Dear Editor,
Primary cardiac lymphomas (CL) are rare and constitute 1% of all cardiac tumors [1]. Initially defined as extra-nodal lymphoma involving only the heart/pericardium, definition was expanded to include those patients of lymphoma with cardiac manifestations where the bulk of disease is in the heart [2]. These are aggressive tumors and usually present with dyspnea, arrhythmia, left ventricular failure or pericardial effusion and rarely with symptoms and signs of superior vena cava syndrome. We present the clinical course of a patient with CL who presented to our center.
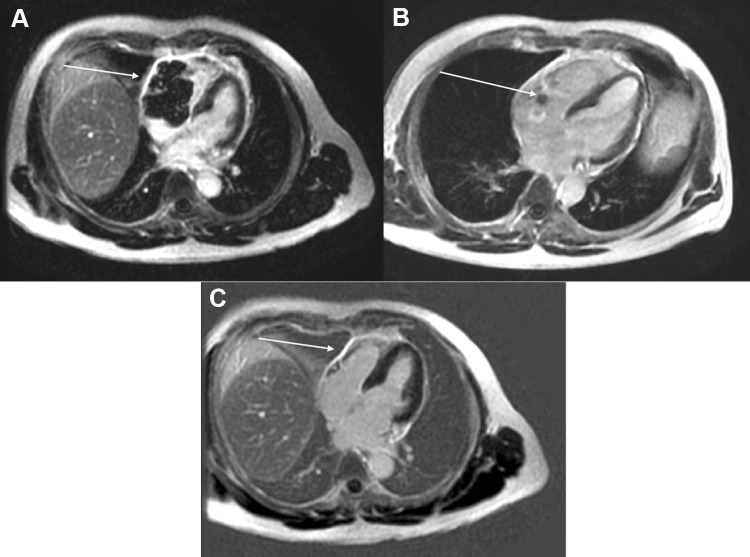
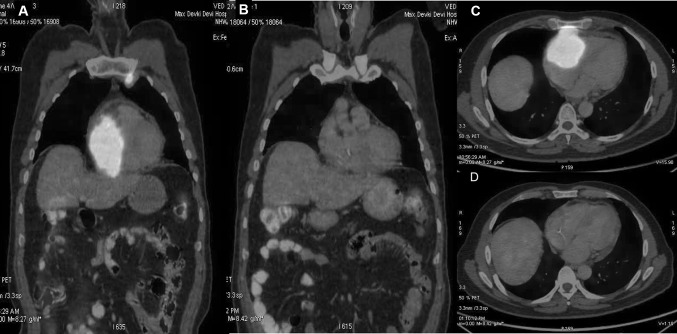
A 51-year-old male presented to cardiologist with a history of progressive shortness of breath and weight loss for 1 month. Echocardiography (Echo) demonstrated large heterogenous lobular mass 5.4 × 2.9 cm in right atrium/right ventricle (RA/RV) involving tricuspid valve and further extending into pericardial cavity. Small pericardial effusion with organized material was seen along RA/RV. No regional wall motion abnormality was noticed. Left ventricular ejection fraction was 55–60%. Electrocardiography showed sinus tachycardia. Cardiac MRI showed large enhancing mass occupying right ventricle extending into the right atrium involving the tricuspid valve obliterating the atrium and ventricle and multiple enlarged mediastinal lymph nodes (Fig. 1a). 18F-Fluorodeoxyglucose PET with CT (FDG PET/CT) was done which showed increased FDG avidity in the cardiac mass lesion (Fig. 2a, c). FDG avid bilateral internal mammary lymph node 2.2 × 1.4 cm (SUV 13.8) and superior mediastinal lymph nodes were seen with faintly FDG avid left level II and IV cervical lymph nodes. Image guided trucut biopsy of FDG avid internal mammary lymph node showed CD45, CD20, CD10 positive and Cyclin D1, Bcl 6 and MUM1 negative diffuse large B cell lymphoma (DLBCL), germinal centre B cell type with Ki-67 labelling index of 90% (Fig. 3). Fluorescent in situ hybridisation for Bcl 2 was negative and c-myc was positive. Bone marrow did not show any lymphomatous infiltration. Patient had an international prognostic index score of 2 (lactate dehydrogenase–412 U/L, performance status—2 and stage IE). A diagnosis of primary cardiac lymphoma was established and he was started on pre-phase with intravenous methylprednisolone 100 mg for 5 days under cardiac monitoring. Following this, he was started on dose adjusted rituximab, etoposide, prednisolone, vincristine, cyclophosphamide and doxorubicin (DA R-EPOCH). PET-CT after three cycles of chemotherapy demonstrated minimal residual non FDG avid lesion in RA/RV region showing complete metabolic response (Fig. 2b, d). Figure 1b shows cardiac MRI findings post therapy. He completed 6 cycles of DA R–EPOCH and is currently in remission on 3 months follow up. From 1947 till 2018, 230 cases of primary cardiac lymphoma have been reported [4]. The median age of presentation is 63 years with male‐to‐female ratio of 1.94. They are usually B cell lymphoma of which most are DLBCL, followed by follicular lymphoma and burkitt lymphoma [2]. Ninety-two percent of patients present with right sided heart involvement [2].The most effective line of management of CL is chemotherapy. Cardiac failure, pulmonary thromboembolism and rupture have been described post chemotherapy [3, 4]. R-EPOCH has been used in the treatment of primary cardiac lymphoma in three patients in literature without any complications [5]. We preferred R-EPOCH as infusional doxorubicin has been associated with lower cardiotoxicity [6], which in turn reduces the risk of cardiac perforation. Other modalities like radiotherapy and surgery has been also used. In patients with hemodynamic instability, surgery has been performed for tumor debulking and any mechanical obstruction [7]. A recent study of aggregate of 197 primary CL patients reported in literature, demonstrated that presence of extra-cardiac disease, left ventricular (LV) involvement, immunodeficiency and absence of arrhythmia had a poor overall survival as compared with those who did not have these risk factors [2]. Our patient was immunocompetent with no LV involvement. He responded well to therapy without any complications.
Fig. 1.
a Cardiac MRI (post contrast images) of patient showing 5.9 × 5.9 cm mass involving the RA/RV prior to treatment (February 2019). b Cardiac MRI (post contrast images) after three cycles of therapy showing a small residual lesion in the RA/RV region (May 2019). c After completion of treatment showing complete resolution of the lesion (August 2019)
Fig. 2.
a, c Fluorodeoxyglucose PET with CT (FDG PET/CT) was done which showed increased FDG avidity in the cardiac mass lesion. b, d PET-CT after three cycles of chemotherapy demonstrated minimal residual non FDG avid lesion in RA/RV region showing complete metabolic response
Fig. 3.
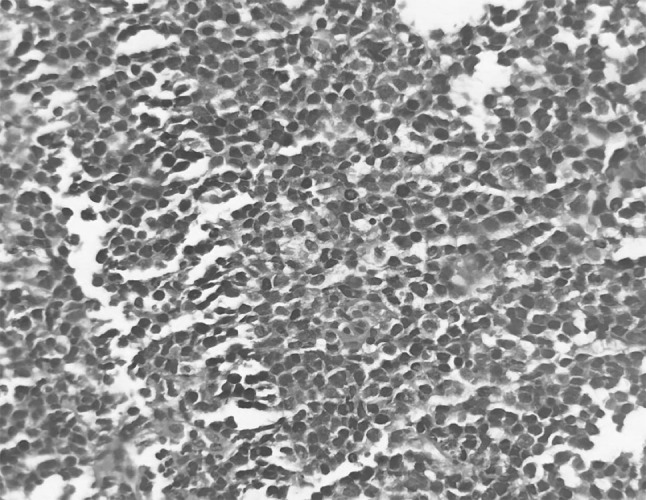
Section of the tumor showing diffuse sheet of large sized lymphoid cells with high N:C ratio, large nuclei and scanty cytoplasm
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Burke A, Virmani R. Atlas of tumor pathology (third series, fascicle 16): tumors of the heart and great vessels. Washington: Armed Forces Institute of Pathology; 1996. pp. 171–179. [Google Scholar]
- 2.Petrich A, Cho S, Billett H. Primary cardiac lymphoma: an analysis of presentation, treatment and outcome patterns. Cancer. 2011;117:581–589. doi: 10.1002/cncr.25444. [DOI] [PubMed] [Google Scholar]
- 3.O’mahony D, Piekarz RL, Bandettini WP, Arai AE, Wilson WH, Bates SE. Cardiac involvement with lymphoma: a review of the literature. Clin Lymphoma Myeloma. 2008;8(4):249–252. doi: 10.3816/clm.2008.n.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miguel CE, Bestetti RB. Primary cardiac lymphoma. Int J Cardiol. 2011;149(3):358–363. doi: 10.1016/j.ijcard.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Anand K, Pingali SR, Trachtenberg B, Iyer SP. Complete response to R-EPOCH in primary cardiac lymphoma. Case Rep Hematol. 2019;2019:7690430. doi: 10.1155/2019/7690430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Legha SS, Benjamin RS, Mackay B, et al. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Ann Intern Med. 1982;96(2):133–139. doi: 10.7326/0003-4819-96-2-133. [DOI] [PubMed] [Google Scholar]
- 7.Parato VM, Muscente F, Scarano M. Primary cardiac lymphoma: a case report. G Ital Cardiol. 2017;18:11. doi: 10.1714/2628.27022. [DOI] [PubMed] [Google Scholar]