Abstract
2-Keto-d-gluconic acid (2KGA) is an important organic acid derived from d-glucose and is used to produce the food antioxidant erythorbic acid. To improve the 2KGA production performance and cell reusability, various carriers such as calcium alginate, k-carrageenan, chitosan, and poly(vinyl alcohol)-alginate were evaluated to immobilize Pseudomonas plecoglossicida JUIM01 resting cells. Calcium alginate was shown to be a suitable carrier since the immobilized cells had the highest number of reuse times and produced the highest 2KGA concentration of 171.77 g/L, with a productivity of 3.58 g/L·h and conversion ratio of 98.38%. The cell concentration, cultivation temperature, aeration rate and initial glucose concentration were further optimized in a 5-L airlift bioreactor to obtain the best 2KGA production performance by calcium alginate-immobilized P. plecoglossicida cells. Under the optimal conditions including a cell concentration of 4.0 g/L, glucose concentration of 126.0 g/L, temperature of 34 °C and aeration rate of 2.8 L/min, 134.45 g/L 2KGA was produced by alginate-immobilized P. plecoglossicida cells within 30 h, with a total productivity of 4.48 g/L·h and yield of 1.07 g/g (conversion ratio of over 99.0%). The immobilized cells maintained a stable conversion capacity after nine reuses and 25 days of storage at 4 °C, which indicated that calcium alginate immobilization of P. plecoglossicida cells had industrial practicability for 2KGA production.
Keywords: Pseudomonas plecoglossicida, 2-Keto-d-gluconic acid (2KGA), Immobilization, Calcium alginate, Airlift bioreactor
Introduction
2-Keto-d-gluconic acid (2KGA) is an important metabolite derived from d-glucose and is widely applied in the cosmetic, pharmaceutical and environmental industries (Li et al. 2016). Currently, 2KGA is the key intermediate for the synthesis of erythorbic acid (isoascorbic acid), which is a safe food additive approved by the Food and Drug Administration (FDA) and used in accordance with Good Manufacturing Practice (GMP) (Aguilar et al. 2016). 2KGA has been prepared by chemical, electrochemical, and biochemical oxidation of glucose or gluconic acid (Baś and Mlynarski 2016; Elseviers et al. 2000; Kokoh et al. 1993; Neidleman et al. 1982; Tanimura et al. 2003). Presently, chemical and enzymatic methods have not been used on an industrial scale mainly because of low volumetric productivity, low yield on glucose, and expensive metal catalysts or enzymes. Microbial fermentation has been proven to produce 2KGA from a high concentration of substrate glucose with a significant performance using Acetobacter species (Kulla et al. 1951), Enterobacter intermedium (Hwangbo et al. 2003), Gluconobacter oxydans (or Gluconobacter oxidans) (Li et al. 2016; Shi et al. 2015), Klebsiella pneumoniae (Wei et al. 2013; Sun et al. 2014), Pseudomonas aeruginosa (Chia et al. 2008), Pseudomonas fluorescens (Sun et al. 2012a, b, 2013, 2015) and Serratia marcescens (Misenheimer et al. 1965). For example, Pseudomonas fluorescens AR4 and Arthrobacter globiformis C224 can tolerate a high glucose concentration of over 140 g/L, with a 2KGA yield of 0.92 (mol/mol). These 2KGA producers have been used in most Chinese erythorbic acid companies (Sun et al. 2012a, 2013, 2015; Teng et al. 2013).
Various strategies including batch, fed-batch, semicontinuous and continuous fermentation have been developed to improve 2KGA production performance by P. fluorescens, A. globiformis, K. pneumoniae and engineered G. oxidans (Shi et al. 2015; Sun et al. 2012a, b, 2013; Teng et al. 2013). For example, a pH-dependent two-stage fermentation strategy was proposed to produce 186 g/L 2KGA by K. pneumoniae, with a conversion ratio of 0.98 (mol/mol) (Sun et al. 2014). By overexpressing the ga2dh gene, G. oxidans could produce approximately 486 g/L 2KGA from 480 g/L gluconic acid (Shi et al. 2015).
Generally, free cell systems have certain limitations, such as poor reusability and relatively rapid loss of activity (Yang et al. 2019). Immobilization of microbial cells has been regarded as a preferred solution to overcome these limitations by reducing substrate consumption, enhancing biocatalyst stability and facilitating its recovery and reuse (Yewale et al. 2016). Previously, most 2KGA producers consumed approximately 10% glucose, with a maximum 2KGA yield below 0.90 (mol/mol) (Sun et al. 2015). It is therefore deduced that in the 2KGA production process, the use of immobilized cells may provide a solution towards reducing the glucose consumption, increasing the 2KGA yield and providing cost savings. Pseudomonas plecoglossicida JUIM01 is another industrial 2KGA producer screened by our group and is currently used by most Chinese erythorbic acid companies. This strain fermented over 140 g/L glucose, with a 2KGA yield of 0.93 (mol/mol), using optimized media compositions of 16.5 g/L corn steep liquor and 0.015 g/L KH2PO4 (Sun et al. 2020). However, immobilization methods for 2KGA production are not available. To address the above issues, the present study attempted to (1) assess 2KGA production by P. plecoglossicida cells immobilized on various carriers, (2) optimize cultivation conditions of immobilized P. plecoglossicida cells with a 5-L airlift fermenter, and (3) check the feasibility of this process compared with the 2KGA batch production process.
Materials and methods
Microorganism and media
P. plecoglossicida JUIM01 was screened and stocked in our laboratory (Sun et al. 2018, 2019, 2020; Wang et al. 2018, 2019). The media for activating and stocking the strain consisted of the following (g/L): peptone 10.0, NaCl 5.0, beef extract 5.0 and agar 20.0, pH 7.0. The media for preparing seeds contained the following (g/L): glucose 20.0, urea 2.0, corn syrup powder 10.0, MgSO4·7H2O 0.5, KH2PO4 2.0 and CaCO3 0.3, pH 7.0. The bioconversion solutions contained 4.0 g/L free or immobilized resting cells, 162.0 g/L glucose and 45 g/L CaCO3.
Resting cell preparation
The seed culture (60 mL/500 mL Erlenmeyer flask) was cultivated at a temperature of 30 °C and rotator speed of 265 rpm for 20 h. Resting cells were harvested by removing the supernatants by centrifugation at 8000×g and 4 °C for 10 min, washed with sterile deionized (DI) water, and resuspended to an OD650 nm of 24.0 (approximate dry cell weight, DCW of 13.8 g/L) for immobilization.
Preparation of immobilized cells
Resting cells of P. plecoglossicida JUIM01 were immobilized by separately using calcium alginate, k-carrageenan, chitosan beads, and poly(vinyl alcohol)-alginate carriers. Briefly, 3.0% (w/v) sodium alginate and 3.0% (w/v) CaCO3 were added to DI water and then mixed with the collected resting cells. The mixture was extruded as small drops through a syringe into a 4.0% (w/v) CaCl2 solution to form beads. The beads were hardened for 12 h at 4 °C and preserved in sterile DI water until use.
The entrapment in k-carrageenan was conducted by adding resting cells to a 4.0% (w/v) sterilized k-carrageenan solution at 55 °C. The suspension was extruded as small drops with a syringe into a stirred solution containing 20 g/L KCl and 0.2 g/L CaCl2 at 4 °C. The beads were collected after 30 min, washed with a 0.9% (w/v) sterile NaCl solution, preserved in a 0.075 M Al(NO3)3 solution for 5 min and washed twice before use.
The immobilization procedure of cells onto chitosan beads was performed by mixing the resting cells with a 4.0% (w/v) chitosan solution and dripping the mixture into a stirred 1.0% (w/v) sodium tripolyphosphate (TPP) solution (pH 9.0) for bead formation. The chitosan solution was prepared with 1.0% acetic acid in DI water. The immobilized beads were collected after 40 min, washed three times with a 0.9% (w/v) sterile NaCl solution, kept in a 0.2% (w/v) sterilized glutaraldehyde solution for 1.0 h and washed three times with a NaCl solution for further use.
The P. plecoglossicida resting cells were entrapped in poly(vinyl alcohol)-alginate by mixing with a solution containing 6% (w/v) poly(vinyl alcohol) (PVA) and 3% (w/v) sodium alginate. Then, the mixture was extruded as small drops through a syringe into a 4% (w/v) CaCl2 solution to form beads. The immobilized beads were collected after curing for 4 h and then washed three times with 0.05 M phosphate buffer (pH 7.0) for further use.
The reuse of the above immobilized cells was carried out by removing the broth through a mesh sieve (No. 35, Sigma-Aldrich., St. Louis, USA) to avoid washing out the carrier particles and feeding with fresh bioconversion media containing 162.0 g/L glucose and 45 g/L CaCO3.
Scanning electron microscopy
Cell-entrapping calcium alginate beads were cut with a sharp blade. Scanning electron microscopy (SEM) analysis was conducted by pre-fixing the specimens with 2.5% (v/v) glutaraldehyde at 4 °C for 1 h, fixing them in 1% OsO4 at 4 °C for 1 h and dehydrating them in increasing concentrations of ethanol (60, 70, 80, 90 and 100%, v/v). The images were obtained using a Hitachi Model TM 3000 scanning electron microscope (Hitachi, Tokyo, Japan) operated under high vacuum with an acceleration voltage of 15 kV and a filament current of 1850 mA.
Performance evaluation studies in a bench fermenter
The bioconversion fermentation was also carried out in a 5-L GRYB-5D airlift fermenter equipped with full automatic control systems (GRCB, Green Bio-engineering Co., Ltd, Zhenjiang, China) under the following conditions: working medium volume of 3.5 L, inoculation volume of 10% (v/v), culture temperature of 30 °C and aeration rate of 3.5 L/min. The experiments continued until the 2KGA content reached its highest values. Samples were withdrawn every 8 h for testing the pH and the 2KGA, residual glucose and cell concentrations.
Analytical methods
The produced 2KGA was determined using HPLC. Briefly, the bioconversion solution was centrifuged at 8000×g for 20 min. The supernatant (50 μL) was subjected to HPLC (Thermo, Massachusetts, USA) equipped with a Hypersil SAX column (4.6 mm × 150 mm, 5 μm, Thermo, Massachusetts, USA). The mobile phase was a KH2PO4 (pH 3.0) solution at a 1.0 mL/min flow rate. The products were identified by their typical retention times using a calcium 2-keto-gluconate standard (Sigma-Aldrich, St. Louis, USA) as the reference.
The residual glucose concentration was determined with a biosensor analyzer (Shandong Academy of Sciences Institute of Biology, Jinan, China) at 25 °C. The cell concentration in immobilized carriers was determined by the initial concentrations of free P. plecoglossicida cells minus the concentrations of free cells after immobilization. The free P. plecoglossicida cell concentration was calculated by optical density (OD650 nm) (Biospec-1601 spectrophotometer, Shimadzu, Kyoto, Japan). An OD650 nm of 1.0 was equivalent to 0.575 g DCW/L by computing with a standard curve equation (DCW (g/L) = 0.595 OD650 nm-0.020) relating OD650 nm and dry cell weight (DCW).
The performance of 2KGA production was evaluated based on the 2KGA concentration (g/L), productivity (g/L·h), yield to glucose (g/g), and conversion ratio (mol/mol) of glucose to 2KGA. 2KGA productivity was defined as the amount of 2KGA produced per hour per litre. The 2KGA yield was calculated by dividing the amount of 2KGA produced (g) by the amount of glucose consumed (g). The conversion ratio (%) was calculated by dividing the amount of 2KGA produced (mol) by the amount of glucose consumed (mol) and multiplying by 100%, and the theoretical value should be 100%.
Statistical analysis
Each experiment was repeated three times using duplicate samples. The results were expressed as the means ± standard deviations and statistically analyzed as described in the literature (Abol-Fotouh et al. 2020). Statistical comparisons were made by one-way analysis of variance (ANOVA), followed by Duncan’s multiple-comparison test using the SAS System (SAS Institute, Cary, NC, USA). Differences were considered significant when the p-values were < 0.05.
Results and discussion
Effect of carrier type on 2KGA production performance by immobilized P. plecoglossicida cells
Gels and gel-like materials including natural (such as alginate, j-carrageenan, agarose, agar and chitosan) and synthetic (such as polyacrylamide, polyacrylate and polyurethane) materials are common carriers for cell immobilization via gel entrapment (Sekoai et al. 2018; Yang et al. 2019; Zdarta et al. 2018). The bead size and porosity directly affect the entrapped cells and catalytic activities. In the present study, calcium alginate, k-carrageenan, chitosan beads, and poly(vinyl alcohol)-alginate were used to entrap the resting cells of P. plecoglossicida JUIM01. K-carrageenan, calcium alginate and chitosan beads had similar sizes with diameters of 2.03 ± 0.06, 2.54 ± 0.10 and 2.66 ± 0.06 mm, respectively, and porosities of 11.97 ± 1.56%, 10.65 ± 1.10% and 9.00 ± 1.06%. However, PVA-alginate beads had a large size with a diameter of 3.33 ± 0.07 mm and a low porosity of 5.90 ± 0.65%.
Table 1 shows the performance of four different immobilized beads on converting glucose to 2KGA compared with that of free resting cells of P. plecoglossicida JUIM01. Free resting cells had the lowest number of reuse times of 3 and produced a 2KGA concentration of 172.59 g/L from 162.00 g/L glucose, with a productivity of 3.62 g/L·h and yield of 1.0715 g/g. K-carrageenan-immobilized cells could be reused seven times and showed a low 2KGA production performance, with a productivity of 3.03 g/L·h, which decreased by 15% compared to that with free resting cells. K-carrageenan tends to solidify at temperatures below 55 °C, which would affect cell viability and activity during bead preparation. In addition, k-carrageenan beads were easily dissolved after long-term use, which resulted in low number of reuse times. Compared to the free cells, chitosan- and alginate-entrapped cells showed similar catalytic activities, regardless of the 2KGA concentration, productivity or yield. However, the number of reuse times of the chitosan beads was only 5, which was limited by their low mechanical strength. Cells immobilized with alginate and PVA-alginate had the highest number of reuse times of 10. Alginate appeared to be the most suitable carrier to immobilize P. plecoglossicida cells, with the highest 2KGA concentration of 171.77 g/L, productivity of 3.58 g/L·h, and conversion ratio of 98.38% (mol/mol) compared to those using other carriers.
Table 1.
2KGA production performance of P. plecoglossicida JUIM01 cells immobilized with different carriers and free cells
| Cell types | Cell concentration (g/L) | Time of the conversion reaction (h) | Initial glucose concentration (g/L) | Residual glucose concentration (g/L) | 2KGA concentration (g/L) | Productivity (g/L·h) | Yield (g/g) | Conversion ratio (%) | Reuse times |
|---|---|---|---|---|---|---|---|---|---|
| Cells immobilized with carrageenan | 4.0 | 56 | 162.00 | 0.08 ± 0.03 | 169.46 ± 2.83 | 3.03 ± 0.05 | 1.0460 ± 0.0175 | 97.06 ± 1.62 | 7 |
| Cells immobilized with alginate | 4.0 | 48 | 162.00 | 0.07 ± 0.02 | 171.77 ± 3.06 | 3.58 ± 0.06 | 1.0603 ± 0.0189 | 98.38 ± 1.75 | 10 |
| Cells immobilized with PVA-alginate | 4.0 | 52 | 162.00 | 0.12 ± 0.02 | 169.52 ± 1.94 | 3.26 ± 0.04 | 1.0464 ± 0.0120 | 97.09 ± 1.11 | 10 |
| Cells immobilized with chitosan | 4.0 | 48 | 162.00 | 0.04 ± 0.01 | 171.03 ± 2.56 | 3.56 ± 0.05 | 1.0557 ± 0.0158 | 97.96 ± 1.46 | 5 |
| Free cells | 4.0 | 48 | 162.00 | 0.03 ± 0.00 | 173.59 ± 1.24 | 3.62 ± 0.03 | 1.0715 ± 0.0077 | 99.42 ± 0.71 | 3 |
Values are expressed as mean ± SD
Scanning electron microscopy (SEM) (Fig. 1) showed the inner morphology and P. plecoglossicida cells entrapped in the alginate cryogel. The cryogels were observed to be highly porous, with large numbers of holes that could easily entrap cells and allow transfer of the substrate glucose and product 2KGA. P. plecoglossicida cells adhered to the carriers, with no significant morphological changes, and were uniformly distributed within the cryogel matrix, indicating proper mixing of the cell suspension and calcium alginate in the composite prior to polymerization.
Fig. 1.
Scanning electron microscopy (SEM) micrographs of immobilized P. plecoglossicida cells on a calcium alginate matrix with the following magnifications: a 22,000 × and b 60,000 × . Scale bars: a 2.0 μm and b 0.5 μm
Effect of the cell concentration on the 2KGA production performance by immobilized P. plecoglossicida cells
P. plecoglossicida cells contained two membrane-bound enzymes glucose dehydrogenase (GDH) and gluconate dehydrogenase (GADH), which consecutively convert glucose to 2KGA (). Hence, the cell concentrations in the reaction system directly affected the catalyst amount, bioconversion speed and efficiency. As summarized in Table 2, the cell concentration of 2.5 g/L had the lowest 2KGA production performance, with a concentration of 164.97 g/L and productivity of 2.95 g/L·h after 56 h of cultivation as well as a theoretical yield of 94.48%. Increasing the cell concentration to 4.0 g/L resulted in a shorter catalytic time of 48 h, with the highest 2KGA concentration of 171.91 g/L, productivity of 3.58 g/L·h, and yield of 1.06 g/g. However, further increasing the cell concentration to 5.5 and 7.0 g/L showed a decreased bioconversion performance, with 2KGA concentrations below 170 g/L. Thus, 4.0 g/L was selected as the optimal P. plecoglossicida cell concentration for the present study.
Table 2.
Effect of cell concentration on 2KGA production performance by immobilized P. plecoglossicida cells
| Cell concentration (g/L) | Time of the conversion reaction (h) | Initial glucose concentration (g/L) | Residual glucose concentration (g/L) | 2KGA concentration (g/L) | Productivity (g/L·h) | Yield (g/g) | Conversion ratio (%) |
|---|---|---|---|---|---|---|---|
| 2.5 | 56 | 162.00 | 0.13 ± 0.02 | 164.97 ± 2.70 | 2.95 ± 0.05 | 1.0183 ± 0.0166 | 94.48 ± 1.54 |
| 4.0 | 48 | 162.00 | 0.10 ± 0.01 | 171.91 ± 2.39 | 3.58 ± 0.05 | 1.0612 ± 0.0148 | 98.46 ± 1.37 |
| 5.5 | 48 | 162.00 | 0.07 ± 0.02 | 168.46 ± 0.60 | 3.51 ± 0.01 | 1.0399 ± 0.0037 | 96.48 ± 0.34 |
| 7.0 | 48 | 162.00 | 0.11 ± 0.03 | 167.98 ± 1.76 | 3.50 ± 0.04 | 1.0369 ± 0.0109 | 96.21 ± 1.01 |
Values are expressed as mean ± SD
Effect of the reaction temperature on the 2KGA production performance by immobilized P. plecoglossicida cells
Generally, temperature significantly affects the activities of glucose dehydrogenase (GDH) and gluconate dehydrogenase (GADH) by accelerating the reaction speed at the optimal temperature or inhibiting/slowing the catalytic reaction due to enzyme structure denaturation at higher temperatures (Daniel and Danson 2013). In most cases, immobilization could improve the cell thermostability to some degree compared to that of free cells. Table 3 shows the effect of six temperatures ranging from 26 to 46 °C on 2KGA production with alginate-immobilized P. plecoglossicida cells. The 2KGA production performance was the highest at a temperature of 34 °C, with a concentration of 173 g/L, productivity of 3.94 g/L·h and yield of 1.074 g/g. Interestingly, an increase in temperature to 42 °C also showed a high 2KGA concentration of 167.03 g/L, productivity of 3.21 g/L·h and yield of 1.03 g/g, which meant that the immobilized cells showed better activity over a broader temperature range than that of free cells. Hence, it could be concluded that immobilized P. plecoglossicida cells had a broad reaction temperature of 26–42 °C, which favors industrial applications with fluctuating operation temperatures.
Table 3.
Effect of temperature on 2KGA production performance by immobilized P. plecoglossicida cells
| Temperature (℃) | Time of the conversion reaction (h) | Initial glucose concentration (g/L) | Residual glucose concentration (g/L) | 2KGA concentration (g/L) | Productivity (g/L·h) | Yield (g/g) | Conversion ratio (%) |
|---|---|---|---|---|---|---|---|
| 26 | 52 | 162.00 | 0.14 ± 0.04 | 169.84 ± 1.71 | 3.27 ± 0.03 | 1.0484 ± 0.0106 | 97.27 ± 0.98 |
| 30 | 48 | 162.00 | 0.09 ± 0.03 | 171.65 ± 1.54 | 3.58 ± 0.03 | 1.0596 ± 0.0095 | 98.31 ± 0.88 |
| 34 | 44 | 162.00 | 0.13 ± 0.03 | 173.51 ± 1.56 | 3.94 ± 0.04 | 1.0710 ± 0.0096 | 99.38 ± 0.89 |
| 38 | 48 | 162.00 | 0.08 ± 0.03 | 168.57 ± 3.71 | 3.51 ± 0.08 | 1.0406 ± 0.0229 | 96.55 ± 2.13 |
| 42 | 52 | 162.00 | 0.11 ± 0.03 | 167.03 ± 2.67 | 3.21 ± 0.05 | 1.0310 ± 0.0165 | 95.66 ± 1.53 |
| 46 | 64 | 162.00 | 0.11 ± 0.03 | 158.94 ± 3.16 | 2.48 ± 0.05 | 0.9811 ± 0.0195 | 91.03 ± 1.81 |
Values are expressed as mean ± SD
Effect of the aeration rate on the 2KGA production performance by immobilized P. plecoglossicida cells
Dissolved oxygen plays a key role in 2KGA production since the two consecutive synthesis steps involve oxidation reactions mediated by GDH and GADH. Aeration rates from 2.1 to 4.2 L/min were used to investigate their effect on the 2KGA production performance by immobilized P. plecoglossicida cells in a 5-L airlift bioreactor with a 3.5-L working volume. Compared to traditional stirred-tank fermenters, the airlift bioreactor has a simpler interior structure without a stirrer, which minimizes shear forces and protects immobilized beads from mechanical damage. As shown in Table 4, the 2.1 L/min aeration rate resulted in the lowest 2KGA concentration of 169.04 g/L, productivity of 3.25 g/L·h and yield of 1.04 g/g after 52 h of reaction. An increase in the aeration rate benefited the 2KGA production performance. An aeration rate of 3.5 L/min resulted in a maximum 2KGA concentration of 172.5 g/L, productivity of 3.59 g/L·h and yield of 1.06 g/g after 48 h of reaction. Interestingly, compared to the 3.5 L/min aeration rate, the 2.8 L/min aeration rate resulted in a slightly lower 2KGA concentration of 171.60 g/L, productivity of 3.58 g/L·h and yield of 1.06 g/g. For the large-scale production of commodity products, the aeration rate, oxygen uptake rate and vessel size have significant capital and operating costs (Humbird et al. 2017). Hence, an aeration rate of 2.8 L/min was acceptable for 2KGA production in the 5-L airlift bioreactor.
Table 4.
Effect of aeration rate on 2KGA production performance by immobilized P. plecoglossicida cells
| Aeration (L/min) | Time of the conversion reaction (h) | Initial glucose concentration (g/L) | Residual glucose concentration (g/L) | 2KGA concentration (g/L) | Productivity (g/L·h) | Yield (g/g) | Conversion ratio (%) |
|---|---|---|---|---|---|---|---|
| 2.1 | 52 | 162.00 | 0.11 ± 0.04 | 169.04 ± 3.09 | 3.25 ± 0.06 | 1.0435 ± 0.0190 | 96.82 ± 1.77 |
| 2.8 | 48 | 162.00 | 0.13 ± 0.03 | 171.60 ± 3.77 | 3.58 ± 0.08 | 1.0593 ± 0.0233 | 98.28 ± 2.16 |
| 3.5 | 48 | 162.00 | 0.09 ± 0.02 | 172.50 ± 2.64 | 3.59 ± 0.05 | 1.0648 ± 0.0163 | 98.80 ± 1.51 |
| 4.2 | 48 | 162.00 | 0.08 ± 0.02 | 172.35 ± 2.42 | 3.59 ± 0.05 | 1.0639 ± 0.0149 | 98.71 ± 1.39 |
Values are expressed as mean ± SD
Effect of the initial glucose concentration on the 2KGA production performance by immobilized P. plecoglossicida cells
Glucose concentrations ranging from 54.0 to 234 g/L were used to investigate their effect on 2KGA production and glucose utilization by alginate-immobilized P. plecoglossicida cells. As presented in Table 5, an increase in the glucose concentration prolonged the 2KGA production time and productivity. The maximum 2KGA production performance with the highest productivity of 4.18 g/L·h and yield of 1.06 g/g could be observed with a glucose concentration of 126.0 g/L after 32 h of bioconversion. The glucose concentration of 234.0 g/L led to the highest 2KGA concentration of 240.93 g/L, while the productivity and yield decreased to 2.51 g/L·h and 1.02 g/g, respectively. As a consequence, a glucose concentration of 126.0 g/L appeared to be optimal for 2KGA production by alginate-immobilized P. plecoglossicida cells.
Table 5.
Effect of initial glucose concentration on 2KGA production performance by immobilized P. plecoglossicida cells
| Initial glucose concentration (g/L) | Time of the conversion reaction (h) | Residual glucose concentration (g/L) | 2KGA concentration (g/L) | Productivity (g/L·h) | Yield (g/g) | Conversion ratio (%) |
|---|---|---|---|---|---|---|
| 54.00 | 16 | 0.03 ± 0.01 | 55.87 ± 1.65 | 3.49 ± 0.10 | 1.0346 ± 0.0305 | 96.00 ± 2.83 |
| 90.00 | 24 | 0.11 ± 0.02 | 95.54 ± 1.14 | 3.98 ± 0.05 | 1.0616 ± 0.0127 | 98.49 ± 1.18 |
| 126.00 | 32 | 0.09 ± 0.03 | 133.84 ± 2.34 | 4.18 ± 0.07 | 1.0622 ± 0.0185 | 98.56 ± 1.72 |
| 162.00 | 48 | 0.07 ± 0.01 | 171.88 ± 2.21 | 3.58 ± 0.05 | 1.0610 ± 0.0137 | 98.44 ± 1.27 |
| 198.00 | 68 | 0.05 ± 0.02 | 208.16 ± 4.08 | 3.06 ± 0.06 | 1.0513 ± 0.0206 | 97.54 ± 1.91 |
| 234.00 | 96 | 0.15 ± 0.04 | 240.93 ± 6.32 | 2.51 ± 0.07 | 1.0296 ± 0.0270 | 95.53 ± 2.51 |
Values are expressed as mean ± SD
2KGA production under optimal conditions by immobilized P. plecoglossicida cells
On the basis of the above results, the optimal conditions for 2KGA production by calcium alginate-immobilized P. plecoglossicida cells, including a cell concentration of 4.0 g/L, glucose concentration of 126.0 g/L, temperature of 34 °C and aeration rate of 2.8 L/min, were used to verify the feasibility in a 5-L fermenter. As shown in Fig. 2, under the optimal conditions, the relative dissolved oxygen (DO) concentration rapidly decreased to approximately 3% at the beginning of the 4-h reaction, remained at a low level of below 30% from the 4th to 20th h, and gradually increased to 80%. Within the 30-h bioconversion process, the glucose concentration gradually decreased to 0.14 g/L and the 2KGA concentration increased to 134.45 g/L, with a total productivity of 4.48 g/L·h and yield of 1.07 g/g (conversion ratio of over 99.0%).
Fig. 2.
Time courses of 2KGA production with calcium alginate-immobilized P. plecoglossicida JUIM01 cells under optimal conditions (cell concentration of 4.0 g/L, initial glucose concentration of 126.0 g/L, temperature of 34 °C and aeration rate of 2.8 L/min) (filled circle: glucose; open triangle: 2KGA determined by HPLC; open square: dissolved oxygen concentration)
Reusability and storage at 4 °C of immobilized cells under the optimal conditions
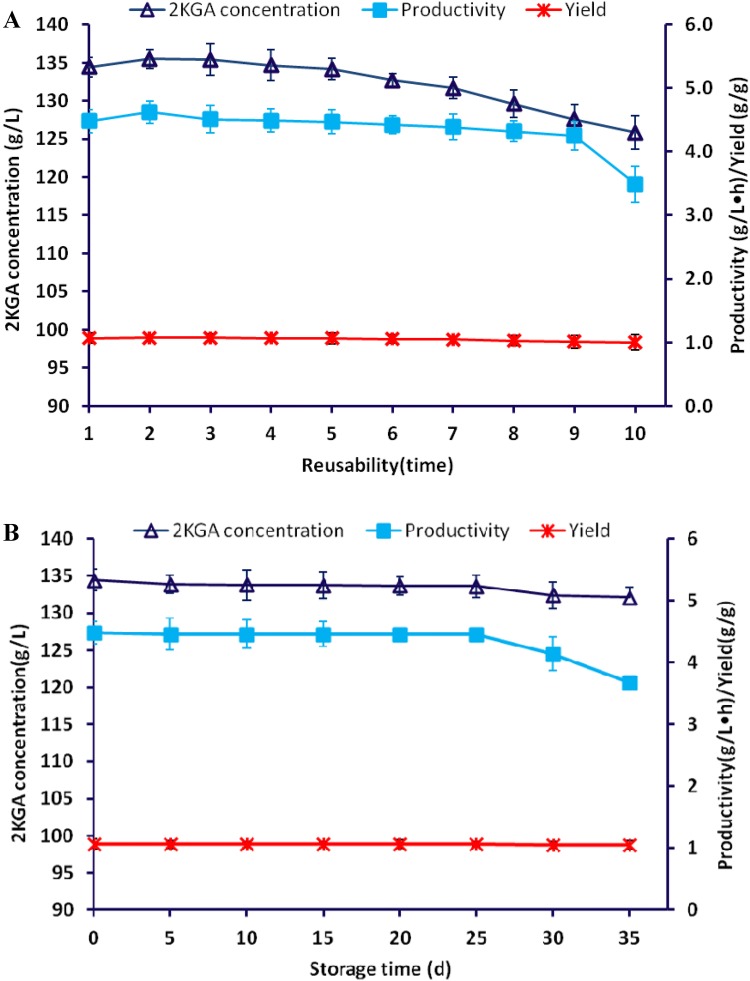
Immobilized cell technology has several advantages including high cell density, remarkable stability, reusability and cost savings (Hua et al. 2019). Among these advantages, reusability is the key factor in evaluating process economics. Hence, after each 30-h cycle under the optimal conditions, the culture broth and immobilized cells were separated using a mesh sieve (No. 35) to avoid washing out the carrier particles. The filtered beads were washed with sterile DI water, and fresh culture broth was fed into fermenter each cycle for 10 recycles. As shown in Fig. 3a, after nine reuses, the 2KGA production remained significant, with stable 2KGA production of 127.56 g/L, productivity of 4.25 g/L·h and yield of 1.02 g/g, which indicated that the immobilization of P. plecoglossicida cells on calcium alginate benefited recovery and reduced cell loss.
Fig. 3.
Effects of number of reuse times (a) and storage time (b) with calcium alginate-immobilized P. plecoglossicida JUIM01 cells on 2KGA production under the following conditions: cell concentration of 4.0 g/L, initial glucose concentration of 126.0 g/L, temperature of 34 °C and aeration rate of 2.8 L/min
For continuous recycling of immobilized P. plecoglossicida cells, the effect of storage time for 35 days at 4 °C on the 2KGA production performance was evaluated. As shown in Fig. 3b, the stored immobilized cells showed a stable 2KGA production capacity, with a productivity of 4.45 g/L·h and yield of 1.06 g/g over 25 days of storage. The 2KGA productivity and yield began to decrease to 4.14 g/L and 1.05 g/g, respectively, after 30 days of storage. The 25-day stability of immobilized P. plecoglossicida cells using calcium alginate as a carrier will contribute to the industrial practicability of 2KGA production.
Conclusions
An industrially feasible process for high 2KGA production performance was proposed by immobilizing P. plecoglossicida cells using calcium alginate as the carrier. Under the optimal conditions including a cell concentration of 4.0 g/L, glucose concentration of 126.0 g/L, temperature of 34 °C and aeration rate of 2.8 L/min in a 5-L airlift fermenter, the immobilized cells stored for 25 days maintained a stable 2KGA production capacity, with a concentration of 133.6 g/L, productivity of 4.45 g/L·h and yield of 1.06 g/g. In conclusion, P. plecoglossicida immobilization could provide a potentially cost-effective and practical approach for 2KGA bioproduction on an industrial scale due to its ease of handling, low energy inputs, decreased production time and increased 2KGA production performance.
Author contributions
W.-J.S. conceived of the study, participated in its design and coordination, and drafted the manuscript. Z.-L.H and L.S performed experiments, analyzed data, and helped to draft the manuscript. D.-M.W., F.-J.C and S.-L.Y performed partial experiments and analyzed data. All authors read and approved the manuscript.
Funding
This work was funded by the Natural Science Foundation of China (31571885), Science and Technology Platform Construction Program of Jiangxi Province (2010DTZ01900), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Zhiliang Hou and Lei Sun are the co-first authors and have the equal contributions.
References
- Abol-Fotouh D, Hassan MA, Shokry H, et al. Bacterial nanocellulose from agro-industrial wastes: low-cost and enhanced production by Komagataeibacter saccharivorans MD1. Sci Rep. 2020;10:3491. doi: 10.1038/s41598-020-60315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar F, Crebelli R, Domenico AD, et al. Scientific opinion on the re-evaluation of erythorbic acid (E 315) and sodium erythorbate (E 316) as food additives. EFSA J. 2016;14(1):4360. [Google Scholar]
- Baś S, Mlynarski J. Synthesis of 2-keto-d-and l-gluconic acid via stereoselective direct aldol reactions. J Org Chem. 2016;81(14):6112–6117. doi: 10.1021/acs.joc.6b01068. [DOI] [PubMed] [Google Scholar]
- Chia M, Van Nguyen TB, Choi WJ. DO-stat fed-batch production of 2-keto-d-gluconic acid from cassava using immobilized Pseudomonas aeruginosa. Appl Microbiol Biotechnol. 2008;78(5):759–765. doi: 10.1007/s00253-008-1374-9. [DOI] [PubMed] [Google Scholar]
- Daniel RM, Danson MJ. Temperature and the catalytic activity of enzymes: a fresh understanding. FEBS Lett. 2013;587(17):2738–2743. doi: 10.1016/j.febslet.2013.06.027. [DOI] [PubMed] [Google Scholar]
- Elseviers M, Coomans SMJ, Lemmens HOJ et al (2000) Process for the production of 2-keto-d-gluconic acid: U.S. Patent 6,018,034. 2000-1-25
- Hua X, Du GL, Xu Y. Cost-practical of glycolic acid bioproduction by immobilized whole-cell catalysis accompanied with compressed oxygen supplied to enhance mass transfer. Bioresour Technol. 2019;283:326–331. doi: 10.1016/j.biortech.2019.03.094. [DOI] [PubMed] [Google Scholar]
- Humbird D, Davis R, McMillan JD. Aeration costs in stirred-tank and bubble column bioreactors. Biochem Eng J. 2017;127:161–166. [Google Scholar]
- Hwangbo H, Park RD, Kim YW, et al. 2-Ketogluconic acid production and phosphate solubilization by Enterobacter intermedium. Curr Microbiol. 2003;47(2):0087–0092. doi: 10.1007/s00284-002-3951-y. [DOI] [PubMed] [Google Scholar]
- Kokoh KB, Parpot P, Belgsir EM, et al. Selective oxidation of d-gluconic acid on platinum and lead adatoms modified platinum electrodes in alkaline medium. Electrochim Acta. 1993;38(10):1359–1365. [Google Scholar]
- Kulka D, Hall AN, Walker TK. Formation of 2-keto-d-gluconic acid, 5-keto-d-gluconic acid, and tartronic acid by Acetobacter species. Nature. 1951;167(4257):905–906. doi: 10.1038/167905b0. [DOI] [PubMed] [Google Scholar]
- Li K, Mao X, Liu L, et al. Overexpression of membrane-bound gluconate-2-dehydrogenase to enhance the production of 2-keto-d-gluconic acid by Gluconobacter oxydans. Microb Cell Fact. 2016;15(1):121. doi: 10.1186/s12934-016-0521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misenheimer TJ, Anderson RF, Lagoda AA, et al. Production of 2-ketogluconic acid by Serratia marcescens. Appl Environ Microbiol. 1965;13(3):393–396. doi: 10.1128/am.13.3.393-396.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidleman SL, Amon Jr WF, Geigert J (1982) Production of 2-keto-d-gluconic acid and hydrogen peroxide: U.S. Patent 4,351,902. 1982-9-28.
- Sekoai PT, Awosusi AA, Yoro KO, et al. Microbial cell immobilization in biohydrogen production: a short overview. Crit Rev Biotechnol. 2018;38(2):157–171. doi: 10.1080/07388551.2017.1312274. [DOI] [PubMed] [Google Scholar]
- Shi Y, Li K, Lin J, et al. Engineered expression vectors significantly enhanced the production of 2-keto-d-gluconic acid by Gluconobacter oxidans. J Agric Food Chem. 2015;63(22):5492–5498. doi: 10.1021/acs.jafc.5b01652. [DOI] [PubMed] [Google Scholar]
- Sun WJ, Liu CF, Yu L, et al. A novel bacteriophage KSL-1 of 2-keto-gluconic acid producer Pseudomonas fluorescens K1005: isolation, characterization and its remedial action. BMC Microbiol. 2012;12(1):127. doi: 10.1186/1471-2180-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WJ, Zhou YZ, Zhou Q, et al. Semi-continuous production of 2-keto-gluconic acid by Pseudomonas fluorescens AR4 from rice starch hydrolysate. Bioresour Technol. 2012;110:546–551. doi: 10.1016/j.biortech.2012.01.040. [DOI] [PubMed] [Google Scholar]
- Sun WJ, Yun QQ, Zhou YZ, et al. Continuous 2-keto-gluconic acid (2KGA) production from corn starch hydrolysate by Pseudomonas fluorescens AR4. Biochem Eng J. 2013;77:97–102. [Google Scholar]
- Sun Y, Wei D, Shi J, et al. Two-stage fermentation for 2-ketogluconic acid production by Klebsiella pneumoniae. J Microbiol Biotechnol. 2014;24(6):781–787. doi: 10.4014/jmb.1401.01038. [DOI] [PubMed] [Google Scholar]
- Sun WJ, Xiao FF, Wei Z, et al. Non-sterile and buffer-free bioconversion of glucose to 2-keto-gluconic acid by using Pseudomonas fluorescens AR4 free resting cells. Process Biochem. 2015;50(4):493–499. [Google Scholar]
- Sun WJ, Alexander T, Man ZW, et al. Enhancing 2-ketogluconate production of Pseudomonas plecoglossicida JUIM01 by maintaining the carbon catabolite repression of 2-ketogluconate metabolism. Molecules. 2018;23(10):2629. doi: 10.3390/molecules23102629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WJ, Wang QH, Luan F, et al. The role of kguT gene in 2-ketogluconate-producing Pseudomonas plecoglossicida JUIM01. Appl Biochem Biotechnol. 2019;187(3):965–974. doi: 10.1007/s12010-018-2843-y. [DOI] [PubMed] [Google Scholar]
- Sun L, Wang DM, Sun W, et al. Two-stage semi-continuous 2-keto-gluconic acid (2KGA) production by Pseudomonas plecoglossicida JUIM01 from rice starch hydrolysate. Front Bioeng Biotechnol. 2020;8:120. doi: 10.3389/fbioe.2020.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura R, Hamada A, Ikehara K, et al. Enzymatic synthesis of 2-keto-d-gluconate and 2-keto-d-galactonate from d-glucose and d-galactose with cell culture of Pseudomonas fluorescens and 2-keto-galactonate from d-galactono 1, 4-lactone with partially purified 2-ketogalactonate reductase. J Mol Catal B Enzym. 2003;23(2–6):291–298. [Google Scholar]
- Teng WH, Sun WJ, Yu B, et al. Continuous conversion of rice starch hydrolysate to 2-keto-d-gluconic acid by Arthrobacter globiformis C224. Biotechnol Bioprocess Eng. 2013;18(4):709–714. [Google Scholar]
- Wang DM, Sun L, Sun WJ, et al. Purification, characterization and gene identification of a membrane-bound glucose dehydrogenase from 2-keto-d-gluconic acid industrial producing strain Pseudomonas plecoglossicida JUIM01. Int J Biol Macromol. 2018;118:534–541. doi: 10.1016/j.ijbiomac.2018.06.097. [DOI] [PubMed] [Google Scholar]
- Wang DM, Sun L, Sun WJ, et al. A membrane-bound gluconate dehydrogenase from 2-keto-d-gluconic acid industrial producing strain Pseudomonas plecoglossicida JUIM01: Purification, characterization, and gene identification. Appl Biochem Biotechnol. 2019;188(4):897–913. doi: 10.1007/s12010-019-02951-0. [DOI] [PubMed] [Google Scholar]
- Wei D, Xu J, Sun J, et al. 2-Ketogluconic acid production by Klebsiella pneumoniae CGMCC 1.6366. J Ind Microbiol Biotechnol. 2013;40(6):561–570. doi: 10.1007/s10295-013-1261-y. [DOI] [PubMed] [Google Scholar]
- Yang SY, Choi TR, Jung HR, et al. Production of glutaric acid from 5-aminovaleric acid by robust whole-cell immobilized with polyvinyl alcohol and polyethylene glycol. Enzyme Microbial Technol. 2019;128:72–78. doi: 10.1016/j.enzmictec.2019.05.003. [DOI] [PubMed] [Google Scholar]
- Yewale T, Panchwagh S, Rajagopalan S, et al. Enhanced xylitol production using immobilized Candida tropicalis with non-detoxified corn cob hemicellulosic hydrolysate. 3Biotech. 2016;6(1):75. doi: 10.1007/s13205-016-0388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdarta J, Meyer AS, Jesionowski T, et al. A general overview of support materials for enzyme immobilization: characteristics, properties, practical utility. Catalysts. 2018;8(2):92. [Google Scholar]