Abstract
Posttransplant lymphoproliferative disorder is an extremely fatal complication arising in transplant recipients as a side effect of immunosuppression. PTLDs are seen after both solid organ and hematopoietic stem cell transplants though the incidence is much higher in the former. Primary Epstein–Barr virus (EBV) infection or reactivation due to a state of immune dysregulation along with intensity of immunosuppression used are of paramount importance in pathogenesis of PTLD. EBV associated PTLDs occur early in the post transplant period whereas late onset lymphomas are usually EBV negative. The uncontrolled B cell proliferation can create a spectrum of histological patterns from nondestructive lesions to destructive polymorphic or more aggressive monomorphic PTLDs. Early detection of seropositivity by serial monitoring in the recipient can prevent PTLD development by starting pre-emptive therapy. The mainstay treatment in established cases remains reduction of immunosuppression. Chemotherapeutic and immunomodulatory agents are added sequentially based on the type of PTLD and based on its response to initial therapy. Despite various treatment options available, the morbidity remains high and achieving state of disease remission without causing graft rejection can be quite challenging. Hence, a better understanding in pathobiology of EBV+ versus EBV− PTLDS may help prevent lymphomagenesis in transplant recipients.
Keywords: Post transplant lymphoproliferative disorder, Epstein–Barr virus (EBV), Immunosuppression, Solid organ transplant, Hematopoietic stem cell transplant, Rituximab
Introduction
Posttransplant Lymphoproliferative Disorders (PTLD) encompass a spectrum of lymphoid proliferations ranging from indolent, benign to malignant population of polyclonal to monoclonal cells. PTLD are defined as lymphomas arising in transplant recipients on immunosuppression. The first case series of five patients was reported in late 1960s by Penn et al. [1, 2]. However, the term PTLD was coined in 1980s and was applied to lymphoproliferations seen in posttransplant patients ranging from uncomplicated infectious mononucleosis to indolent polyclonal population and to aggressive malignant clones.
These are potentially fatal complications that develop in recipients of both solid organ and Hematopoietic stem cell transplant (HSCT) and is mostly EBV related. The oncogenic EBV causes abnormal lymphoid proliferation in 50–80% of PTLDs especially in early onset disease (< 2 year after transplantation) [3–5]. Various risk factors predisposing to PTLDs are viral infections, type of allograft, degree of immunosuppression and host and recipient related factors [6–8]. Majority of PTLDs are associated with EBV infection either acquired after transplant in a seronegative recipient or reactivation of latent EBV virus due to immunosuppression. The pathogenesis of these EBV related lymphoproliferative disorders is complex, that involves transformation and immortalization of B cells causing uncontrolled proliferation. However, 20–40% of PTLDs are EBV negative and the pathogenesis in these remains unclear.
The latest classification of PTLD’s was incorporated in WHO 2017 and includes four histologic types as follows (a) early lesions (b) polymorphic PTLD’s (c) monomorphic PTLD and (d) classic Hodgkin’s lymphoma like PTLD (Table 1). As per the classification PTLDs can be polymorphic or monomorphic. The majority (> 90%) are of B cell origin, but PTLDs can also be of T cell origin/null cell origin, more so in late onset disease. Though histologically same, lymphomas developing in post transplant patients differ from lymphomas occurring in immunocompetent individuals in many ways (Table 2) [9]. Also, studies have shown that EBV driven PTLDs in transplant recipients do not harbor the same genetic aberrations as noted in Non Hodgkin’s Lymphoma (NHL) developing in immunocompetent individuals. In this paper, we have reviewed the existing literature and recommendations for diagnosis and treatment of PTLDs and summarized the data under different heads. The etiology, pathogenesis of EBV+ and EBV− PTLDS, clinical manifestations, pathological classification and latest inputs on diagnosis and treatment available has been incorporated in the review.
Table 1.
WHO classification of posttransplant lymphoproliferative disorders
| Non destructive PTLDs |
| Plasmacytic hyperplasia |
| Infectious mononucleosis |
| Florid follicular hyperplasia |
| Polymorphic PTLD |
| Monomorphic PTLDs (classify according to lymphoma they resemble) |
| B-cell neoplasm |
| Diffuse large B-cell lymphoma |
| Burkitt lymphoma |
| Plasma cell myeloma |
| Plasmacytoma |
| Othersa |
| T cell neoplasm |
| Peripheral T-cell lymphoma, NOS |
| Hepatosplenic T-cell lymphoma |
| Others |
| Classic Hodgkin lymphoma PTLD |
aIndolent small B-cell lymphomas arising in transplant recipients are not included among the PTLDs, with the exception of EBV-positive marginal zone lymphomas
Table 2.
Difference between PTLD and lymphomas developing in immunocompetent individuals
| Incidence | EBV association | Site of disease involvement | Type of lymphomas | Treatment modality | |
|---|---|---|---|---|---|
| Lymphomas in immunocompetent | .2–4% of general population | Rare | Nodal > extranodal |
NHL (B > T) HL |
Chemotherapy Rituximab HSCT |
| PTLDs | 20% of transplant recipients | 10–75% of all cases | Extranodal > nodal |
Early lesions Polymorphic Monomorphic Hodgkin’s like |
Reducing immunosuppression Rituximab Chemotherapy Adoptive therapy Immunomodulatory agents |
NHL non Hodgkin lymphoma, HL Hodgkin’s lymphoma, HSCT hematopoietic stem cell transplant
Clinical Features
PTLDs can have myriad ways, hence familiarity and high index of suspicion is critical for making a diagnosis. Because of heterogeneity of presentation differentiating it from acute graft failure or infectious complication becomes difficult. The symptoms are nonspecific like fever, weight loss, anorexia, night sweats and allograft dysfunction or it may be related to lymphoid proliferation in organs or specific sites i.e. symptoms of GIT, liver, lungs, brain or kidneys [10–12]. Extranodal involvement of various organs i.e. Gastrointestinal tract (30%), followed by CNS (10–15%), lungs (4%) and liver (5–12%) is more common. So, any recipient manifesting with unexplained fever, weight loss associated with mass lesions in non allograft organ should be evaluated for PTLD at the earliest [13–18]. Involvement of allograft is seen in up to 15% of kidney recipients and this may be higher in cardiothoracic organs. Other signs that could raise a trigger for PTLD may be nonspecific like generalized lymphadenopathy, Hemophagocytic Lymphohistiocytosis, or be more localized like headache or confusion. Solid organ transplant PTLDs (SOT-PTLDs) are usually of recipient origin as against HSCT-PTLDs which are of donor origin [19, 20].
Epidemiology and Risk Factors
The incidence of PTLDs, following SOT is much higher as compared to HSCT. After SOT, PTLDs are the second commonest malignancy (20%) following non melanotic skin cancers [21–23]. On the other hand the incidence of PTLD following HSCT is 01–2.5% though its associated with disseminated disease and carries higher chance of mortality [24–27]. The various risk factors associated with PTLD are enumerated in Table 3.
Table 3.
Risk factors for PTLD
| Risk factors for SOT | Risk factors for HSCT |
|---|---|
| EBV seromismatch (R−/D+) | HLA mismatch (haploidentical) MMURD > MUD > MRD |
| Type of SOT | T cell depleted graft/use of antiCD3 antibodies |
| Intensity and type of immunosuppression | Duration of immunosuppression |
| Underlying disorders (HCV, Cystic fibrosis, autoimmune hepatitis) | Underlying disorders (PID, advanced Hodgkin’s Lymphoma) |
EBV Epstein barr virus, MMUD mismatched unrelated donor, MUD matched unrelated donor, MRD matched related donor, PID primary immunodeficiency disorder
The most important risk factor implicated in development of PTLD is seronegativity for EBV in recipients at the time of transplant. Seronegativity increases the risk of development of PTLD by 10–75%. So, any recipient who is seronegative at the time of transplant and acquires the infection posttransplant, progress to PTLD. Hence, this is more common in children (< 10 years) as about 50% of them are seronegative at the time of transplant [28, 29]. Other viral infections, viruses implicated in PTLD are concomitant infection with CMV, Hepatitis C and HHV-8. Over last decade, there has been a rise in EBV negative PTLDs. Though the pathogenesis remains unclear and various postulated hypothesis includes persistence of subclinical viral infection, combination of immunosuppression reagents used and viral oncogenicity.
The incidence in SOT, varies and depends on the type of organ transplanted. This difference may be related to amount of lymphoid tissue transplanted and the amount of immunosuppression given to the recipient. The involvement of various organs in descending order is multivisceral transplant (12–17%), recipient of lung (6–10%), heart (3–5%), liver (2–3%) and kidney (1.5–2.5%).
The combination of immunosuppressive agents used and its intensity serve as important risk factors for both SOT and HSCT PTLDs. Antilymphocyte agents like ATG, anti-CD3 monoclonal antibodies, azathioprine and other drugs that cause profound T cell depletion increase the risk of PTLD by 25% as compared to normal individuals [30–35]. Use of mammalian target of rapamycin inhibitors (mTOR) inhibitors on the other hand has been shown to reduce PTLD incidence [33, 36]. In some retrospective studies, tacrolimus as compared to cyclosporine A has been shown to increase the risk of PTLD, whereas in other studies this finding has been refuted [37].
HSCT patients have lower risk (< 2%) of developing PTLD. PTLD after SOT follows a bimodal peak before 02 years (early onset) of transplant and second peak after 5–10 years (late onset). Early onset PTLDs are usually EBV positive whereas, the late onset PTLDs are frequently EBV negative.
PTLDs post HSCT occurs very early in the transplant course. The high incidence of early onset PTLDs seen post HSCT is suggested to be due to delay in immune reconstitution of anti EBV cytotoxic T lymphocytes (CTLs) after myeloablation and homing of donor stem cells [38]. In a study by Landgren et al. of 21,686 HSCT patients, use of ATG and T cell depleting agents for Graft versus host disease (GVHD) were identified as major risk factors for PTLDs. Other important predisposing factors were HLA mismatch, second transplant and elderly patients [22]. In another study conducted by Sundin et al., they observed HLA mismatch, splenectomy and EBV seronegativity as significant risk factors associated with PTLD [21].
Pathobiology of EBV Related PTLDS
EBV is transmitted through contact with the saliva of carriers and they enter and lodge in the subepithelial B cells of nasopharyngeal tract. The virus establishes a lytic infection associated with production of new versions which can be transmitted to a new host. The viral genome can also get integrated with the host genome establishing a latent infection in host memory B cells [39–45].
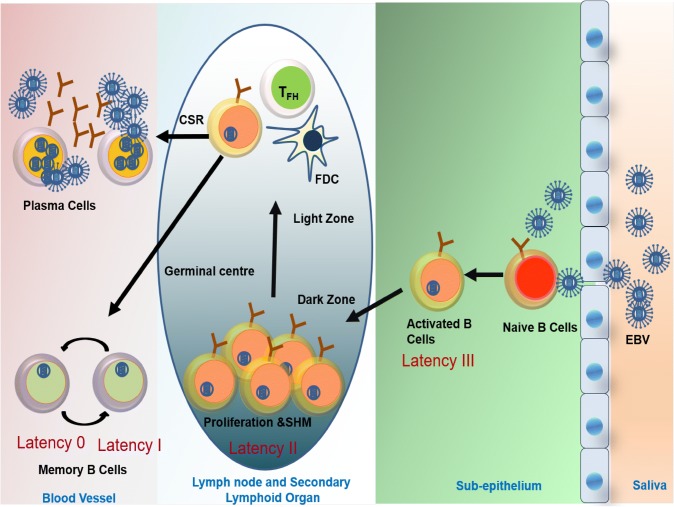
As the EBV infected naïve B cells proliferate and transform, the viral programme called latency expression changes from III-II-I-0. This latency profile is based on expression of viral EBV proteins and these are associated with different stages of EBV B cell infection. The naïve B cells in the subepithelium of nasopharynx are activated by EBV binding to CD21 receptors. The activated B cells either differentiate into memory B cells or plasma cell via the T cell dependent or independent pathway. The activated B cells express latency III viral encoded proteins (namely 6 EBV nuclear antigens-EBNA-1, 2, 3A-C and EBNA leader protein) and 03 latent membrane proteins (LMP-1, 2A-B). This latency III proteins or growth promotors trigger initial proliferation of viral infected B cells. Once the B cells enter the germinal center of a lymph node or secondary lymphoid organs the default program called latency II is activated characterized by expression of EBNA1, LMP1, LMP2A and EBERs. As the B cells mature from centroblasts to centrocytes they undergo somatic hypermutation and class switch recombination. After leaving the germinal center they differentiate mostly into memory B cells and plasma cells. The memory B cells express latency I (EBNA1) or latency 0 i.e. no viral proteins (Fig. 1) [46, 47].
Fig. 1.
Pathophysiology of Epstein–Barr positive PTLDs developing in solid organ transplant recipients
As EBV infected B cells proliferate and express Epstein Barr nuclear antigen (EBNA) and latent membrane protein (LMP) host cytotoxic T lymphocyte (CTL) immune response checks the proliferation of the EBV infected B cells. Some of these infected cells escape the immune check and enter a latent phase. The memory B cells downregulate EBV encoded proteins but there remains enough antigenic expression to mount a secondary CTL immune response. Therefore, a balance is maintained and the EBV infected B cells are unable to progress to PTLD and persist in circulation leading to a subclinical infection. With immunosuppression, the balance is shifted towards B cell proliferation, leading to EBV driven lymphoproliferation.
Dissimilarities Between EBV− Versus EBV+ PTLD
PTLD occurring in EBV+ recipients is a known fact, however there are 33–48% cases of PTLD occurring in EBV seronegative individuals. The underlying pathophysiology in these cases remains ambiguous. Various hypothesis proposed are chronic immune triggering post transplant, infection due to other organisms and hit and run EBV infection [48–50]. However there is limited evidence to support these theories. EBV− cases tend to occur much later compared to EBV+ cases [51]. Molecular studies have detected similar genetic defects in PTLDs arising in EBV− individuals as seen in classical lymphoma developing in immunocompetent individuals [52]. Hence, there is a school of thought not to place them in PTLD category. The mere fact that they respond well to reduced immunosuppression necessitates them to be classified under PTLDs.
Pathologic Classification
The commonly used classification system of PTLD is as per the WHO classification 2017 [53], which divides PTLD into early lesions, polymorphic PTLD, monomorphic and Hodgkin’s like PTLD. The monomorphic and classic Hodgkin’s lymphoma types of PTLD are further categorized as in immunocompetent individuals. The indolent lymphomas with the exception of EBV positive MALT lymphomas are not considered a type of PTLD.
Early Lesions—These include lesions with features of infectious mononucleosis and plasmacytic hyperplasia with preservation of tissue architecture. These lesions are the first morophological variants seen in the spectrum of PTLDs. Diffuse follicular hyperplasia or paracortical expansion with infectious mononucleosis like features is typical of the early lesions. They are composed of a mixed cell population consisting primarily of small lymphocytes with scattered plasma cells and immunoblasts that exhibit minimal cytological atypia. Rarely small clonal or oligoclonal population may be apparent [54].
Polymorphic PTLDs—These lesions show predominantly effacement and destruction of tissue and architecture and show malignant histological features such as nuclear pleomorphism, necrosis and mitosis. They are composed of small and medium sized lymphocytes, atypical immunoblasts, mature plasma cells and occasional Reed Sternberg (RS) like cells. These lesions are mostly monoclonal and about 15% reveal cytogenetic abnormality.
Monomorphic PTLDs—These are composed of monotonous population of transformed lymphoid cells or plasmacytic cells showing marked nuclear atypia/pleomorphism. The monomorphic PTLDs are commonly of B cell origin and include Diffuse large B cell lymphoma (DLBCL), Burkitts lymphoma or a plasma cell neoplasm. The indolent B cell lymphomas are not included under the classification of M-PTLDs. The T/NK cell type of PTLD includes the entire spectrum of T or NK cell neoplasm with peripheral T cell lymphoma NOS, hepatosplenic T cell lymphoma, extranodal NK/T cell lymphoma nasal type noted in descending sequence. In M-PTLDs, Ig G rearrangement in B cell lymphomas and clonal T cell receptor gene rearrangement in T cell lymphoma is present virtually in all cases.
Hodgkin’s Lymphoma—Hodgkin’s lymphoma is a rare type of PTLD, usually seen in post renal transplant recipients. The classical histological feature is presence of RS like cells in an inflammatory background. It is imperative to employ a panel of immunohistochemical markers to distinguish it from polymorphic PTLD/IM like PTLD in which EBV infected cells may show RS like features.
Diagnosis of PTLD
Early diagnosis of PTLDs requires a high index of suspicion as most of them manifest with nonspecific signs and symptoms, which may mimic more commoner complications like acute rejections after solid organ transplant or Graft versus host disease (GVHD) after HSCT. Hence, the first step towards timely diagnosis is a detailed physical examination. This has to be supported by various imaging studies and laboratory investigations.
The role of EBV in pathogenesis of PTLDs is well established, hence, serial monitoring of EBV load post SOT/HSCT is crucial to determine the risk for development of PTLD and initiate preemptive treatment. It is also necessary to differentiate an episode of acute rejection from PTLD, especially when there is involvement of the allograft. So, a step wise approach to detect EBV load during the post transplant period may prevent PTLDs. Various laboratory tests available are:
Serological markers: Antibody titers for IgG and IgM antibodies against antiviral capsid antigen, early antigen (EA) and Epstein Barr nuclear antigen are helpful to determine EBV infection in immunocompetent individuals [55]. But in immunocompromised patients, antibody serological titer monitoring is not a reliable investigation because of delayed humoral response. Also, as most of these transplant recipients are transfusion dependent, passive transfer of antibodies renders EBV IgG detection difficult. The role of serological assessment of EBV status is limited to pretransplant workup only.
Detection of EBV nuclei acids or proteins in tissue: EBV specific nuclei acid can be detected in tissue by in situ hybridization of EBV encoded small nuclear RNA (EBNA). This is a highly sensitive test as EBER is expressed in all stages of latency. Other EBV associated proteins that can be detected in paraffin embedded tissue using immunohistochemistry are EBNA1, EBNA2, LMP1, however, these are less sensitive than in situ hybridization [56, 57].
-
Viral load determination: Quantitative polymerase chain reaction (PCR) is recommended for detecting viral load in serum, plasma or whole blood.
Preemptive treatment to prevent PTLD occurrence is started if the EBV viral load is high [58–61]. The major drawback of this technique is lack of international consensus on frequency of performing the test and nonexistence of standard cutoffs for positivity.
The monitoring of EBV viral load should be part of any transplant centre as it can prevent overt PTLDs. However, non availability of molecular laboratories, high cost associated with frequent monitoring and technical expertise needed often limits its applicability.
Treatment of PTLD
Once the diagnosis of PTLD is established, the aim is to reduce the lymphoma disease bulk while preserving allograft function. This is achieved by use of systematic treatment approach that involves firstly reducing the immunosuppression. Depending on the disease type and response achieved, treatment with anti CD20 monoclonal antibody and chemotherapy, either in combination or sequentially is initiated. Patients with localized disease may be treated with other adjunct therapies like surgery or radiation. Use of newer modalities like EBV specific cytotoxic T lymphocytes is being explored.
Reduction of immunosuppression (RI) in PTLD: Reducing the immunosuppression has been the mainstay treatment for PTLDs. Most studies or case series on PTLD however document a variable response to RI alone. The response of patients to RI depends on large number of other factors like subtype of histological disease and localized versus disseminated disease. The optimal dose reduction is not standardized and certain guidelines recommend 25–50% reduction in calcineurin inhibitor and or replacement by mTOR inhibitors. RI may cause acute rejection flare up in the patient causing graft failure and hence its use in isolation has not shown much benefit [6, 62–64].
Rituximab monoclonal therapy: Chimeric anti CD20 monoclonal antibody has proven to be efficacious in most cases of B cell PTLDs either alone or in conjunction with CHOP regime. It is administered once a week for 4 weeks at a dosage of 375 mg/m2. This is followed by 4 cycles of CHOP therapy, in patients who don’t achieve complete remission with initial dosage of rituximab. Jeyanthi et al. conducted a detailed pooled analysis of available literature on use of rituximab as a single agent for PTLDs. They concluded that rituximab is highly efficacious in treating less aggressive PTLDs which don’t respond to RI alone [62, 65–67].
Chemotherapy: CHOP regime (cyclophosphamide, doxorubicin, vincristine, prednisone) is used to treat high grade Non-Hodgkin’s lymphoma along with Rituximab and RI. Although the complete remission rate is higher with these drugs up to 42–90% [68], it is fraught with higher treatment related mortality (TRM) mostly due to infectious complications. In spite of the major side effects, treatment with chemotherapeutic agents remains an important component of treatment paradigm for PTLD.
Adoptive Immunotherapy—EBV specific cytotoxic T lymphocytes are being employed to target the viral antigens expressed in EBV positive malignancies after both SOT and HSCT. The cytotoxic activity of these ex vivo T cells decreases from tumors expressing latency III to II to I. Latency III proteins are the ones which are expressed in severely immunocompromised individuals, HIV patients etc. Though it is a promising treatment modality, the major obstacle is the cost and time required to clone cell lines against recipient EBV latent proteins. It has shown promising results in PTLDs post HSCT patients as the disease is donor derived but experience in SOT is limited as the disease is recipient derived in SOT. The HLA matched unrelated cytotoxic lymphocytes have been used in multicentric trial successfully by Haque et al. [69–72].
Immunomodulatory/Cytokine therapy: Newer treatment therapy using immunomodulatory agents are still in very nascent stage and require more research in this field. Interferon alfa, IL-6 are the two cytokines proposed for use of PTLD treatment [73–76].
CNS disease: CNS PTLD is relatively a rare entity and as of now no standard treatment protocols are available. The recommended management guidelines include whole brain irradiation or use of high dose methotrexate as first line therapy, similar to what is used in immunocompetent patients with primary CNS lymphoma. However, these therapies are associated with severe neurological as well as systemic toxicity causing high morbidity [77]. Use of rituximab in such cases has been restricted as it does not cross blood brain barrier. However, Patrick et al. in their series of primary CNS PTLDs have shown the effectiveness of using high dose intravenous rituximab given in dose escalation protocol in these patients, leading to complete remission [78]. Newer agents like anti CD30 antibodies, checkpoint inhibitors, proteasome inhibitors and anti BTK antibodies with high efficacy and minimal side effects need to be explored to treat patients resistant to initial therapy [79].
Conclusion
PTLDs are a major cause of increased mortality and morbidity in patients of solid organ transplant. Deep insight in the pathogenesis of post transplant lymphomas over the years has considerably improved the diagnosis and management of this entity. However, PTLDs are rare diseases and hence a high index of suspicion on part of clinicians can help clinch the diagnosis in early stages and improve the outcome. Reduced immunosuppression along with additional chemotherapy and use of newer adoptive and immunomodulatory agents has definitely improved the outcome. Use of Rituximab in B cell NHL has been standardized however, multicentric collaborations and research involving gene sequencing and gene expression profile can provide better opportunities for targeted therapy. Efforts are also needed to formalize the use of newer biological agents like checkpoint inhibitors and in vitro expanded EBV specific CTLs as therapeutic modality for PTLDs.
Funding
None.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Devika Gupta, Email: devikalives5h@gmail.com.
Satish Mendonca, Email: drsatishmendonca@gmail.com.
Sushmita Chakraborty, Email: sushmita.pahari@gmail.com.
Tathagata Chatterjee, Email: ctathagata@hotmail.com.
References
- 1.Doak PB, Montgomerie JZ, North JD, Smith F. Reticulum cell sarcoma after renal homotransplantation and azathioprine and prednisone therapy. Br Med J. 1968;4:746–748. doi: 10.1136/bmj.4.5633.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penn I, Hammond W, Brettschneider L, Starzl TE. Malignant lymphomas in transplantation patients. Transpl Proc. 1969;1:106–112. [PMC free article] [PubMed] [Google Scholar]
- 3.Ghobrial IM, Habermann TM, Macon WR, et al. Differences between early and late posttransplant lymphoproliferative disorders in solid organ transplant patients: are they two different diseases? Transplantation. 2005;79:244–247. doi: 10.1097/01.tp.0000144335.39913.5c. [DOI] [PubMed] [Google Scholar]
- 4.Johnson LR, Nalesnik MA, Swerdlow SH. Impact of Epstein–Barr virus in monomorphic B cell posttransplant lymphoproliferative disorders: a histogenetic study. Am J Surg Pathol. 2006;30(12):1604–1612. doi: 10.1097/01.pas.0000213317.59176.d2. [DOI] [PubMed] [Google Scholar]
- 5.Nourse JP, Jones K, Gandhi MK. Epstein–Barr Virus-related post-transplant lymphoproliferative disorders: pathogenetic insights for targeted therapy. Am J Transpl. 2011;11(5):888–895. doi: 10.1111/j.1600-6143.2011.03499.x. [DOI] [PubMed] [Google Scholar]
- 6.Knight JS, Tsodikov A, Cibrik DM, Ross CW, Kaminski MS, Blayney DW. Lymphoma after solid organ transplantation: risk, response to therapy, and survival at a transplantation center. J Clin Oncol. 2009;27(20):3354–3362. doi: 10.1200/JCO.2008.20.0857. [DOI] [PubMed] [Google Scholar]
- 7.Taylor AL, Marcus R, Bradley JA. Post-transplant lymphoproliferative disorders (PTLD) after solid organ transplantation. Crit Rev Oncol Hematol. 2005;56:155–167. doi: 10.1016/j.critrevonc.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Cockfield SM. Identifying the patient at risk for post-transplant lymphoproliferative disorder. Transpl Infect Dis. 2001;3:70–78. doi: 10.1034/j.1399-3062.2001.003002070.x. [DOI] [PubMed] [Google Scholar]
- 9.Bates WD, Gray DW, Dada MA, et al. Lymphoproliferative disorders in oxford renal transplant recipients. J Clin Pathol. 2003;56:439–446. doi: 10.1136/jcp.56.6.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leblond V, Dhedin N, Mamzer Bruneel MF, Choquet S, Hermine O, Porcher R, et al. Identification of prognostic factors in 61 patients with posttransplantation lymphoproliferative disorders. J Clin Oncol. 2001;19(3):772–778. doi: 10.1200/JCO.2001.19.3.772. [DOI] [PubMed] [Google Scholar]
- 11.Ghobrial IM, Habermann TM, Maurer MJ, Geyer SM, Ristow KM, Larson TS, et al. Prognostic analysis for survival in adult solid organ transplant recipients with post-transplantation lymphoproliferative disorders. J Clin Oncol. 2005;23(30):7574–7582. doi: 10.1200/JCO.2005.01.0934. [DOI] [PubMed] [Google Scholar]
- 12.Dotti G, Fiocchi R, Motta T, Mammana C, Gotti E, Riva S, et al. Lymphomas occurring late after solid-organ transplantation: influence of treatment on the clinical outcome. Transplantation. 2002;74(8):1095–1102. doi: 10.1097/00007890-200210270-00007. [DOI] [PubMed] [Google Scholar]
- 13.Buell JF, Gross TG, Hanaway MJ, Trofe J, Roy-Chaudhury P, First MR, et al. Posttransplant lymphoproliferative disorder: significance of central nervous system involvement. Transpl Proc. 2005;37(2):954–955. doi: 10.1016/j.transproceed.2004.12.130. [DOI] [PubMed] [Google Scholar]
- 14.Penn I, Porat G. Central nervous system lymphomas in organ allograft recipients. Transplantation. 1995;59(2):240–244. [PubMed] [Google Scholar]
- 15.Cavaliere R, Petroni G, Lopes MB, Schiff D, International Primary Central Nervous System Lymphoma Collaborative G Primary central nervous system post-transplantation lymphoproliferative disorder: an International Primary Central Nervous System Lymphoma Collaborative Group report. Cancer. 2010;116(4):863–870. doi: 10.1002/cncr.24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boubenider S, Hiesse C, Goupy C, Kriaa F, Marchand S, Charpentier B. Incidence and consequences of post-transplantation lymphoproliferative disorders. J Nephrol. 1997;10(3):136–145. [PubMed] [Google Scholar]
- 17.Caillard S, Lelong C, Pessione F, Moulin B, French PTLD Working Group Post-transplant lymphoproliferative disorders occurring after renal transplantation in adults: report of 230 cases from the French registry. Am J Transpl. 2006;6:2735–2742. doi: 10.1111/j.1600-6143.2006.01540.x. [DOI] [PubMed] [Google Scholar]
- 18.Oton AB, Wang H, Leleu X, et al. Clinical and pathological prognostic markers for survival in adult patients with post-transplant lymphoproliferative disorders in solid transplant. Leuk Lymphoma. 2008;49:1738–1744. doi: 10.1080/10428190802239162. [DOI] [PubMed] [Google Scholar]
- 19.Kinch A, et al. Donor or recipient origin of posttransplant lymphoproliferative disorders following solid organ transplantation. Am J Transpl. 2014;14:2838–2845. doi: 10.1111/ajt.12990. [DOI] [PubMed] [Google Scholar]
- 20.Sanz J, et al. EBV-associated post-transplant lymphoproliferative disorder after umbilical cord blood transplantation in adults with hematological diseases. Bone Marrow Transpl. 2014;49:397–402. doi: 10.1038/bmt.2013.190. [DOI] [PubMed] [Google Scholar]
- 21.Doycheva I, Amer S, Watt KD. De novo malignancies after transplantation: risk and surveillance strategies. Med Clin North Am. 2016;100(3):551–567. doi: 10.1016/j.mcna.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348(17):1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 23.Mihalov ML, Gattuso P, Abraham K, Holmes EW, Reddy V. Incidence of post-transplant malignancy among 674 solid-organ-transplant recipients at a single center. Clin Transpl. 1996;10(3):248–255. [PubMed] [Google Scholar]
- 24.Sundin M, Le Blanc K, Ringden O, et al. The role of HLA mismatch, splenectomy and recipient Epstein–Barr virus seronegativity as risk factors in post-transplant lymphoproliferative disorder following allogeneic hematopoietic stem cell transplantation. Haematologica. 2006;91:1059–1067. [PubMed] [Google Scholar]
- 25.Landgren O, Gilbert ES, Rizzo JD, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113:4992–5001. doi: 10.1182/blood-2008-09-178046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juvonen E, Aalto SM, Tarkkanen J, et al. High incidence of PTLD after non-T-cell-depleted allogeneic haematopoietic stem cell transplantation as a consequence of intensive immunosuppressive treatment. Bone Marrow Transpl. 2003;32:97–102. doi: 10.1038/sj.bmt.1704089. [DOI] [PubMed] [Google Scholar]
- 27.Trofe J, et al. Analysis of factors that influence survival with post-transplant lymphoproliferative disorder in renal transplant recipients: the Israel Penn International Transplant Tumor Registry experience. Am J Transpl. 2005;5:775–780. doi: 10.1111/j.1600-6143.2005.00776.x. [DOI] [PubMed] [Google Scholar]
- 28.Sampaio MS, Cho YW, Shah T, Bunnapradist S, Hutchinson I. Impact of Epstein–Barr virus donor and recipient serostatus on the incidence of post-transplant lymphoproliferative disorder in kidney transplant recipients. Nephrol Dial Transpl. 2012;27:2971–2979. doi: 10.1093/ndt/gfr769. [DOI] [PubMed] [Google Scholar]
- 29.Allen U, Hébert D, Moore D, Dror Y, Wasfy S, the Canadian PTLD Survey Group—1998 (2001) Epstein–Barr virus-related post-transplant lymphoproliferative disease in solid organ transplant recipient, 1988–97: a Canadian multi-centre experience. Pediatr Transpl 5:198–203 [DOI] [PubMed]
- 30.Opelz G, Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transpl. 2004;4(2):222–230. doi: 10.1046/j.1600-6143.2003.00325.x. [DOI] [PubMed] [Google Scholar]
- 31.Lange A, Klimczak A, Dlubek D, Dybko J. B-cell lymphoproliferative syndrome and peripheral blood CD20+ cells expansion after hematopoietic stem cell transplantation: association with fludarabine and anti-thymocyte globulin containing conditioning regimen. Transpl Proc. 2003;35(8):3093–3095. doi: 10.1016/j.transproceed.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 32.Schubert S, Renner C, Hammer M, et al. Relationship of immunosuppression to Epstein–Barr viral load and lymphoproliferative disease in pediatric heart transplant patients. J Heart Lung Transpl. 2008;27(1):100–105. doi: 10.1016/j.healun.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 33.Caillard S, Dharnidharka V, Agodoa L, Bohen E, Abbott K. Posttransplant lymphoproliferative disorders after renal transplantation in the United States in era of modern immunosuppression. Transplantation. 2005;80(9):1233–1243. doi: 10.1097/01.tp.0000179639.98338.39. [DOI] [PubMed] [Google Scholar]
- 34.Duvoux C, Pageaux GP, Vanlemmens C, et al. Risk factors for lymphoproliferative disorders after liver transplantation in adults: an analysis of 480 patients. Transplantation. 2002;74(8):1103–1109. doi: 10.1097/00007890-200210270-00008. [DOI] [PubMed] [Google Scholar]
- 35.Opelz G, Naujokat C, Daniel V, Terness P, Dohler B. Disassociation between risk of graft loss and risk of non-Hodgkin lymphoma with induction agents in renal transplant recipients. Transplantation. 2006;81(9):1227–1233. doi: 10.1097/01.tp.0000219817.18049.36. [DOI] [PubMed] [Google Scholar]
- 36.Krams SM, Martinez OM. Epstein–Barr virus, rapamycin, and host immune responses. Curr Opin Organ Transpl. 2008;13(6):563–568. doi: 10.1097/MOT.0b013e3283186ba9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao S, Cox KL, Berquist W, et al. Long-term outcomes in pediatric liver recipients: comparison between cyclosporin A and tacrolimus. Pediatr Transpl. 1999;3(1):22–26. doi: 10.1034/j.1399-3046.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 38.Uhlin M, et al. Risk factors for Epstein–Barr virus-related post-transplant lymphoproliferative disease after allogeneic hematopoietic stem cell transplantation. Haematologica. 2014;99:346–352. doi: 10.3324/haematol.2013.087338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Babcock GJ, Hochberg D, Thorley-Lawson AD. The expression pattern of Epstein–Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity. 2000;13:497–506. doi: 10.1016/s1074-7613(00)00049-2. [DOI] [PubMed] [Google Scholar]
- 40.Langerak AW, Moreau E, van Gastel-Mol EJ, van der Burg M, van Dongen JJ. Detection of clonal EBV episomes in lymphoproliferations as a diagnostic tool. Leukemia. 2002;16:1572–1573. doi: 10.1038/sj.leu.2402519. [DOI] [PubMed] [Google Scholar]
- 41.Middeldorp JM, Brink AA, van den Brule AJ, Meijer CJ. Pathogenic roles for Epstein–Barr virus (EBV) gene products in EBV-associated proliferative disorders. Crit Rev Oncol Hematol. 2003;45:1–36. doi: 10.1016/s1040-8428(02)00078-1. [DOI] [PubMed] [Google Scholar]
- 42.Delecluse HJ, Bartnizke S, Hammerschmidt W, Bullerdiek J, Bornkamm GW. Episomal and integrated copies of Epstein–Barr virus coexist in Burkitt lymphoma cell lines. J Virol. 1993;67:1292–1299. doi: 10.1128/jvi.67.3.1292-1299.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohshima K, Suzumiya J, Kanda M, Kato A, Kikuchi M. Integrated and episomal forms of Epstein–Barr virus (EBV) in EBV associated disease. Cancer Lett. 1998;122:43–50. doi: 10.1016/s0304-3835(97)00368-6. [DOI] [PubMed] [Google Scholar]
- 44.Reisinger J, Rumpler S, Lion T, Ambros PF. Visualization of episomal and integrated Epstein–Barr virus DNA by fiber fluorescence in situ hybridization. Int J Cancer. 2006;118:1603–1608. doi: 10.1002/ijc.21498. [DOI] [PubMed] [Google Scholar]
- 45.Chaganti S, et al. Epstein–Barr virus colonization of tonsillar and peripheral blood B-cell subsets in primary infection and persistence. Blood. 2009;113:6372–6381. doi: 10.1182/blood-2008-08-175828. [DOI] [PubMed] [Google Scholar]
- 46.Petrara MR, Giunco S, Serraino D, Dolcetti R, De Rossi A. Post-transplant lymphoproliferative disorders: from epidemiology to pathogenesis-driven treatment. Cancer Lett. 2015;369(1):37–44. doi: 10.1016/j.canlet.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Luskin MR, Heil DS, Tan KS, et al. The impact of EBV status on characteristics and outcomes of posttransplantation lymphoproliferative disorder. Am J Transpl. 2015;15(10):2665–2673. doi: 10.1111/ajt.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimizu N, Tanabe-Tochikura A, Kuroiwa Y, Takada K. Isolation of Epstein–Barr virus (EBV)-negative cell clones from the EBV-positive Burkitt’s lymphoma (BL) line Akata: malignant phenotypes of BL cells are dependent on EBV. J Virol. 1994;68(9):6069–6073. doi: 10.1128/jvi.68.9.6069-6073.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ambinder RF. Gammaherpesviruses and “hit-and-run” oncogenesis. Am J Pathol. 2000;156(1):1–3. doi: 10.1016/S0002-9440(10)64697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Capello D, Rossi D, Gaidano G. Post-transplant lymphoproliferative disorders: molecular basis of disease histogenesis and pathogenesis. Hematol Oncol. 2005;23(2):61–67. doi: 10.1002/hon.751. [DOI] [PubMed] [Google Scholar]
- 51.Nelson BP, Nalesnik MA, Bahler DW, Locker J, Fung JJ, Swerdlow SH. Epstein–Barr virus-negative post-transplant lymphoproliferative disorders: a distinct entity? Am J Surg Pathol. 2000;24(3):375–385. doi: 10.1097/00000478-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Rinaldi A, Capello D, Scandurra M, et al. Single nucleotide polymorphism-arrays provide new insights in the pathogenesis of post-transplant diffuse large B-cell lymphoma. Br J Haematol. 2010;149(4):569–577. doi: 10.1111/j.1365-2141.2010.08125.x. [DOI] [PubMed] [Google Scholar]
- 53.Swerdlow SH (2017) World Health Organization. WHO classification of tumours of haematopoietic and lymphoid tissues. 5th, Lyon, France: International Agency for Research on Cancer. International Agency for Research on Cancer
- 54.Parker A, Bowles K, Bradley JA, Emery V, Featherstone C, Gupte G, et al. Diagnosis of posttransplant lymphoproliferative disorder in solid organ transplant recipients—BCSH and BTS Guidelines. Br J Haematol. 2010;149(5):675–692. doi: 10.1111/j.1365-2141.2010.08161.x. [DOI] [PubMed] [Google Scholar]
- 55.Carpentier L, Tapiero B, Alvarez F, et al. Epstein–Barr virus (EBV) early-antigen serologic testing in conjunction with peripheral blood EBV DNA load as a marker for risk of posttransplantation lymphoproliferative disease. J Infect Dis. 2003;188:1853–1864. doi: 10.1086/379834. [DOI] [PubMed] [Google Scholar]
- 56.Young L, Alfieri C, Hennessy K, et al. Expression of Epstein–Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N EngI J Med. 1989;321:1080–1085. doi: 10.1056/NEJM198910193211604. [DOI] [PubMed] [Google Scholar]
- 57.Fanaian N, Cohen C, Waldrop S, et al. EBER: automated in situ hybridization (ISH) vs. manual ISH and immunohistochemistry (IHC) for detection of EBV in pediatric lymphoproliferative disorders. Pediatr Dev Pathol. 2009;12:195–199. doi: 10.2350/07-07-0316.1. [DOI] [PubMed] [Google Scholar]
- 58.Hayden RT, Hokanson KM, Pounds SB, et al. Multicenter comparison of different real-time PCR assays for quantitative detection of Epstein–Barr virus. J Clin Microbiol. 2008;46:157–163. doi: 10.1128/JCM.01252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Preiksaitis JK, Pang XL, Fox JD, et al. Inter-laboratory comparison of Epstein–Barr virus (EBV) viral load assays. Am J Transpl. 2009;9:269–279. doi: 10.1111/j.1600-6143.2008.02514.x. [DOI] [PubMed] [Google Scholar]
- 60.Hakim H, Gibson C, Pan J, et al. Comparison of various blood compartments and reporting units for the detection and quantification of Epstein–Barr virus (EBV) in peripheral blood. J Clin Microbiol. 2007;45:2151–2155. doi: 10.1128/JCM.02308-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Paoli P, Pratesi C, Bortolin MT. The Epstein Barr virus DNA levels as a tumor marker in EBV-associated cancers. J Cancer Res Clin Oncol. 2007;133:809–815. doi: 10.1007/s00432-007-0281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghobrial IM, Habermann TM, Ristow KM, Ansell SM, Macon W, Geyer SM, et al. Prognostic factors in patients with post-transplant lymphoproliferative disorders (PTLD) in the rituximab era. Leuk Lymphoma. 2005;46(2):191–196. doi: 10.1080/10428190400012011. [DOI] [PubMed] [Google Scholar]
- 63.Swinnen LJ, Mullen GM, Carr TJ, Costanzo MR, Fisher RI. Aggressive treatment for postcardiac transplant lymphoproliferation. Blood. 1995;86(9):3333–3340. [PubMed] [Google Scholar]
- 64.Reshef R, Vardhanabhuti S, Luskin MR, Heitjan DF, Hadjiliadis D, Goral S, et al. Reduction of immunosuppression as initial therapy for posttransplantation lymphoproliferative disorder. Am J Transpl. 2011;11(2):336–347. doi: 10.1111/j.1600-6143.2010.03387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evens AM, David KA, Helenowski I, Nelson B, Kaufman D, Kircher SM, et al. Multicenter analysis of 80 solid organ transplantation recipients with post-transplantation lymphoproliferative disease: outcomes and prognostic factors in the modern era. J Clin Oncol. 2010;28(6):1038–1046. doi: 10.1200/JCO.2009.25.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trappe R, Oertel S, Leblond V, Mollee P, Sender M, Reinke P, et al. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012;13(2):196–206. doi: 10.1016/S1470-2045(11)70300-X. [DOI] [PubMed] [Google Scholar]
- 67.Ramanarayanan Jeyanthi, Krishnan Ganapathy S, Czuczman Myron S, Hernandez-Ilizaliturri Francisco J. Efficacy and safety of rituximab in post transplant lymphoproliferative disorders (PTLD): pooled analysis and review of literature. Blood. 2007;110:1084. [Google Scholar]
- 68.Evens AM, David KA, Helenowski I, et al. Multicenter analysis of 80 solid organ transplantation recipients with post-transplantation lymphoproliferative disease: outcomes and prognostic factors in the modern era. J Clin Oncol. 2010;28:1038–1046. doi: 10.1200/JCO.2009.25.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haque T, Wilkie GM, Jones MM, Higgins CD, Urquhart G, Wingate P, et al. Allogeneic cytotoxic T cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110(4):1123–1131. doi: 10.1182/blood-2006-12-063008. [DOI] [PubMed] [Google Scholar]
- 70.Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein–Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92(5):1549–1555. [PubMed] [Google Scholar]
- 71.Gustafsson A, Levitsky V, Zou JZ, Frisan T, Dalianis T, Ljungman P, et al. Epstein–Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood. 2000;95(3):807–814. [PubMed] [Google Scholar]
- 72.Gottschalk Stephen, Rooney Cliona. Adoptive T-cell immunotherapy. Curr Top Microbiol Immunol. 2015;391:427–454. doi: 10.1007/978-3-319-22834-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haddad E, Paczesny S, Leblond V, Seigneurin JM, Stern M, Achkar A, et al. Treatment of B-lymphoproliferative disorder with a monoclonal anti-interleukin-6 antibody in 12 patients: a multicenter phase 1–2 clinical trial. Blood. 2001;97(6):1590–1597. doi: 10.1182/blood.v97.6.1590. [DOI] [PubMed] [Google Scholar]
- 74.Davis CL, Wood BL, Sabath DE, Joseph JS, Stehman-Breen C, Broudy VC. Interferon-alpha treatment of posttransplant lymphoproliferative disorder in recipients of solid organ transplants. Transplantation. 1998;66(12):1770–1779. doi: 10.1097/00007890-199812270-00035. [DOI] [PubMed] [Google Scholar]
- 75.Komrokji RS, Oliva JL, Zand M, Felgar R, Abboud CN. Mini-BEAM and autologous hematopoietic stem cell transplant for treatment of post-transplant lymphoproliferative disorders. Am J Hematol. 2005;79(3):211–215. doi: 10.1002/ajh.20334. [DOI] [PubMed] [Google Scholar]
- 76.Williams KM, Higman MA, Chen AR, Schwartz CL, Wharam M, Colombani P, et al. Successful treatment of a child with late-onset T cell post-transplant lymphoproliferative disorder/lymphoma. Pediatr Blood Cancer. 2008;50(3):667–670. doi: 10.1002/pbc.21171. [DOI] [PubMed] [Google Scholar]
- 77.Cavaliere R, Petroni G, Lopes MB, Schiff D, The International Primary Central Nervous System Lymphoma Collaborative Group Primary central nervous system post-transplantation lymphoproliferative disorder. Cancer. 2010;116:863–870. doi: 10.1002/cncr.24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patrick A, Wee A, Hedderman A, Wilson D, Weiss J, Govani M. High-dose intravenous rituximab for multifocal, monomorphic primary central nervous system posttransplant lymphoproliferative disorder. J Neuro Oncol. 2011;103:739–743. doi: 10.1007/s11060-010-0425-0. [DOI] [PubMed] [Google Scholar]
- 79.Daan D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med. 2018;378:549–562. doi: 10.1056/NEJMra1702693. [DOI] [PubMed] [Google Scholar]