted hydrolysis of coconut oil. Lipase from Thermomyces lanuginosus (TLL), lipase from Rhizomucor miehei (RML), and lipase B from Candida antarctica (CALB) were evaluated as biocatalysts in different combinations. The best conversion (33.66%) was achieved using only RML; however, the best lipase cocktail (75% RML and 25% CALB) proposed by the triangular response surface was used to achieve higher conversions. At the best lipase cocktail, reaction parameters [temperature, biocatalyst content and molar ratio (water/oil)] were optimized by a Central Composite Design, allowing to obtain more than 98% of conversion in the hydrolysis of coconut oil in 3 h of incubation at 37 kHz, 300 W and 45 °C by using 20% of the lipase cocktail (w/w) and a molar ratio of 7.5:1 (water/oil). The lipase cocktail retained about 50% of its initial activity after three consecutive cycles of hydrolysis. To the authors’ knowledge, up to date, this communication is the first report in the literature for the ultrasound-assisted hydrolysis of coconut oil catalyzed by a cocktail of lipases. Under ultrasound irradiation, the concept of lipase cocktail was successfully applied, and this strategy could be useful for the other types of reactions using heterogeneous substrates.
Keywords: Lipase cocktail, Ultrasound irradiation, Hydrolysis, Coconut oil
Introduction
Although chemical catalysts are widely used in industrial processes, the use of enzymes as biocatalysts provides advantages, such as high selectivity and specificity under mild reaction conditions (Wohlgemuth 2010; Souza et al. 2016; Pinheiro et al. 2018; Pinheiro et al. 2019a, b; Fonseca et al. 2020). Lipases (triacylglycerol acyl hydrolases, E.C. 3.1.1.3 – IUPAC) have been gaining increasing attention worldwide due to their wide range of scientific and industrial applications (Yadav and Borkar 2009; Santos et al. 2015; Rosenthal and Lütz 2018; Rios et al. 2018, 2019; Reis et al. 2019; de Oliveira et al. 2019; Monteiro et al. 2019, 2020). Lipases’ physiological function is to hydrolyze oils, and therefore, lipases have been used in breakdown reactions, in the presence of sufficient amount of water, forming monoglycerides, diglycerides, Free Fatty Acids (FFAs), and glycerol (Al-Zuhair et al. 2003; Hasan et al. 2009; Choi et al. 2015; Verdasco-Martín et al. 2016; Bonazza et al. 2018; Lima et al. 2017; dos Santos et al. 2017; Melo et al. 2017; de Oliveira et al. 2018).
Free Fatty Acids (FFAs), which can be produced, for example, by the enzymatic hydrolysis of oils, are used as raw materials in the a wide range of applications in industrial processes, such as in the production of chemical oils, alcohols, amines, and esters (Al-Zuhair et al. 2003). However, the main fatty acid present in oils represents 70% or less of fatty acid composition; therefore, the hydrolysis of oils catalyzed by specific lipases may result in low conversions due to the heterogeneity of the substrates and the specificity of lipases (Alves et al. 2014).
Natural oils and fats contain mixtures of triglycerides, diglycerides, monoglycerides, and FFAs in their composition, and a combination of lipases with different specificities may be required for complete hydrolysis of these heterogenous substrates (Rodrigues and Ayub 2011). Extra virgin coconut oil (Cocos nucifera L.) has its main fatty acid lauric acid (49%), which may be used as a natural food preservative due to its ability to convert to monolaurin and destroy the lipid membrane of bacteria (Stillwell and Stillwell 2016).
Lipase B from Candida antarctica (CALB) is a versatile lipase produced in genetically modified organisms (Anderson et al. 1998). There is a commercial immobilized preparation of CALB, which is named Novozym® 435 (Ortiz et al. 2019), and is the most used lipase in biotransformation (Anderson et al. 1998; Verdasco-Martín et al. 2016; Santos et al. 2017; Pinheiro et al. 2019a, b). Once CALB has a very small lid, making it unable to completely isolate the active site from reaction medium, it has a high degree of stereospecificity (see Fig. 1a) (Uppenberg et al. 1995). Lipase from Rhizomucor miehei (RML), commercially available as Lipozyme® RM–IM, has great applicability in the esterification of oils and fats (see Fig. 1b) (Foresti et al. 2005). Similarly, lipase from Thermomyces lanuginosus (TLL) (see Fig. 1c) is commercially available in its immobilized form as Lipozyme® TL–IM (Fernandez-Lafuente 2010).
Fig. 1.
Representation of lipases 3D structures: a lipase B from Candida antarctica (CALB) (PDB code: 1TCA) with active site residues SER105 (red), ASP187 (green), HIS224 (blue); b lipase from Rhizomucor miehei (RML) (PDB: 4TGL), with active site residues SER144 (red), ASP203 (green), HIS258 (blue); c lipase from Thermomyces lanuginosus lipase (TLL) (PDB: 1DT3), with active site residues SER146 (red), ASP201 (green), and HIS258 (blue). All 3D structures were obtained from the Protein Data Bank (PDB) using pymol vs educational
Ultrasound technology has been used in reactions, such as those catalyzed by lipases, once it is a green technology of high efficiency, economic performance, and low instrumental need (Sá et al. 2017). At appropriate frequencies, ultrasound technology facilitates mass transfer and creates super active free radicals (Sancheti and Gogate 2017). Furthermore, in ultrasound-assisted processes, the phenomenon generated by the rarefaction and compression of sound waves, which is known as cavitation, enables the formation and expansion of water bubbles, generating localized supercritical effects of temperature and pressure (Ho et al. 2016; Sá et al. 2017).
The search for chemical and/or biochemical processes optimization goes beyond the choice of a specific catalyst and/or biocatalyst. Researchers are increasingly using computer simulations to reduce the time and costs for the optimization of experiments (Garud et al. 2017). The Design of Experiments (DoE) allows the optimization of the main reaction parameters by the proposed models (Hibbert 2012). Among the most widely used DoEs, the Central Composite Design (CCD) is a widely used model for studying the main effects and possible interactions between them to find the optimal reaction conditions (Djoudi et al. 2007).
In this work, combinations of three commercial immobilized lipases, Novozym®, Lipozyme® TL-IM, and Lipozyme® RL-IM were evaluated using a three-factor mixture design and triangular surface analysis to determine the best lipase cocktail for the production of FFAs from coconut oil under ultrasound irradiation. Once the best lipase cocktail was determined, hydrolysis parameters (enzyme content, molar ratio, and temperature) were optimized by CCD. To the authors’ knowledge, up to date, this communication is the first report in the literature for the ultrasound-assisted hydrolysis of coconut oil catalyzed by a cocktail of lipases.
Materials and methodology
All experiments were performed at least in duplicate, and the results are presented as the average of these values and the standard deviation (generally below 5%).
Materials
Lipase from T. lanuginosus (TLL immobilized on a silicate support, Lipozyme® TL–IM), lipase from R. miehei (RML immobilized on an anion-exchange resin, Lipozyme® RM–IM), and lipase B from Candida antarctica (CALB immobilized on a macroporous resin, Novozym® 435) were kindly donated by Novozymes (Madrid, Spain). The heterogeneous substrate used was commercial refined coconut oil, with a maximum acidity of 0.3%, and it was purchased at a local market, and it is composed of caproic acid (0.38%), caprylic acid (5.56%), capric acid (4.99%), lauric acid (45.78%), myristic acid (18.56%), palmitic acid (8.85%), stearic acid (3.39%), oleic acid (5.65%), and linoleic acid (0.94%), as it was reported by the manufacturer. Oleic acid, anhydrous ethanol 99.9%, and hexane were purchased from Vetec (São Paulo, Brazil). All other chemicals were of analytical grade and they were used without any further purification.
Ultrasound equipment setup
The equipment used in all experiments was an ultrasonic bath (Unique Inc., model USC 2800A, Brazil). The equipment presents the capacity volume of 9.5 L with the following dimensions: 300 × 240 × 150 mm (length × width × height). Two disk transducers were placed at the bottom of the reactor. The ultrasonic frequency was 37 kHz and the total ultrasonic power 300 W. Additionally, the equipment has temperature control.
Methodology
Lipase activity
The activity of the lipases (TLL, RML, and CALB) was quantified by the hydrolysis of olive oil as proposed by Soares et al. (1999). The substrate (oil-gum Arabic emulsion) was composed of a mixture of 50 g of olive oil and 150 g of gum Arabic solution (7%, w/v in water). The reaction medium, which was composed of 5 mL of the emulsion, 5 mL of 0.1 mol/L phosphate buffer (pH 7.0), and the biocatalyst, was incubated under mechanical stirring for 5 min at 37 °C and 200 r/min. The reaction products were titrated with 0.025 mol/L KOH solution, using three drops of phenolphthalein as indicator. One unit of enzyme activity was defined as the amount of enzyme that produces 1 µmol of fatty acid per minute under test conditions (Soares et al. 1999).
Lipase cocktail
To determine the best combination of lipases (TLL, RML, and CALB) for the hydrolysis of coconut oil, a three-factor mixture design and triangular surface analysis was performed using Statistica® (Statsoft, USA). For the hydrolysis of coconut oil, it was considered a 2:1 molar ratio (water/oil) and a biocatalyst content of 10% of the coconut oil mass. The reactions were conducted at ultrasound equipment for 1 h at 30 °C (Alves et al. 2014), with some modifications.
Hydrolysis of coconut oil
Once the optimal lipase cocktail was determined, the hydrolysis of coconut oil was conducted for different molar ratio of water (5:1–10:1) added to 1.4 mmol of coconut oil into 10 mL Erlenmeyer flasks. The biocatalyst content (the best lipase cocktail) was varied from 10 to 20% of the coconut oil mass. The resulting mixture of water, coconut oil, and biocatalyst was incubated in an ultrasound bath for 3 h and temperature varying from 35 to 55 °C (Table 1), according to the methodology described in the literature (Alves et al. 2014), with modifications. To monitor the progress of hydrolysis, 0.3 g of sample was titrated using NaOH (10 mM), 5 mL ethanol, and three drops of phenolphthalein (Alves et al. 2014). The conversion yield for hydrolysis was determined by the method reported in the literature (Rooney and Weatherley 2001; Ramos et al. 2015).
Table 1.
Variables and their levels for the Central Composite Design for the optimization of the hydrolysis of coconut oil
| Variable | Name | Coded levels | ||
|---|---|---|---|---|
| −1 | 0 | + 1 | ||
| X1 | Temperature (°C) | 35 | 45 | 55 |
| X2 | Enzyme content (%)a | 10 | 15 | 20 |
| X3 | Molar ratio (water/oil) | 5:1 | 7.5:1 | 10:1 |
aRelated to the coconut oil mass
Central composite design (CCD)
To determine the optimal conditions for the hydrolysis of coconut oil, three variables CCD was carried out. Table 4 shows 18 treatments of the three variables, each at three levels. The second-order polynomial equation for the variables is as follows:
| 1 |
Table 4.
Experimental design for the optimization of the hydrolysis of coconut oil, using the best lipase cocktail (75% RML and 25% CALB)
| Run | Temperature (°C) | Biocatalyst content (%) | Molar ratio (water/oil) | Conversion (%) |
|---|---|---|---|---|
| 1 | 35 (−1) | 10 (−1) | 5:1 (−1) | 58.92 ± 1.10 |
| 2 | 35 (−1) | 10 (−1) | 10:1 (+ 1) | 61.49 ± 0.92 |
| 3 | 35 (−1) | 20 (+ 1) | 5:1 (−1) | 21.42 ± 0.83 |
| 4 | 35 (−1) | 20 (+ 1) | 10:1 (+ 1) | 60.87 ± 1.06 |
| 5 | 55 (+ 1) | 10 (−1) | 5:1 (−1) | 55.59 ± 0.69 |
| 6 | 55 (+ 1) | 10 (−1) | 10:1 (+ 1) | 62.54 ± 0.24 |
| 7 | 55 (+ 1) | 20 (+ 1) | 5:1 (−1) | 87.67 ± 0.93 |
| 8 | 55 (+ 1) | 20 (+ 1) | 10:1 (+ 1) | 96.16 ± 1.12 |
| 9 | 35 (−1) | 15 (0) | 7.5:1 (0) | 71.96 ± 0.77 |
| 10 | 55 (+ 1) | 15 (0) | 7.5:1 (0) | 81.40 ± 0.57 |
| 11 | 45 (0) | 10 (−1) | 7.5:1 (0) | 79.28 ± 0.66 |
| 12 | 45 (0) | 20 (+ 1) | 7.5:1 (0) | 98.68 ± 0.19 |
| 13 | 45 (0) | 15 (0) | 5:1 (-1) | 96.94 ± 0.85 |
| 14 | 45 (0) | 15 (0) | 10:1 (+ 1) | 87.15 ± 1.31 |
| 15 | 45 (0) | 15 (0) | 7.5:1 (0) | 78.53 ± 2.23 |
| 16(C) | 45 (0) | 15 (0) | 7.5:1 (0) | 78.70 ± 2.78 |
| 17(C) | 45 (0) | 15 (0) | 7.5:1 (0) | 79.28 ± 2.35 |
| 18(C) | 45 (0) | 15 (0) | 7.5:1 (0) | 79.66 ± 2.14 |
Further details are given on the “Materials and methodology” section
In which, Y is the response variable, β0 is the constant, βi, βii, and βij are the coefficients for the linear, quadratic, and for the interaction effects, respectively, and Xi and Xj are the coded levels of variables Xi and Xj. The above quadratic equation was used to plot surfaces for all variables.
Statistical analysis
The softwares Statistica® 10 (Statsoft, USA) and OriginPro 2017 (OriginLab, USA) were used for the experimental design and statistical analysis of the hydrolysis of coconut oil. The statistical analysis of the model was performed by the analysis of variance (ANOVA). The significance of the regression coefficients and the associated probabilities, p(t), were determined using Student’s t test. The variance explained by model was given by the multiple determination coefficient, R2. For each variable, the quadratic models were represented as contour plots (2D).
Operational stability
Using the best lipase cocktail and under optimized reaction conditions, after the hydrolysis of coconut oil, the lipase cocktail was separated from the reaction medium by vacuum filtration using a sintered glass funnel. The lipase cocktail was washed three times with hexane to dissolve and remove any residual reaction substrate and product from the support, and allowed to stay in the vacuum system for 30 min to ensure its dryness (dos Santos et al. 2017).
Results and discussion
Lipase activity
The commercial biocatalysts (TLL, RML, and CALB) used in this study were evaluated as biocatalysts in the hydrolysis of olive oil in a solvent-free medium. The substrate was an olive-gum Arabic emulsion and the reaction medium was incubated for 5 min at 37 °C and 200 r/min. Under these conditions, the hydrolysis activity of the lipases used in this study are listed (Table 2).
Table 2.
Hydrolytic activity of the lipases under study
| Lipase | Activity (Ug−1) |
|---|---|
| CALB | 766.5 ± 38.33 |
| RML | 4154 ± 68.36 |
| TLL | 1078 ± 53.9 |
Oleic acid is the major FFA constituent of olive oil, and therefore, the results obtained from the analysis of the activity of the lipases suggest that the highest yield will be achieved by the samples with RML as biocatalyst. Despite having the second largest catalytic activity among the studied lipases, TLL does not perform satisfactorily in hydrolysis reactions, being more indicated its use in transesterification and esterification reactions (Rodrigues and Ayub 2011).
Coconut oil hydrolysis optimization
Lipase cocktail
With the purpose to find the best combination of TLL, RML, and CALB for the hydrolysis of coconut oil, it was performed a three-factor mixture design. The conversions obtained for the mixture design are shown in Table 3 and graphically represented in Fig. 2.
Table 3.
Experimental design for the determination of the best lipase cocktail
| Run | TLL | RML | CALB | Conversion (%) |
|---|---|---|---|---|
| 1 | 1.000 | 0.000 | 0.000 | 27.06 ± 0.94 |
| 2 | 0.000 | 1.000 | 0.000 | 33.66 ± 1.46 |
| 3 | 0.000 | 0.000 | 1.000 | 14.17 ± 1.19 |
| 4 | 0.500 | 0.500 | 0.000 | 30.86 ± 0.65 |
| 5 | 0.500 | 0.000 | 0.500 | 16.90 ± 0.12 |
| 6 | 0.000 | 0.500 | 0.500 | 32.00 ± 0.43 |
| 7 | 0.333 | 0.333 | 0.333 | 28.05 ± 2.12 |
| 8 | 0.667 | 0.167 | 0.167 | 25.21 ± 1.00 |
| 9 | 0.167 | 0.667 | 0.167 | 30.68 ± 1.95 |
| 10 | 0.167 | 0.167 | 0.667 | 26.24 ± 0.98 |
Further details are given on the “Materials and methodology” section
Fig. 2.
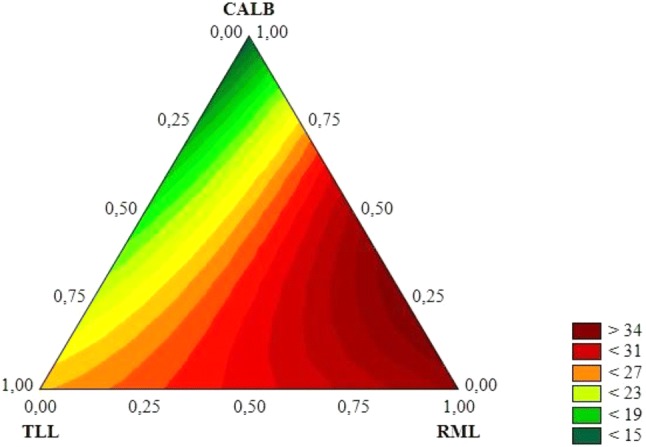
Triangular response surface for experimental design, indicating the best lipase cocktail. Reaction medium: 2:1 molar ratio (water/oil), biocatalyst content of 10% of the coconut oil mass. The reactions were conducted in an ultrasound bath (37 kHz and 300 W) for 1 h and 30 °C
As it can be seen, the lowest conversions were obtained using CALB alone, whereas the highest conversions were obtained using higher amounts of RML, either alone or in combination. Mixtures of RML and CALB improved the conversions, but this behavior was not observed for mixtures of TLL and CALB, or for TLL and RML. Therefore, it is possible to propose that the best combination of lipases, among those under study, for the hydrolysis of coconut oil is the combination of 75% of RML and 25% of CALB (see Fig. 2).
When it was used as a single lipase, both TLL and RML led to the highest conversions, but their combination did not improve the conversions, probably because of their similar substrate specificities, which may have led to an inhibition of catalytic activity between RML and TLL. However, when CALB was combined with RML, the conversion was more than doubled, when compared to the use of CALB alone. In a previous work, Rodrigues and Ayub (2011) found that 65% of TLL and 35% of RML were the most effective mixture of lipases for vegetable oil hydrolysis (Rodrigues and Ayub 2011). The difference between the results obtained in this study and those from the previously cited study might be explained by the diverse TLL preparations. Rodrigues and Ayub (2010) used a TLL preparation immobilized onto Lewatit support activated with aldehyde groups, whereas it was used a TLL preparation immobilized by adsorption onto an anion-exchange support in this study (Rodrigues and Ayub 2011). The differences concerning the nature of support and immobilization strategies are known to largely affect the catalytic activity of enzymes (Mateo et al. 2007).
Reaction parameters
At certain levels, temperature, biocatalyst content, and substrate molar ratio may further hydrolyze coconut oil. Among these three parameters, temperature had the highest effect, while the content of biocatalyst had the lowest. From Table 4, comparing the experiments in which the only parameter that changes temperature (1–5, 2–6, 3–7, 4–8 and 9–10), the conversions have increased almost 1.24-fold along with the temperature. As it will be discussed in further details later, increasing temperature increases the catalytic activity, once it allows greater solubility of the substrate to the active site of the biocatalysts (Waghmare and Rathod 2016).
At a confidence interval of 90%, the statistical analysis of the model was done by the Fisher’s statistical test for Analysis of Variance (ANOVA). The computed F value (2.38) was significant (p < 0.0001). The model fitting was checked by the determination coefficient (R2). The determination coefficient (R2 = 0.82) implies that sample variation of up 80% is attributed to the independent variables, and can be explained by the model. The model polynomial equation is given as follows:
In which, Y is the conversion (%) of hydrolysis, and X1, X2 and X3 are the coded values of temperature, biocatalyst content, and molar ratio, respectively.
For the hydrolysis of coconut oil catalyzed by the best lipase cocktail (75% RML and 25% CALB), the optimal conditions were found to be 20% of biocatalyst content (related to the oil mass), 10:1 (water/oil) after 3 h of incubation at 50 °C and 37 kHz/300 W, obtaining a theoretical value of 100% for the conversion predicted by the model after 3 h of incubation at 50 °C and 37 kHz/300 W. However, the experimental monitored value under optimal conditions was 98.68% ± 0.42.
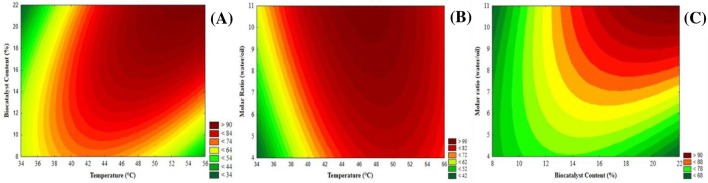
The interactions between temperature, biocatalyst content, and substrate molar ratio, and their effect on the conversions are shown in Fig. 3. Figure 3a shows the possible effect of temperature on the conversions, being the optimal temperature of 50 °C, considering the best biocatalyst content (20%) was close to the central point. The interactions between substrate molar ratio and temperature strongly suggest that increasing until certain level (10:1; water/oil) the amount of water in the reaction medium positively affects the conversion (see Fig. 3b, c). Water, as one of the substrates, is an important factor in maintaining the enzyme activity and stability. However, lipases perform better at microaqueous environment, so higher contents of water in the reaction medium may prejudice its performance by competitive inhibition (Sharma et al. 2013).
Fig. 3.
Contour plots for the experimental design for the hydrolysis of coconut oil. a Temperature versus biocatalyst content; b molar ratio (water/oil) versus biocatalyst content; c temperature versus molar ratio (water/oil). Reaction medium: 5:1–10:1 molar ratio (water/oil) and biocatalyst content of 10–20% of the coconut oil mass. The reactions were conducted in an ultrasound bath (37 kHz and 300 W) for 3 h and 35–55 °C (See Table 4). In each figure, the variable that is not represented was fixed at its central value
Effect of parameters on the hydrolysis of coconut oil
Temperature
Temperature is a key parameter in enzyme-catalyzed processes. Although increasing temperature may enhance reaction rates to some extent, higher level of temperature may lead to the denaturation of enzymes (Waghmare and Rathod 2016). In this work, to obtain the optimal process temperature, it was varied from 35 °C to 55 °C. From Table 4, it is possible to observe that the higher conversions are associated with an increase in temperature. In fact, the best experimental conversion value (98.68%) was found at 45 °C. Increasing in conversions with increasing in temperature is associated with a decrease in coconut oil viscosity which, combined with ultrasound captivation waves, improves mass transfer, so that the substrates may interact with the active site of the lipases more easily and efficiently without facing significant energy losses due to viscosity resistance in mass transfer (Waghmare and Rathod 2016).
Molar ratio
The hydrolysis of coconut oil was performed under a solvent-free medium. The reaction medium was composed of two phases, oil and water, which has two main roles in the reaction as it helps in hydrating the lipases and acts as one of the substrates. To determine the influence on the conversion, molar ratio was studied ranging from 5:1 to 10:1 (water/oil), as it is shown in Table 4. Under this range, it was observed that the conversion increases with the molar ratio, which means that the amount of water offered was enough to guarantee the hydration of the lipases, favoring their stability and activity, and act as a substrate.
On the other hand, higher concentrations of oil may increase reaction medium viscosity, limiting mass transfer of the substrates to the lipase active site. Therefore, the concentration of the oil remained the same for all runs of this work. Similarly, increasing viscosity reduces the effect of cavitation, which is responsible for the catalytic effect of ultrasound irradiation. Therefore, only the amount of water in the reaction medium was evaluated to analyze how the hydrolysis conversion rate is affected. The ultrasound irradiation feeds emulsification between substrates and also feeds free electron production (Liu et al. 2008), decreasing the limitations of mass transfer between substrates and, therefore, physically catalyze the reaction (Waghmare and Rathod 2016).
Biocatalyst content
Biocatalyst content is one of the parameters to be considered to evaluate the viability of any process, once it has considerable impact on the economic viability of the process. To determine the most appropriate biocatalyst content of the best lipase cocktail (75% RML and 25% CALB), the biocatalyst content was varied from 10 to 20% of the oil mass. As can be seen in Table 4, increasing biocatalyst content increases the conversions. In fact, the best experimental conversion (98.68%) was found at the highest biocatalyst content (20%). Increasing biocatalyst content increases the biocatalyst active surface area concentration; that is, there will be a higher biocatalyst concentration in the reaction medium that may catalyze a higher concentration of substrates (Waghmare and Rathod 2016).
Generally, ultrasound-assisted enzymatic reactions are performed at low frequencies (20–50 kHz), because high irradiation frequencies may denature enzymes (Bansode and Rathod 2017). Accordingly, the effect of ultrasound irradiation on coconut oil hydrolysis was evaluated using a frequency of 37 kHz with the biocatalyst content ranging from 10 to 20% of the oil mass. It was observed that the conversion remained stable throughout the study period and in all of the experimental runs (see Table 4), having a nearly linear behavior. Similarly, at a high power, as the value used in this study (300 W), there is the emergence of a large number of cavitation bubbles in the reaction medium, favoring the conversion and suggesting that the power of the equipment used in this study has an positive effect for enhancing the conversions (Csoka et al. 2011).
Time course
At the best lipase cocktail (75% RML and 25% CALB) and under optimized reactional conditions (20% of biocatalyst content, 10:1 (water/oil), 3 h of incubation at 50 °C and 37 kHz/300 W), the influence of mechanical stirring and ultrasound irradiation were evaluated in the hydrolysis of coconut oil, as it is in Fig. 4.
Fig. 4.
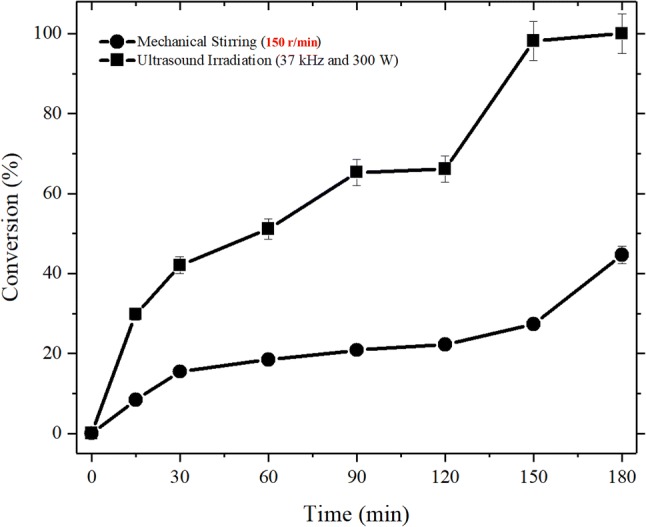
Conversions versus time in different reaction conditions (ultrasonic irradiation and mechanical stirring) in the hydrolysis of coconut oil catalyzed by the lipase cocktail (75% RML and 25% CALB). Reaction medium: 10:1 molar ratio (water/oil) and biocatalyst content of 20% of the coconut oil mass. The reactions were conducted in an ultrasound bath (37 kHz and 300 W) for 3 h at 50 °C
As can be seen in Fig. 4, the experimental runs performed under ultrasound irradiation were significantly faster than those performed under mechanical stirring. Indeed, after 15 min of reaction, the conversion of the sample incubated in the ultrasound bath was 70.36% higher than that incubated in the mechanical agitation equipment and, over the time, the samples incubated in the ultrasound bath show higher conversions than that observed for mechanical agitation equipment.
Ultrasound irradiation by the cavitation phenomena improves mass transfer in heterogeneous reaction systems (Sancheti and Gogate 2017), such as the enzyme-catalyzed hydrolysis of this work. Moreover, the ultrasound irradiation improves mixing and interaction between phases, due to the collapse of bubbles in aqueous solutions, and it may increase the reaction rates, decreasing the reaction time, and reduce the limitations of mass transfer between enzyme and substrate (Galgali et al. 2018).
Using a lipase cocktail similar to this study (80% RML and 20% CALB), Alves et al. (2014) achieved a conversion of approximately 80% after 20 h of reaction (Alves et al. 2014). In this work, a maximum conversion of 98.68% was achieved after 3 h of reaction under ultrasonic irradiation. Kulkarni and Pandit (2005), using only lipase from Aspergillus oryzae, achieved a maximum conversion of 93.18% after 24 hours of reaction for the hydrolysis of castor oil under ultrasonic irradiation (Kulkarni and Pandit 2005). Therefore, from the results obtained in this work, the combination of lipases and ultrasonic irradiation is better than individual lipase and mechanical stirring for the hydrolysis of coconut oil.
Operational stability
Industrial applicability of lipases requires stability and maintenance of the catalytic activity, allowing the repeated use of these biocatalysts. To evaluate the operational stability of the best lipase cocktail (75% RML and 25% CALB) under optimized reactional conditions (20% of lipase cocktail, 10:1 (water/oil), 3 h of incubation at 50 °C and 37 kHz/300 W), consecutive cycles of hydrolysis of coconut oil were performed. Prior to each cycle, the lipase cocktail was washed with hexane to dissolve and remove any residual reaction product (dos Santos et al. 2017). The operational stability of the lipase cocktail is shown in Fig. 5, which presents that the reuse of the lipase cocktail is possible for three consecutive cycles maintaining 50% of its initial activity, suggesting that one of the enzymes of the lipase cocktail loses its activity/stability throughout the consecutive cycles of hydrolysis.
Fig. 5.
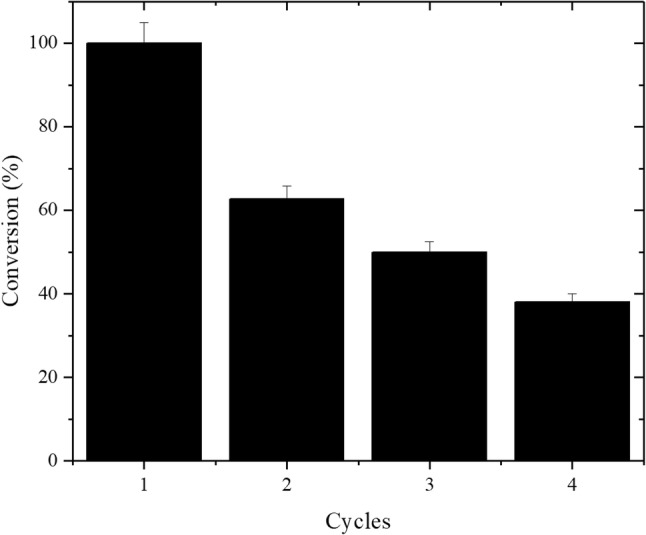
Operational stability of the lipase cocktail (75% RML and 25% CALB) in consecutive coconut oil hydrolysis. Reaction medium: 10:1 molar ratio (water/oil) and biocatalyst content of 20% of the coconut oil mass. The reactions were conducted in an ultrasound bath (37 kHz and 300 W) for 3 h at 50 °C
It is important to note that RML presents low operational stability; in contrast, CALB presents high operational stability. Therefore, this may explain the decrease in the conversion after consecutive cycles of hydrolysis of coconut oil. The low stability of RML may be due to fractures in the acrylic resin in which it is immobilized. When the support exhibits stuttering defects, the immobilization of the enzyme is compromised and, as a consequence, the enzyme is more prone to denaturation and conformational changes. Therefore, the defects in the structure of the support provide undesirable enzyme-support binding sites, which makes biocatalysts unstable (Wenlei and Ning 2009). These embryogenic enzyme-supported bonds added to consecutive cycles with consecutive solvent washes may have caused RML to become unstable during reuse. In the case of CALB, other found that Novozym® 435 maintains its operational stability for up to five cycle studies (Foresti and Ferreira 2005; Xie and Ma 2010). Nevertheless, in this work, as CALB represents only 25% of the amount of lipase in the lipase cocktail, this operational stability is not as significant as RML, which represents 75% of the cocktail.
Conclusion
The recent concept of combining different lipases to catalyze reactions with heterogeneous substrate was applied in this work. The lipase cocktail improved the ultrasound-assisted hydrolysis of coconut oil, and, by triangular surface, the best lipase cocktail was found to be 75% RML and 25% CALB. The reaction parameters [temperature, biocatalyst content, and molar ratio (water/oil)] were also optimized by response surface methodology, achieving almost the complete conversion (up to 98%) of the substrate into free fatty acids. The lipase cocktail has retained approximately half of its initial activity after three consecutive cycles of coconut oil hydrolysis, mainly due to the low stability of RML, the main component of the lipase cocktail. The operational stability of RML in consecutive reaction cycles was the limiting factor in this study. To the authors’ knowledge, up to date, this communication is the first report in the literature for the ultrasound-assisted hydrolysis of coconut oil catalyzed by a cocktail of lipases. Especially under ultrasonic irradiation, the combination of lipases might be useful for the hydrolysis of other vegetable oils and could bring better performances in the food industry.
Acknowledgements
We gratefully acknowledge the financial support of Brazilian Agencies for Scientific and Technological Development, Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP), project number BP3-0139-00005.01.00/18, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), project number 422942/2016-2 and 408790/2016-4, Coordenação de Aperfeiçoamento de Ensino Superior (CAPES). In addition, we gratefully acknowledge the supply of Lipases by Mr. Martinez (Novozymes, Spain).
Author contributions
Conceptualization, validation, formal analysis, investigation and methodology: JESS, RRCM, TGR, AKSB and JCSS; Software: RRCM; data curation, writing—original draft preparation: JESS, RRCM, TGR and KSM and FTTC; resources: AKSB, MCMS and JCSS; Writing—review and editing, visualization, supervision, project administration:, RRCM, MCMS and JCSS.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest related to this study.
Contributor Information
Maria C. M. de Souza, Email: mariacristiane@unilab.edu.br
José C. S. dos Santos, Email: jcs@unilab.edu.br
References
- Aga SÁ, de Meneses AC, de Araújo PHH, de Oliveira D. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci Technol. 2017;69:95–105. [Google Scholar]
- Alves JS, Vieira NS, Cunha AS, et al. Combi-lipase for heterogeneous substrates: a new approach for hydrolysis of soybean oil using mixtures of biocatalysts. RSC Adv. 2014;4:6863–6868. [Google Scholar]
- Al-Zuhair S, Hasan M, Ramachandran KB. Kinetics of the enzymatic hydrolysis of palm oil by lipase. Process Biochem. 2003;38:1155–1163. [Google Scholar]
- Anderson EM, Larsson KM, Kirk O. One biocatalyst many applications: the use of Candida antarctica B-lipase in organic synthesis. Biocatal and Biotransformation. 1998;16:181–204. [Google Scholar]
- Bansode SR, Rathod VK. An investigation of lipase catalysed sonochemical synthesis: a review. Ultrason Sonochem. 2017;38:503–529. doi: 10.1016/j.ultsonch.2017.02.028. [DOI] [PubMed] [Google Scholar]
- Bonazza HL, Manzo RM, Santos JCS, Mammarella EJ. Operational and thermal stability analysis of Thermomyces lanuginosus lipase covalently immobilized onto modified chitosan supports. Appl Biochem Biotechnol. 2018;184:182–196. doi: 10.1007/s12010-017-2546-9. [DOI] [PubMed] [Google Scholar]
- Choi JM, Han SS, Kim HS. Industrial applications of enzyme biocatalysis: current status and future aspects. Biotechnol Adv. 2015;33:1443–1454. doi: 10.1016/j.biotechadv.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Csoka L, Katekhaye SN, Gogate PR. Comparison of cavitational activity in different configurations of sonochemical reactors using model reaction supported with theoretical simulations. Chem Eng J. 2011;178:384–390. [Google Scholar]
- Cunha AG, Besteti MD, Manoel EA, et al. Preparation of core–shell polymer supports to immobilize lipase B from Candida antarctica: Effect of the support nature on catalytic properties. J Mol Catal B Enzym. 2014;100:59–67. [Google Scholar]
- da Fonseca AM, dos Santos JCS, de Souza MCM, et al. The use of new hydrogel microcapsules in coconut juice as biocatalyst system for the reaction of quinine. Ind Crops Prod. 2020;145:111890. [Google Scholar]
- de Oliveira UMF, Lima de Matos LJB, de Souza MCM, et al. Effect of the presence of surfactants and immobilization conditions on catalysts’ properties of Rhizomucor miehei lipase onto chitosan. Appl Biochem Biotechnol. 2018;184:1263–1285. doi: 10.1007/s12010-017-2622-1. [DOI] [PubMed] [Google Scholar]
- de Oliveira UMF, Lima de Matos LJB, de Souza MCM, et al. Efficient biotechnological synthesis of flavor esters using a low-cost biocatalyst with immobilized Rhizomucor miehei lipase. Mol Biol Rep. 2019;46:597–608. doi: 10.1007/s11033-018-4514-z. [DOI] [PubMed] [Google Scholar]
- de Souza TC, de Fonseca T, Costa JA, et al. Cashew apple bagasse as a support for the immobilization of lipase B from Candida antarctica: application to the chemoenzymatic production of (R)-Indanol. J Mol Catal B Enzym. 2016;130:58–69. [Google Scholar]
- Djoudi W, Aissani-Benissad F, Bourouina-Bacha S. Optimization of copper cementation process by iron using central composite design experiments. Chem Eng J. 2007;133:1–6. [Google Scholar]
- dos Santos JCS, Rueda N, Gonçalves LRB, Fernandez-Lafuente R. Tuning the catalytic properties of lipases immobilized on divinylsulfone activated agarose by altering its nanoenvironment. Enzyme Microb Technol. 2015;77:1–7. doi: 10.1016/j.enzmictec.2015.05.001. [DOI] [PubMed] [Google Scholar]
- dos Santos JCS, Bonazza HL, de Matos LJBL, et al. Immobilization of CALB on activated chitosan: Application to enzymatic synthesis in supercritical and near-critical carbon dioxide. Biotechnol Rep. 2017;14:16–26. doi: 10.1016/j.btre.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lafuente R. Lipase from Thermomyces lanuginosus: uses and prospects as an industrial biocatalyst. J Mol Catal B Enzym. 2010;62:197–212. [Google Scholar]
- Foresti ML, Ferreira ML. Solvent-free ethyl oleate synthesis mediated by lipase from Candida antarctica B adsorbed on polypropylene powder. Catal Today. 2005;107–108:23–30. [Google Scholar]
- Galgali A, Gawas SD, Rathod VK. Ultrasound assisted synthesis of citronellol laurate by using Novozym 435. Catal Today. 2018;309:133–139. [Google Scholar]
- Garud SS, Karimi IA, Kraft M. Design of computer experiments: a review. Comput Chem Eng. 2017;106:71–95. [Google Scholar]
- Hasan F, Shah AA, Hameed A. Methods for detection and characterization of lipases: a comprehensive review. Biotechnol Adv. 2009;27:782–798. doi: 10.1016/j.biotechadv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hibbert DB. Experimental design in chromatography: a tutorial review. J Chromatogr B. 2012;910:2–13. doi: 10.1016/j.jchromb.2012.01.020. [DOI] [PubMed] [Google Scholar]
- Ho WWS, Ng HK, Gan S. Advances in ultrasound-assisted transesterification for biodiesel production. Appl Therm Eng. 2016;100:553–563. [Google Scholar]
- Kulkarni SR, Pandit AB. Enzymatic hydrolysis of castor oil: an approach for rate enhancement and enzyme economy. Indian J Biotechnol. 2005;4:241–245. [Google Scholar]
- Lima GV, da Silva MR, de Sousa FT, et al. Chemoenzymatic synthesis of (S)-Pindolol using lipases. Appl Catal A Gen. 2017;546:7–14. [Google Scholar]
- Liu Y, Jin Q, Shan L, et al. The effect of ultrasound on lipase-catalyzed hydrolysis of soy oil in solvent-free system. Ultrason Sonochem. 2008;15:402–407. doi: 10.1016/j.ultsonch.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Mateo C, Palomo JM, Fernandez-Lorente G, et al. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol. 2007;40:1451–1463. [Google Scholar]
- Melo A, Silva F, dos Santos JC, et al. Synthesis of benzyl acetate catalyzed by lipase immobilized in nontoxic chitosan-polyphosphate beads. Molecules. 2017;22:2165. doi: 10.3390/molecules22122165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro RRC, Lima PJM, Pinheiro BB, et al. Immobilization of Lipase A from Candida antarctica onto Chitosan-Coated Magnetic Nanoparticles. Int J Mol Sci. 2019;20:4018. doi: 10.3390/ijms20164018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro RRC, Virgen-Ortiz JJ, Berenguer-Murcia Á, et al. Biotechnological relevance of the lipase A from Candida antarctica. Catal Today. 2020 doi: 10.1016/j.cattod.2020.03.026. [DOI] [Google Scholar]
- Ortiz C, Ferreira ML, Barbosa O, et al. Novozym 435: the “perfect” lipase immobilized biocatalyst? Catal Sci Technol. 2019;9:2380–2420. [Google Scholar]
- Pinheiro MP, Rios NS, de Fonseca T, et al. Kinectic resolution of drug intermediates catalyzed by lipase B from Candida antarctica immobilized on immobead-350. Biotech Progress. 2018;34:878–889. doi: 10.1002/btpr.2630. [DOI] [PubMed] [Google Scholar]
- Pinheiro BB, Rios NS, Rodríguez Aguado E, et al. Chitosan activated with divinyl sulfone: a new heterofunctional support for enzyme immobilization. Application in the immobilization of lipase B from Candida antarctica. Int J Biol Macromol. 2019;130:798–809. doi: 10.1016/j.ijbiomac.2019.02.145. [DOI] [PubMed] [Google Scholar]
- Pinheiro MP, Monteiro RRC, Silva FFM, et al. Modulation of Lecitase properties via immobilization on differently activated Immobead-350: Stabilization and inversion of enantiospecificity. Process Biochem. 2019;87:128–137. [Google Scholar]
- Ramos EZ, Júnior RHM, De Castro PF, et al. Production and immobilization of Geotrichum candidum lipase via physical adsorption on eco-friendly support: Characterization of the catalytic properties in hydrolysis and esterification reactions. J Mol Catal B Enzym. 2015;118:43–51. [Google Scholar]
- Reis CL, de Sousa EYA, Serpa JF, et al. Design of immobilized enzyme biocatalysis: drawbacks and opportunities. Quim Nova. 2019;42:768–783. [Google Scholar]
- Rios NS, Pinheiro BB, Pinheiro MP, et al. Biotechnological potential of lipases from Pseudomonas: Sources, properties and applications. Process Biochem. 2018;75:99–120. [Google Scholar]
- Rios NS, Morais EG, dos Galvão W, et al. Further stabilization of lipase from Pseudomonas fluorescens immobilized on octyl coated nanoparticles via chemical modification with bifunctional agents. Int J Biol Macromol. 2019;141:313–324. doi: 10.1016/j.ijbiomac.2019.09.003. [DOI] [PubMed] [Google Scholar]
- Rodrigues RC, Ayub MAZ. Effects of the combined use of Thermomyces lanuginosus and Rhizomucor miehei lipases for the transesterification and hydrolysis of soybean oil. Process Biochem. 2011;46:682–688. [Google Scholar]
- Rooney D, Weatherley LR. The effect of reaction conditions upon lipase catalysed hydrolysis of high oleate sunflower oil in a stirred liquid-liquid reactor. Process Biochem. 2001;36:947–953. [Google Scholar]
- Rosenthal K, Lütz S. Recent developments and challenges of biocatalytic processes in the pharmaceutical industry. Curr Opin Green Sustain Chem. 2018;11:58–64. [Google Scholar]
- Sancheti SV, Gogate PR. A review of engineering aspects of intensification of chemical synthesis using ultrasound. Ultrason Sonochem. 2017;36:527–543. doi: 10.1016/j.ultsonch.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Sharma A, Chaurasia SP, Dalai AK. Enzymatic hydrolysis of cod liver oil for the fatty acids production. Catal Today. 2013;207:93–100. [Google Scholar]
- Soares CMF, Castro HF, Moraes FF, et al. Characterization and utilization of Candida rugosa lipase immobilized on controlled pore silica. Appl Biochem Biotechnol. 1999;79:745–757. doi: 10.1385/abab:79:1-3:745. [DOI] [PubMed] [Google Scholar]
- Stillwell W (2016) Introduction to Biological Membranes. In: An introduction to biological membranes. Elsevier, pp 3–15. 10.1016/B978-0-444-63772-7.00001-4
- Uppenberg J, Ohmer N, Norin M, et al. Crystallographic and molecular-modeling studies of lipase B from Candida antarctica reveal a stereospecificity pocketfor secondary alcohols. Biochemistry. 1995;34:16838–16851. doi: 10.1021/bi00051a035. [DOI] [PubMed] [Google Scholar]
- Verdasco-Martín CM, Villalba M, dos Santos JCS, et al. Effect of chemical modification of Novozym 435 on its performance in the alcoholysis of camelina oil. Biochem Eng J. 2016;111:75–86. [Google Scholar]
- Waghmare GV, Rathod VK. Ultrasound assisted enzyme catalyzed hydrolysis of waste cooking oil under solvent free condition. Ultrason Sonochem. 2016;32:60–67. doi: 10.1016/j.ultsonch.2016.01.033. [DOI] [PubMed] [Google Scholar]
- Wenlei X, Ning M. Immobilized lipase on Fe3O4 nanoparticles as biocatalyst for biodiesel production. Energy Fuels. 2009;23:1347–1353. [Google Scholar]
- Wohlgemuth R. Biocatalysis-key to sustainable industrial chemistry. Curr Opin Biotechnol. 2010;21:713–724. doi: 10.1016/j.copbio.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Xie W, Ma N. Enzymatic transesterification of soybean oil by using immobilized lipase on magnetic nano-particles. Biomass Bioenergy. 2010;34:890–896. [Google Scholar]
- Yadav GD, Borkar IV. Synthesis of n-butyl acetamide over immobilized lipase. J Chem Technol Biotechnol. 2009;84:420–426. [Google Scholar]