Abstract
Introduction
The aim of this study was to describe the real-word treatment and associated healthcare resource use (HCRU) of multiple sclerosis (MS) patients, as stratified by different MS subtypes.
Methods
All patients with MS continuously insured by two German statutory healthcare insurance funds from 2011 to 2015 were enrolled. These patients were categorized into four subgroups according to their MS type as follows: clinically isolated syndrome (CIS); relapsing remittent MS (RRMS); primary progressive MS (PPMS); and secondary progressive MS (SPMS). Sociodemographic characteristics, treatments, and HCRU for 2015 were analyzed. Treatment cascades for treatment-naïve patients were also determined.
Results
A total of 13,333 patients with MS were identified. The largest proportion of patients had RRMS (41.9%), followed by PPMS (17.1%). Mean age of the enrolled patients was 50.2 years, and 70.7% were female. Among all patients, 38.3% of those with CIS, 22.4% with PPMS, 69.6% with RRMS, and 33.9% with SPMS received a prescription of a disease-modifying immunomodulatory agent, with interferon beta-1a being the most frequently prescribed agent. Likewise, 14.5, 18.5, 19.9, and 21.5% of patients with CIS, PPMS, RRMS, and SPMS, respectively, received a flare-up treatment with glucocorticoids. MS-associated overall costs, including indirect costs for MS-associated days absent from work, were € 16,433, with costs related to MS medication (€ 8770; 53.4%) being the main driver of costs in all subgroups. MS-associated costs according to MS subtypes were € 12,427 for CIS patients, € 14,459 for PPMS patients, € 20,583 for RRMS patients, and € 17,554 for SPMS patients.
Conclusion
Among the four MS subtypes, RRMS patients most often received a disease-modifying immunomodulatory treatment. Consequently, healthcare costs were highest for patients with this MS subtype. Contrary to the treatment guideline, a substantial percentage of patients with CIS, RRMS, and SPMS did not receive any disease-modifying immunomodulatory treatment.
Electronic Supplementary Material
The online version of this article (10.1007/s40120-019-00172-5) contains supplementary material, which is available to authorized users.
Keywords: Claims data, Costs, Germany, HCRU, Multiple sclerosis, Real-world treatment
Key Summary Points
| Why carry out this study? |
| Multiple sclerosis (MS) is a chronic and progressive autoimmune disease of the central nervous system, the prevalence of which is increasing in Germany. |
| Although disease severity is known to be a cost driver, far less is known about whether healthcare resource use (HCRU) and costs are similar among MS subtypes or whether specific MS subtypes are associated with substantially higher HCRU/costs. |
| The aim of this study was to identify real-world treatment patterns, patient characteristics, MS-related HCRU, and direct/indirect costs of patients diagnosed with unselected clinically isolated syndrome (CIS), relapsing remittent MS (RRMS), primary progressive MS (PPMS), or secondary progressive MS (SPMS) and treated in Germany. |
| What was learned from the study? |
| Of all enrolled patients, 41.9% had from RRMS, 17.1% had PPMS, 15.6% had SPMS, and 10.6% had CIS. |
| RRMS patients experienced the highest drug prescription quotas and, consequently, healthcare costs were highest for patients with this MS subtype. |
| The general rates of MS patients not receiving any disease-modifying immunomodulatory agent or any glucocorticoid treatment were high. |
Introduction
Multiple sclerosis (MS) is a chronic and progressive autoimmune disease of the central nervous system [1, 2]. Data from 2009 and 2010 shows that there are approximately 143,000–200,000 patients with MS in Germany, with a trend towards an increasing prevalence [3, 4].
MS patients suffer from diverse symptoms, such as spasms, ataxia, fatigue, bladder dysfunction, sexual dysfunction, depression, pain, paresis, sensory deficits, visual impairment, and intestinal disorders [2, 5–7]. With the appearance of the first symptoms, the typical initial diagnosis of physicians is clinically isolated syndrome (CIS), which indicates the possibility of MS disease course. After confirmation of an MS diagnosis, the most commonly diagnosed type of MS is relapsing–remitting MS (RRMS), which presents with an unpredictable course of relapses followed by a period of remission and recovery [2, 5, 6]. When an initial relapsing–remitting phase is followed by a progressive phase, the MS is classified as secondary progressive MS (SPMS). The proportion of patients with RRMS who develop SPMS increases with longer disease duration, and the majority of RRMS patients (up to 90% after 20–25 years) ultimately develop SPMS [3, 4, 8, 9]. It has been reported that about 10–15% of patients suffer from primary progressive MS (PPMS), which is the fourth subtype of MS and characterized by continuous clinical disability progression without remissions [3, 4, 8, 9].
Current treatment guidelines recommend long-term disease-modifying immunomodulatory treatment for patients with CIS, RRMS, and SPMS and additional short-term treatment when flare-ups occur in patients with those disease subtypes [2, 5, 6]. MS symptoms should be treated with appropriate medicinal/nonmedicinal therapies. To date, there is only one (recently) approved and recommended immunomodulatory treatment available for PPMS [10, 11]. Beyond that, patients should receive symptomatic treatment in the manner of best supportive care (BSC) [2, 5, 6].
Previous publications have reported substantial total annual MS-associated healthcare costs in Europe of € 22,600–62,700 per person per year [12–21] and in the US population of up to $18,829–26,520 [7, 22, 23]. While disease severity is known to be a driver of cost, far less is known about whether healthcare resource use (HCRU) and costs are similar among treatments for the different MS subtypes or whether specific subtypes are associated with substantially higher HCRU/costs. In an attempt to clarify this uncertainty, we initiated a study aimed at identifying real-world treatment patterns, patient characteristics, MS-related HCRU, and direct/indirect costs of patients diagnosed with unselected CIS, RRMS, SPMS, or PPMS and treated in Germany.
Methods
Data Source and Patient Samples
A retrospective cohort analysis was carried out based on anonymized claims data provided by two German statutory public healthcare insurance funds that represent approximately 10% of the statutorily insured German population (AOK PLUS: 3.2 million insured persons; AOK Baden-Wuerttemberg: 3.9 million insured persons). This large sample size was assumed to have achieved representativity of the MS population.
This was a non-interventional, retrospective study analyzing anonymized data and, consequently, ethical approval and informed consent from patients were not required in accordance with German law and the policy of the institutions conducting the analysis (Institute for Pharmacoeconomics and Medication Logistics [IPAM], AOK PLUS, and AOK Baden-Württemberg). However, the study was evaluated by a scientific steering committee to which all the authors belonged and was based on a pre-defined study protocol to which all members of the steering committee consented. The analysis included all continuously insured persons with at least one diagnosis of MS (ICD-10 code: G35.-; https://www.who.int/classifications/icd/icdonlineversions/en/) from 01 January 2011 until the end of data availability (31 December 2015) or time of death.
A patient was defined as MS-prevalent if at least two outpatient diagnoses of MS (ICD-10 code: G35.-) were documented by a neurologist in two separate quarters of 1 year and/or if at least one inpatient MS diagnosis was documented in the database. In addition, five patient subgroups according to the diagnosed MS subtype (unspecified MS being the fifth type) were identified (Table 1), whereby a patient could be assigned to more than one group, taking into account that a change in specific subtype over time is clinically plausible.
Table 1.
Sample definition of patients with multiple sclerosis enrolled in the study
| MS patient categories and subtypes | Inclusion criteria | Start of observation (index date) | End of observation |
|---|---|---|---|
| MS-prevalent patients | At least two outpatient MS diagnoses (ICD-10 G35.-) documented by a neurologist in two different quarters of 1 year and/or at least one inpatient MS diagnosis (ICD-10 G35.-) between 01 January 2011 and 31 December 2015 | First documented MS diagnosis | End of 2015 or death |
| MS-incident patients | At least two outpatient MS diagnoses (ICD-10 G35.-) documented by a neurologist in two different quarters of 1 year and/or at least one inpatient MS diagnosis (ICD-10 G35.-) between 01 January 2012 and 31 December 2015 without any previous MS diagnosis (minimum pre-index period of 12 months) | First documented MS diagnosis | End of 2015 or death |
| Therapy-naïve MS-incident patients | After incident MS diagnosis, at least one prescription of an agent of interest (disease-modifying immunomodulatory agent) between 01 January 2012 and 31 December 2013 and no previous prescription of these agents (minimum pre-index period of 12 months) | First prescription of a disease-modifying immunomodulatory agent | End of 2015 or death |
| PPMS patients | At least two outpatient PPMS diagnoses (ICD-10 G35.2) documented by a neurologist in two different quarters of 1 year and/or at least one inpatient PPMS diagnosis (ICD-10 G35.2) between 01 January 2011 and 31 December 2015 | First documented MS diagnosis | End of 2015 or death |
| SPMS patients | At least two outpatient SPMS diagnoses (ICD-10 G35.3) documented by a neurologist in two different quarters of 1 year and/or at least one inpatient SPMS diagnosis (ICD-10 G35.3) and no PPMS (G35.2) diagnosis between 01 January 2011 and 31 December 2015 | First documented SPMS diagnosis | End of 2015 or death |
| RRMS patients | At least two outpatient RRMS diagnoses (ICD-10 G35.1) documented by a neurologist in two different quarters of 1 year and/or at least one inpatient RRMS diagnosis (ICD-10 G35.1) and no PPMS (G35.2) or SPMS diagnosis (G35.3) between 01 January 2011 and 31 December 2015 | First documented RRMS diagnosis | End of 2015 or SPMS diagnosis or death |
| CIS patients | At least two outpatient CIS diagnoses (ICD-10 G35.0) documented by a neurologist in two different quarters of 1 year and/or at least one inpatient CIS diagnosis (ICD-10 G35.0) between 01 January 2011 and 31 December 2015 and no PPMS (G35.2), SPMS (G35.3) or RRMS (G35.1) diagnosis between 01 January 2011 and 31 December 2015 | First documented CIS diagnosis | End of 2015 or SPMS or RRMS diagnosis or death |
MS Multiple sclerosis, PPMS primary progressive MS, SPMS secondary progressive MS, RRMS relapsing–remitting MS, CIS clinically isolated syndrome, ICD International Statistical Classification of Diseases and Related Health Problems by the World Health Organization
This table shows the criteria used to identify patients that belong to the corresponding MS subtypes. A patient could be assigned to different MS subtypes during the observation time. Reassignment was allowed from MS subtypes of lower severity to those of higher severity, starting with the lowest severity (CIS) and progressing, in order of increasing severity, to RRMS, SPMS, and PPMS. Reassignment was “prohibited” for reassignment to MS subtypes of reduced severity; thus, patients remained assigned to a more severe subtype even if, after such a diagnosis, a less severe MS subtype was diagnosed. Patients that fulfilled the criteria for PPMS at any given time were considered to be PPMS patients for the whole observational period
MS-incident patients as a subsample of the MS-prevalent patients defined in preceding text were defined as having received two outpatient MS diagnoses in two different quarters of 1 year by a neurologist and one inpatient MS diagnosis between 01 January 2012 and 31 December 2015 without any previous diagnosis of MS in a pre-index period of at least 12 months. These persons were considered to be treatment-naïve MS-incident patients if they received at least one prescription of an MS agent after their incident MS diagnosis between 01 January 2012 and 31 December 2013 without having received any such prescription in a pre-index period (12 months).
Observational Periods
Analyses of the treatment, HCRU, and costs were carried out for all MS-prevalent patients using data for 2015. Only MS-prevalent patients still alive on 01 January 2015 were included in this analysis. If a patient had been diagnosed with MS later than this date, the period between the date of that initial diagnosis up to the end of 2015 (or death, whatever came first) was taken as the time period for the analysis.
In a separate analysis of therapy cascades (longitudinal analysis), all incident and therapy-naïve patients were observed for at least 24 months from the date of their first respective prescription, with death before the end of this period being the only exception to the rule.
Outcomes
Patient characteristics of MS-prevalent patients were described as of the 01 January 2015 time point, and those for MS-incident/newly-treated patients were described as of the date of their first diagnosis/first prescription of an MS-related agent. In addition to the main characteristics of age, gender, and comorbidity status (based on the Charlson Comorbidity Index; see Electronic Supplementary Material [ESM] file 1), we evaluated the number of visits to a general practitioner (GP), the number of visits to a specialist, and the number of hospitalizations in the 12-month pre-index period.
As a general principle, all outcomes related to treatment, HRCU, and related costs were calculated per observed patient year (PY), with the exception of the percentage of patients who received at least one prescription of specific disease-modifying and/or flare-up treatments in 2015. For patients who received at least one prescription of a respective agent in 2015, the prescribed dosage of that therapy per observed PY, expressed in defined daily dosages (DDD) per PY, was reported [24, 25]. A total of 13 different disease-modifying immunomodulatory agent groups1 and two flare-up treatments2 were considered in the analysis of MS-related treatments.
The items assessed in the HCRU analysis were: prescriptions of MS therapy as defined above; number of visits as outpatient to the GP and neurologist (with MS diagnosis); inpatient stays in acute and rehabilitation care (with MS being the main diagnosis); MS-associated prescriptions of medical device; and days absent from work due to MS. The treatment of MS patients with respect to the following pre-selected MS-associated symptoms was also included in the HCRU analysis: spasms, ataxia, fatigue, bladder problems, sexual dysfunction, depression, pain, and bowel problems. These HCRU items had been identified in the respective treatment guidelines [2, 5, 6] and subsequently discussed and defined in clinical expert workshops (ESM file 2).
Direct and indirect healthcare costs were also calculated. Direct costs included the costs of drug and medical device prescriptions, which were based on list prices; the costs for inpatient stays, which were based on the DRG (diagnosis-related group)-based reimbursement of the specific stays; and the costs for outpatient treatment by GPs and neurologists, which were based on the sum of documented treatment costs. Indirect costs were calculated by multiplying the number of days of absence from work due to sickness absence by € 193, the mean daily salary of a working person in Germany in 2015 [26].
For the analysis of treatment cascades, the percentage of MS-incident patients who received a first disease-modifying immunomodulatory agent, an additional second-agent, and third-agent treatment was assessed. Respective agents for these therapy lines were also reported. Using Kaplan–Meier analysis covering a follow-up period of at least 24 months, we assessed time until discontinuation (gap > 90 days of coverage taking stockpiles into consideration) or change to another therapy, censoring for death and end of observation during the follow-up period.
No group comparisons were made, as our analysis was descriptive in nature. All evaluations were executed with Microsoft SQL Server 2008 and Microsoft Excel 2016 (Microsoft Corp., Redmond, WA, USA). All other statistical analyses were performed with STATA version 13.1 (StataCorp, College Station, TX, USA).
Results
Identified MS Patient Populations
The search of the databases of the two German public healthcare insurance funds identified 13,133 MS-prevalent patients alive on 01 January 2015 (Table 2). Of these, 1398 patients had been diagnosed with CIS, 5498 with RRMS, 2247 with PPMS, and 2042 with SPMS. A large number of patients (n = 2203) could not be assigned to a specific subgroup because they did not satisfy the sample criteria; these patients remained in the MS-prevalent sample but were not included in the subgroup-specific analysis. Within the MS-prevalent subgroup, 255 patients were diagnosed with more than one subtype during the observational period. Patients with SPMS or PPMS (median age of 57 and 59 years, respectively) were substantially older than those with CIS or RRMS (median age of 39 and 45 years, respectively). This difference in age between subgroups corresponded with a higher comorbidity score and a higher number of hospitalizations in the pre-index period, whereas the number of outpatient physician visits was similar across the subgroups. The proportion of female patients was considerably lower in the PPMS group than in the other subgroups (64.1 vs. 70.6–73.1%; Table 2).
Table 2.
Baseline characteristics of all patients with multiple sclerosis included in the study and according to subgroup
| Characteristics | MS-prevalent patients | CIS patients | RRMS patients | PPMS patients | SPMS patients | Incident-MS patients | Newly-treated, MS-incident patients |
|---|---|---|---|---|---|---|---|
| N | 13,133 | 1398 | 5498 | 2247 | 2042 | 8026 | 1750 |
| Mean length of follow-up, days (median | SD) | 339.1 (365 | 73.7) | 314.8 (365 | 96.8) | 345.5 (365 | 62.1) | 329.9 (365 | 85.9) | 350.6 (365 | 56.5) | 733.9 (715 | 423.7) | 990.9 (1011 | 288.2) |
| Mean age, yearsa (median | SD) | 50.2 (50 | 15.1) | 40.0 (39 | 13.8) | 44.6 (45 | 12.8) | 58.9 (59 | 13.0) | 57.7 (57 | 12.2) | 48.4 (48 | 16.1) | 42.5 (43 | 12.9) |
| Female gender, n (%) | 9278 (70.7%) | 989 (70.7%) | 4018 (73.1%) | 1440 (64.1%) | 1441 (70.6%) | 5606 (69.8%) | 1255 (71.7%) |
| Mean CCI (median | SD) | 2.6 (2 | 2.8) | 1.6 (1 | 2.3) | 2.2 (1 | 2.6) | 3.5 (3 | 2.9) | 3.4 (3 | 2.8) | 3.0 (2 | 3.1) | 2.8 (2 | 2.9) |
| Mean number of GP visitsb per PY (median | SD)c | 15.0 (14 | 7.8) | 12.6 (12 | 7.6) | 15.5 (15 | 8.1) | 15.2 (14 | 7.6) | 15.5 (15 | 7.3) | 17.6 (16 | 9.3) | 18.6 (17 | 9.1) |
| Mean number of neurologist visits per PYc (median | SD) | 1.3 (0 | 1.7) | 0.6 (0 | 1.2) | 1.7 (1 | 1.8) | 1.3 (0 | 1.8) | 1.4 (0 | 1.8) | 0.9 (0 | 1.4) | 1.3 (1 | 1.6) |
| Mean number of hospitalizations per PY (median | SD)c | 0.8 (0 | 1.4) | 0.6 (0 | 1.0) | 0.6 (0 | 1.3) | 1.3 (1 | 1.8) | 1.0 (0 | 1.6) | 1.2 (1 | 1.2) | 1.2 (0 | 1.0) |
SD Standard deviation, CCI Charlson Comorbidity Index, GP general practitioner, PY person year
aAge is calculated as of the index date (01 January 2015 for the prevalent sample and respective subsample in terms of the different MS subtypes or date of first diagnosis/first prescription for incident/newly-treated patients)
bAll GP visits
cNumber of GP visits/neurologist visits per PY and inpatient hospitalizations per PY were calculated based a 12-month pre-index period
For the inclusion period from 01 January 2012 to 31 December 2015, we identified 8026 patients without any previous MS diagnosis in a 12-month pre-index period. Of these, 1750 patients started a disease-modifying treatment between 01 January 2012 and 31 December 2013. The characteristics of MS-incident patients were similar to those in the MS-prevalent sample. However, newly-treated MS-incident patients were characterized by a younger age and a higher rate of outpatient GP/neurologist and inpatient visits in the pre-index period (Table 2).
Drug Treatment of MS-Prevalent Patients in 2015
Among the agents for disease-modifying MS therapy, the most frequently prescribed agents for all MS-prevalent patients were interferon beta-1a (11.3% of patients with at least one prescription), glatiramer acetate (8.2%), dimethyl fumarate (7.9%), and methylprednisolone (8.1%) or other glucocorticoids (12.2%) for the treatment of MS flare-up events (Fig. 1). RRMS patients generally had the highest prescription quotas of disease-modifying drugs, whereas prescription quotas were lowest for MS patients with the PPMS and SPMS subtypes. Among all subtypes, 61.7% of CIS patients, 77.6% of PPMS patients, 30.4% of RRMS patients, and 66.1% of SPMS patients did not receive any prescription of a disease-modifying immunomodulatory agent, and 85.5% of CIS patients, 81.5% of PPMS patients, 80.1% of RRMS patients, and 78.5% of SPMS patients did not receive any flare-up treatment with observed glucocorticoids.
Fig. 1.
Description of drug treatments for all multiple sclerosis (MS)-prevalent patients and the respective MS subtype groups in 2015. The percentage of patients who received different MS agents (at least one prescription in 2015) and the prescribed defined daily dosage (DDD) per person year based on patients who received at least one prescription of a respective agent are shown. The disease-modifying immunomodulatory agents alemtuzumab, mitoxantrone, ofatumumab, and rituximab were not included in this figure due to the low number of patients receiving each drug. Specific numbers referring to this figure are available in ESM file 3. CIS clinically isolated syndrome, RRMS relapsing–remitting MS, PPMS primary progressive MS, SPMS secondary progressive MS
With two exceptions (peginterferon beta-1a at an exceptionally high prescribed dosage, and azathioprine at an exceptionally low dosage), prescribed dosages per PY among patients receiving the respective treatments were very similar between MS subtypes as well as between different agents, ranging from 254.8 to 350.6 prescribed DDDs per PY. Among MS flare-up glucocorticoid treatments, dosages differed between MS subtypes, with the highest observed dosages for other glucocorticoids being observed in SPMS patients (up to 485.7 DDDs per PY, which is equal to about 10 mg of prednisolone on average per observed patient day).
Pharmacological treatment of the MS symptoms (BSC) was mostly observed in patients with SPMS and PPMS (see ESM file 5). In particular, agents to treat or ease spasticity, pain, or depression were prescribed more often for patients with these subtypes of MS (between approx. 30 and 50% of the patients with at least one prescription of a respective agent in 2015) than for those with RRMS.
Disease-Modifying Immunomodulatory Agent Treatment Cascades of MS-Incident, Newly-Treated Patients
Among treatment-naïve MS-incident patients, treatment cascades since first prescription of the respective agent for a mean follow-up period of 990.9 days were observed (Table 2). The most commonly prescribed agents for disease-modifying therapy were interferon beta-1a (40.6% of patients) and glatiramer acetate (26.9% of patients). In total, 599 patients (34.2%) received a second agent during the follow-up period, and 93 patients (5.3%) received a third agent. The most commonly prescribed agent as second-line therapy was dimethyl fumarate, with 33.9% of patients receiving a second-agent as therapy received this drug. For third-line therapy, dimethyl fumarate and glatiramer acetate (20.4/20.4%) were the most commonly prescribed agents (see ESM file 4).
A complete treatment cascade of patients who were initially treated with interferon beta-1a is described in Fig. 2. Of the 710 patients starting this therapy, 278 patients (39.2%) received a second agent, and 41 (5.8%) received a third agent. Consequently, if a change of therapy was observed, most of the patients switched to the new agent instead of adding the new agent to the current therapy. Of those receiving a second agent, the minority (14 of 278 patients, 5.0%) received this agent as an add-on drug (after the first prescription for the second agent, another prescription for the first agent was documented).
Fig. 2.
Treatment cascade of MS-incident patients who started interferon beta-1a therapy. The treatment cascade is shown for treatment-naïve MS-incident patients who started their first therapy with interferon beta-1a, the most commonly prescribed first-line agent (note: treatment cascades for less common first-line agents are presented in ESM file 4), between 01 January 2012 and 31 December 2013 without prior prescription of an MS treatment (minimum pre-index period of 12 months). An agent was classified as “add-on” if there was at least one prescription for the previous agent after the prescription of the second-line agent. Third-line agents were not further stratified by agent due to low patient numbers
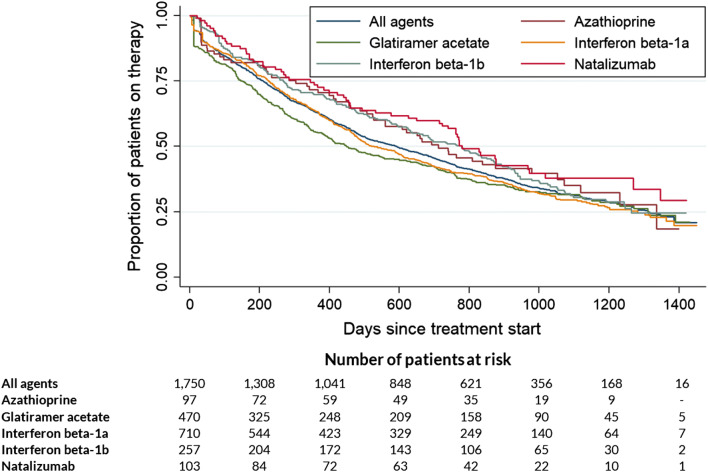
Persistence to the index treatment for all 1750 treatment-naïve MS-incident patients who started a disease-modifying immunomodulatory treatment and for the patient subgroups receiving the most frequently prescribed agents is shown in Fig. 3. In general, no major differences in persistence between these agents were observed. Of all patients, 62.9% continued their disease-modifying immunomodulatory therapy for the first year; after 2 and 3 years of observation, the respective persistence was 44.2 and 28.9%.
Fig. 3.
Persistence to MS index treatment in treatment-naïve MS-incident patients. Kaplan–Meier curves of the percentage of patients without changes in treatment or non-persistence for the five most commonly prescribed immunomodulatory agents are shown. Censored events include death and end of observational period. Uncensored events are specified as agent changes, the addition of another agent, or a drug availability gap of > 90 days
HCRU and Direct/Indirect Healthcare Costs
In 2015, based on the total MS-prevalent population and calculated using observed PYs, patients visited a GP a mean of 8.7 times, saw a neurologist a mean of 1.4 times, received a mean of 2.6 prescriptions of a disease-modifying immunomodulatory agent and a mean of 0.4 prescriptions of glucocorticoid MS flare-up treatments, experienced a mean of 0.5 acute hospitalizations with a mean length of 3.7 days, and missed, on average, 7.1 working days due to MS. Respective numbers for MS subtypes are shown in Table 3. The mean number of GP/neurologist visits per PY was similar among MS subtypes and ranged from 6.5 to 10.4 visits to a GP and from 1.2 to 2.0 visits to a neurologist. CIS patients had a higher hospitalization risk with a substantially longer hospitalization duration than MS patients with the other MS subypes (mean of 1.8 visits per PY, mean duration 11.5 days; see Table 3). The mean number of missed working days due to MS was the highest among patients with RRMS or CIS patients (10.6–11.3 days per PY), compared to those with PPMS or SPMS (3.1–3.6 days per PY).
Table 3.
Healthcare resource use by multiple sclerosis-prevalent patients in 2015
| Healthcare resource use | MS-prevalent patients | CIS patients | RRMS patients | PPMS patients | SPMS patients |
|---|---|---|---|---|---|
| N | 13,133 | 1398 | 5498 | 2247 | 2042 |
| Mean number of GP visitsa per PY (median | SD) | 8.7 (8.2 | 5.4) | 6.5 (4.8 | 11.4) | 9.6 (9.0 | 5.3) | 9.6 (9.0 | 5.1) | 10.4 (10.0 | 5.0) |
| Mean number of neurologist visits per PY (median | SD) | 1.4 (0.0 | 1.9) | 1.2 (0.0 | 4.5) | 2.0 (2.0 | 2.1) | 1.3 (0.0 | 1.9) | 1.5 (0.0 | 2.0) |
| Mean number of hospitalizations per PY (median | SD) | 0.5 (0.0 | 3.0) | 1.8 (0.0 | 7.6) | 0.6 (0.0 | 6.1) | 0.5 (0.0 | 2.0) | 0.6 (0.0 | 1.4) |
| Mean duration of hospitalizations per PY, days (median | SD) | 3.7 (0.0 | 19.9) | 11.5 (0.0 | 48.6) | 3.8 (0.0 | 33.0) | 4.8 (0.0 | 15.2) | 6.5 (0.0 | 21.3) |
| Number of patients receiving an immunomodulatory agent (%) | 6020 (45.8%) | 536 (38.3%) | 3826 (69.6%) | 504 (22.4%) | 693 (33.9%) |
| Mean number of prescriptions of an immunomodulatory agent per PY (median | SD) | 2.6 (0.0 | 3.6) | 2.6 (0.0 | 3.8) | 4.0 (4.0 | 3.8) | 1.3 (0.0 | 2.9) | 1.9 (0.0 | 3.2) |
| Number of patients receiving a flare-up treatment (%) | 2488 (18.9%) | 203 (14.5%) | 1093 (19.9%) | 415 (18.5%) | 439 (21.5%) |
| Number of patients receiving at least two flare-up treatments (%) | 1175 (9.0%) | 81 (5.8%) | 460 (8.4%) | 226 (10.1%) | 246 (12.0%) |
| Mean number of prescriptions of a flare-up treatment agent per PY (median | SD) | 0.4 (0.0 | 1.4) | 0.4 (0.0 | 1.6) | 0.4 (0.0 | 1.2) | 0.5 (0.0 | 1.6) | 0.6 (0.0 | 1.5) |
| Mean number of days absent from work due to MS per PY (median | SD) | 7.1 (0.0 | 32.5) | 11.3 (0.0 | 43.0) | 10.6 (0.0 | 39.3) | 3.1 (0.0 | 20.3) | 3.6 (0.0 | 22.3) |
| Mean number of inpatient rehabilitations per patient year (median | SD) | 0.3 (0.0 | 1.3) | 0.8 (0.0 | 3.9) | 0.4 (0.0 | 5.4) | 0.4 (0.0 | 1.3) | 0.5 (0.0 | 1.2) |
| Mean duration of inpatient rehabilitation per patient year, days (median | SD) | 3.3 (0.0 | 17.3) | 5.6 (0.0 | 25.0) | 3.1 (0.0 | 31.0) | 5.2 (0.0 | 24.5) | 7.3 (0.0 | 25.4) |
This table shows the healthcare resource use of observed MS-prevalent patients in 2015, per observed PY
aMS-related GP visits
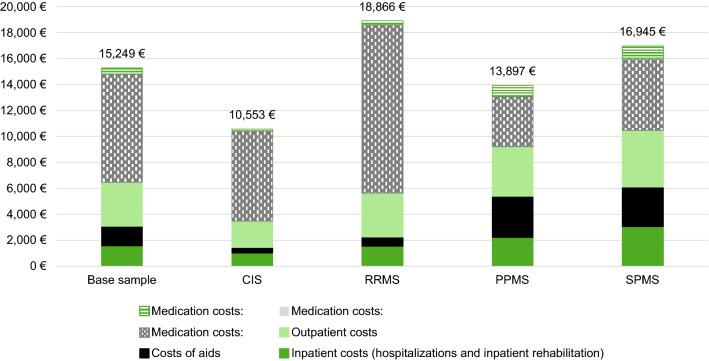
The overall observed direct MS-associated treatment costs, including medicinal/non-medicinal treatment for pre-selected MS symptoms, are shown in Fig. 4. Overall, mean MS-associated healthcare costs per observed PY were € 15,249 for the overall sample, which is more than fivefold the mean sum of healthcare costs per insured person in Germany.3 Medication costs constituted 57.5% of this total. The highest costs were observed for RRMS patients (€18,866), with medication expenses being the main driver (€ 13,330; 70.1%), whereas the lowest expenditure was observed for CIS patients (€ 10,553), with the difference mainly driven by lower medication costs (€ 7073; 67.0%). Compared to the costs of the disease-modifying therapy, the expenses related to the medication used for the alleviation of symptoms were negligible (ESM files 5–8 provide details on medication costs and on specific costs associated with other treatments of MS symptoms).
Fig. 4.
Total healthcare cost of MS-prevalent patients per person year (PY) in 2015. MS-associated healthcare costs are shown per observed PY for MS-prevalent patients, stratified by MS subtype, and separately for different healthcare components. Further details are available in ESM files 5 and 6
In addition, indirect costs due to days absent from work were assessed and found to amount to € 1183 for the overall sample, ranging across all samples from € 562 (PPMS) to € 1874 (CIS).
Discussion
Study Objectives and Main Results
The aim of our study was to assess real-world treatment and associated HCRU and costs generated by German MS patients by MS subtype using using data compiled in German healthcare claims dataset. To our knowledge, this is the first study of its kind. An additional aim was to describe observable disease-modifying immunomodulatory treatment cascades related to treatment-naïve MS-incident patients. Our results may provide important information for both the assessment of the real-world treatment of MS patients as well as for future health-economic models, which typically do not deal with a general MS population but with an MS subtype [27]. The main strength of our analysis is the real-world nature of the dataset; there is no selection bias present in this dataset and, consequently, our study results have a very high generalizability. The coverage of all healthcare sectors, including days absent from work, the detailed nature of our healthcare cost reporting, and the reporting of costs associated with MS treatment only instead of reporting all-cause cost/HCRU as reported by physicians or patients, is also unique.
A total of 13,133 MS patients were observed in our study, with the majority of these identified as having RRMS (41.9%) or PPMS (17.1%), which is in line with previously reported prevalence rates of MS subtypes [28]. The different gender ratio and age of PPMS patients compared to those of patients with the other MS subtypes are also in line with existing evidence [29–31]. In our study, the proportion of females was considerably lower among patients with PPMS versus the other MS subtypes, and mean patient age was higher.
Our data showed that about 38.3% of patients with CIS, 69.6% with RRMS, 33.9% with SPMS, and 22.4% with PPMS received disease-modifying immunomodulatory drugs, with interferon beta-1 being the most frequently prescribed treatment. Based on our overall sample of MS patients, 45.8% of patients received a disease-modifying immunomodulatory agent, which is in line with other German (healthcare research) studies (range 37.6–49% [4, 12]). A recent US study reported that 73.5% of RRMS patients and 50.0% of SPMS patients receive disease-modifying treatments, which is slightly higher than the values in our analysis [32].
The high percentage of RRMS patients receiving disease-modifying immunomodulatory agents, as compared to patients with CIS, SPMS and PPMS, respectively, was expected, and is in line with guideline recommendations. However, we observed a substantial percentage of patients with PPMS also receiving disease-modifying immunomodulatory therapy. As all currently available agents have been shown to be ineffective in this patient group, this observation either indicates diagnosis documentation errors by treating physicians or a deviation from guideline recommendations. As we only used neurologist or hospital diagnoses for the assignment of patients to a MS subgroup and based on current knowledge that such diagnoses are very reliable, we interpret our data as showing the latter. In support of this reasoning, we assumed that a patient had suffered from PPMS from the first observed MS diagnosis onwards (regardless of the subtypes/any MS diagnosis) whenever PPMS was subsequently diagnosed. However, in a post hoc analysis we examined treatment patterns only for periods after the date of the first PPMS diagnosis for these patients and observed very similar treatment patterns.
Our analysis of treatment cascades in MS-incident treatment-naïve patients showed that only 1750 of 8026 patients (21.8%) received a disease-modifying treatment after the first MS diagnosis during the observational period, even though guidelines recommend an early start of this therapy [2, 5, 6]. Of these 1750 patients, about one-third moved on to a second-agent therapy, which is in line with the expected rates of treatment failure [33].
Our data also showed that within 1 year of the initial diagnosis, 14.5% of patients with CIS, 19.9% of those with RRMS, 21.5% of those with SPMS, and 18.5% of those with PPMS received at least one prescription of glucocorticoids as a treatment for flare-ups (see ESM file 3; respective number for at least two prescriptions: 5.8% of CIS patients, 8.4% of RRMS patients, 12.0% of SPMS patients, and 10.1% of PPMS patients). These values reveal the high importance of MS flare-up treatment and the substantial flare-up frequency in these patients, even though we expected a lower percentage of both in PPMS patients. Overall, 18.9% of our overall MS sample patients received a flare-up treatment, which is slightly lower than previously reported (23.4% [12]).
A substantial number of our patients received a treatment for their symptoms, of which the most important were spasms, depression, and pain, with > 30% of patients receiving a treatment. The most commonly prescribed non-medicinal treatments were endurance workouts and physiotherapy (see ESM file 4). To our knowledge, our study is the first to report data on this aspect of MS treatment.
MS-associated overall costs, including indirect costs due to missed working days, were estimated to be € 16,433 per PY, ranging from € 12,427 (CIS patients) to € 20,583 (RRMS patients). MS-associated medication expenses were the main driver of these costs. These costs are lower than those previously reported for Germany (€ 28,200 for Expanded Disability Status Scale [EDSS] stage 0–3; €44,000 for EDSS stage 4–6.5; € 62,700 for EDSS stage 7–9 [21]), Sweden (the average difference in cost of illness between MS patients and matched controls was SEK 225,923 [about €20,800] in 2012 [34]), and Spain (€ 24,272 [35]) and higher than those reported for France (€ 12,296 in 2014 [36]). One possible explanation for the higher costs in some of these earlier studies might be a selection bias in those studies based on data derived from patient interviews [35, 37]. These patients might have received an above-average treatment with more expensive drugs. Moreover, in the earlier German study, patients were interviewed on HCRU and treatment but no differentiation was made between MS-associated versus all-cause HCRU [37]. In our study, we limited our reporting of cost to MS-associated items; for example, days absent from work were included only in the case of an associated MS diagnosis. The French study assessed only direct costs, which consequently resulted in a reporting of lower amounts compared to our study [36].
Our data showed that CIS and RRMS patients were absent from work for more days than SPMS and PPMS patients. Our interpretation of this finding is that it is mainly explained by the higher age of SPMS/PPMS patients and by an associated lower percentage of patients still working. The number of patients not actively working due to their MS could not be calculated; therefore, the mean number of days absent from work is potentially underestimated.
Our separate analysis of treatment persistence for patients beginning a disease-modifying immunomodulatory treatment showed that about 37% of patients discontinued or switched their treatment after 12 months, with 56% having discontinued/switched their index treatment after 24 months. The treatment cascade analysis proved that prescribing an add-on therapy is very uncommon if a second or third agent is prescribed. Our reported non-persistence numbers are generally in line with those reported in previous studies. In a German claims data analysis, overall persistence was 44.2% at the end of the 24-month observation period [33], and in a US study, 12-month discontinuation rates relating to different agents were reported to be 27.9–43.7% [38]. Clearly, a substantial percentage of observed patients discontinued their therapy early. As there might be varying agent-, disease- or patient-associated reasons for this, further research is needed to explore the aspects related to an early discontinuation of therapy. Another important aspect of undertreatment is late treatment initiation, but that was not the subject of this investigation. Reasons for delayed therapy and appropriate countermeasures are various and have been well discussed [39, 40]. We were unable to find any analyses of the extent of delayed therapy, indicating that further research is needed in this area.
Limitations
There is a number of limitations to our analysis. Firstly, the assessment of MS subtypes in our study was based on documented confirmed specialist/hospital diagnoses. Nevertheless, both a substantial number of patients with an unspecified MS type and our unexpected results with respect to the drug treatment of PPMS patients show that a specific uncertainty around MS subtype diagnoses might exist. Secondly, due to nature of the analyzed data, we could not differentiate between different stages of MS disease severity. Thirdly, due to longitudinal limitations of our dataset at the time of analysis, we were only able to observe a maximum of 48 months after the first prescription of an MS treatment agent in therapy-naïve MS-incident patients. Further research is needed to describe longer-term persistence to MS therapy. Fourthly, in our NP analysis, we defined NP as a treatment gap being > 90 days or a change to another therapy. While the 90-day threshold is widely used in general persistence literature, validation in the clinical setting has been rare to date. In terms of costs, the scope of measuring indirect costs was restricted to estimating costs associated with days absent from work. Although we recognize that there are other indirect cost components, such as caregiver costs, we were bounded by data availability on these aspects. Additionally, it needs to be noted that a bottom–up approach could have potentially provided more accurate cost estimates than the top–down approach used in our analysis. However, claims data are a comprehensive and reliable basis that have become an important data source for health economic evaluations [41, 42]. Furthermore, we only analyzed filled prescriptions in our database. We may have underestimated the percentage of patients who received respective prescriptions because specific patients may not have had their prescriptions filled. Moreover, we included some agents (i.e., azathioprine, rituximab) that are not approved for the treatment of MS but which are supposed to be used off-label; however, it is possible that these agents were used to treat of comorbidities rather than MS. Finally, some symptomatic treatment drugs (e.g. treatment for sexual dysfunction) are not covered by German statutory healthcare insurance.
Conclusions and Implications for Practice
Multiple sclerosis is associated with substantial HCRU and costs. Among MS subtypes, patients with RRMS more often receive a disease-modifying immunomodulatory treatment than do patients with other MS subtypes. Consequently, healthcare costs are highest for the treatment of this MS subtype, even when RRMS patients are younger than those with PPMS and SPMS. About 20% of MS patients receive glucocorticoid flare-up treatment in the course of 1 year. In contradiction to treatment guidelines, we observed that a substantial percentage of patients with CIS, RRMS, or SPMS did not receive any disease-modifying immunomodulatory treatment, whereas more than 20% of PPMS patients did receive such a treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the employees of AOK PLUS and AOK Baden-Württemberg for their support in providing the anonymized dataset.
Funding
This work, including the journal’s Rapid Service Fee, was financially supported by Roche Pharma AG.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors made substantial contributions to all of the following: (1) conception and design, acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; (3) final approval of the version to be published; and (4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Specifically, the main tasks the authors were engaged in were: TW: project lead; participated in writing all parts of the paper. SM/TH: statistical analysis, validation of the database; AF/AP/KE: interpretation and discussion of results; SB/GL: preparation of the study protocol, discussion of the results, and participation in writing the Discussion section.
Disclosures
TW has received honoraria from several pharmaceutical/consultancy companies (Novo Nordisk, Abbvie, Merck, GSK, BMS, LEO Pharma, Astra Zeneca, Bayer, Boehringer Ingelheim, Eisai). SM participated in this study as a staff member of IPAM; IPAM work in this study was sponsored by Roche Pharma AG. TH participated in this study as a staff member of IPAM; IPAM work in this study was sponsored by Roche Pharma AG. TH is now employed at GWQ PLUS. AF conducted consultancy work for Bayer Pharma AG in 2016 and is employed by AOK PLUS. AP does not have any conflicts of interest except those potentially related to their employers AOK PLUS and AOK Baden-Württemberg. KE does not have any conflicts of interest except those potentially related to their employers, AOK PLUS and AOK Baden-Württemberg. GL does not have any conflicts of interest except those potentially related to their employer, Roche Pharma AG.
Compliance with Ethics Guidelines
This was a non-interventional, retrospective study analyzing anonymized data. Thus, ethical approval and informed consent from patients were not required in accordance with the German law and the policy of the institutions conducting the analysis (IPAM, AOK PLUS and AOK Baden-Württemberg). However, the study was evaluated by a scientific steering committee to which all the authors belonged and was based on a pre-defined study protocol, to which all members of the steering committee consented.
Data Availability
German data protection law (SGB X) does not allow us to distribute the analyzed dataset.
Footnotes
Interferon beta-1a (ATC code: L03AB07), interferon beta-1b (ATC code: L03AB08), glatiramer acetate (ATC code: L03AX13), peginterfon beta-1a (ATC code: L03AB13), dimethyl fumarate (ATC code: N07XX09), teriflunomide (ATC code: L04AA31), fingolimod (ATC code: L04AA27), natalizumab (ATC code: L04AA23), alemtuzumab (ATC codes: L01XC04, L04AA34), ofatumumab (ATC code: L01XC10), rituximab (ATC code: L01XC02), mitoxantrone (ATC code: L01DB07), azathioprine (ATC code: L04AX01).
Methylprednisolone (ATC codes: H02AB04, H02AB54), other glucocorticoids (ATC codes: H02, except methylprednisolone).
Mean healthcare cost per insured person: 2,856.67 €; Source: AOK Report “Zahlen und Fakten 2017” GKV-Leistungsausgaben je Versicherten.
Tobias Heidler: Formerly at Institute for Pharmacoeconomics and Medication Logistics (IPAM), University of Wismar, Alter Holzhafen 19, 23966 Wismar, Germany.
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.10298483.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 2.Deutsche Gesellschaft für Neurologie, editor. Leitlinien für Diagnostik und Therapie in der Neurologie: Diagnose und Therapie der Multiplen Sklerose. Berlin: Deutsche Gesellschaft für Neurologie
- 3.Höer A, Schiffhorst G, Zimmermann A, et al. Multiple sclerosis in Germany: data analysis of administrative prevalence and healthcare delivery in the statutory health system. BMC Health Serv Res. 2014;14:381. doi: 10.1186/1472-6963-14-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen G, Wittmann R, Arndt V, Göpffarth D. Epidemiologie der Multiplen Sklerose in Deutschland: regionale Unterschiede und Versorgungsstruktur in Abrechnungsdaten der gesetzlichen Krankenversicherung. Nervenarzt. 2014;85:990–998. doi: 10.1007/s00115-014-4097-4. [DOI] [PubMed] [Google Scholar]
- 5.Söllner. DGN/KKNMS Leitlinie zur Diagnose und Therapie der MS. http://www.dmsg.de/dokumentearchiv/aktualisierung_herbst_dgnkknms_msll_20140813.pdf.
- 6.Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, Dissemination, and implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:777–788. doi: 10.1212/WNL.0000000000005347. [DOI] [PubMed] [Google Scholar]
- 7.Asche CV, Singer ME, Jhaveri M, Chung H, Miller A. All-cause health care utilization and costs associated with newly diagnosed multiple sclerosis in the United States. J Manag Care Pharm. 2010;16:703–712. doi: 10.18553/jmcp.2010.16.9.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan O, Miller AE, Tornatore C, Phillips JT, Barnes CJ. Practice patterns of US neurologists in patients with SPMS and PPMS: a consensus study. Neurol Clin Pract. 2012;2:58–66. doi: 10.1212/CPJ.0b013e31824cb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tornatore C, Phillips JT, Khan O, Miller AE, Barnes CJ. Practice patterns of US neurologists in patients with CIS, RRMS, or RIS: a consensus study. Neurol Clin Pract. 2012;2:48–57. doi: 10.1212/cpj.0b013e31824cb09b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24:96–120. doi: 10.1177/1352458517751049. [DOI] [PubMed] [Google Scholar]
- 11.Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 12.Windt R, Glaeske G, Hoffmann F. Treatment of multiple sclerosis in Germany: an analysis based on claims data of more than 30,000 patients. Int J Clin Pharm. 2013;35:1229–1235. doi: 10.1007/s11096-013-9857-x. [DOI] [PubMed] [Google Scholar]
- 13.Lebrun-Frenay C, Kobelt G, Berg J, Capsa D, Gannedahl M. New insights into the burden and costs of multiple sclerosis in Europe: results for France. Mult Scler. 2017;23:65–77. doi: 10.1177/1352458517708125. [DOI] [PubMed] [Google Scholar]
- 14.Calabrese P, Kobelt G, Berg J, Capsa D, Eriksson J. New insights into the burden and costs of multiple sclerosis in Europe: results for Switzerland. Mult Scler. 2017;23:192–203. doi: 10.1177/1352458517708685. [DOI] [PubMed] [Google Scholar]
- 15.Dubois B, Kobelt G, Berg J, Capsa D, Gannedahl M. New insights into the burden and costs of multiple sclerosis in Europe: results for Belgium. Mult Scler. 2017;23:29–40. doi: 10.1177/1352458517708100. [DOI] [PubMed] [Google Scholar]
- 16.Thompson A, Kobelt G, Berg J, Capsa D, Eriksson J, Miller D. New insights into the burden and costs of multiple sclerosis in Europe: results for the United Kingdom. Mult Scler. 2017;23:204–216. doi: 10.1177/1352458517708687. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen PV, Kobelt G, Berg J, Capsa D, Gannedahl M. New insights into the burden and costs of multiple sclerosis in Europe: results for Denmark. Mult Scler. 2017;23:53–64. doi: 10.1177/1352458517708118. [DOI] [PubMed] [Google Scholar]
- 18.Berger T, Kobelt G, Berg J, Capsa D, Gannedahl M. New insights into the burden and costs of multiple sclerosis in Europe: results for Austria. Mult Scler. 2017;23:17–28. doi: 10.1177/1352458517708099. [DOI] [PubMed] [Google Scholar]
- 19.Battaglia M, Kobelt G, Ponzio M, Berg J, Capsa D, Dalén J. New insights into the burden and costs of multiple sclerosis in Europe: results for Italy. Mult Scler. 2017;23:104–116. doi: 10.1177/1352458517708176. [DOI] [PubMed] [Google Scholar]
- 20.Kobelt G, Thompson A, Berg J, Gannedahl M, Eriksson J. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler. 2017;23:1123–1136. doi: 10.1177/1352458517694432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flachenecker P, Kobelt G, Berg J, Capsa D, Gannedahl M. New insights into the burden and costs of multiple sclerosis in Europe: results for Germany. Mult Scler. 2017;23:78–90. doi: 10.1177/1352458517708141. [DOI] [PubMed] [Google Scholar]
- 22.Carroll CA, Fairman KA, Lage MJ. Updated cost-of-care estimates for commercially insured patients with multiple sclerosis: retrospective observational analysis of medical and pharmacy claims data. BMC Health Serv Res. 2014;14:286. doi: 10.1186/1472-6963-14-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reese JP, John A, Wienemann G, et al. Economic burden in a German cohort of patients with multiple sclerosis. Eur Neurol. 2011;66:311–321. doi: 10.1159/000331043. [DOI] [PubMed] [Google Scholar]
- 24.WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index 2017. Oslo: WHO Collaborating Centre for Drug Statistics Methodology.
- 25.Tagesdosen A-t-c-Km. Amtliche Fassung des ATC-Index mit DDD-Angaben für die Bundesrepublik Deutschland im Jahr 2016. Köln: Deutsches Institut für Medizinische Dokumentation und Information (DIMDI); 2016. https://www.dimdi.de/dynamic/.downloads/arzneimittel/atcddd/atc-ddd-amtlich-2016.pdf
- 26.Bundesanstalt für Arbeitsschutz und Arbeitsmedizin (BAuA). Volkswirtschaftliche Kosten durch Arbeitsunfähigkeit 2015. Dortmund: BAuA.
- 27.Owens GM, Olvey EL, Skrepnek GH, Pill MW. Perspectives for managed care organizations on the burden of multiple sclerosis and the cost-benefits of disease-modifying therapies. J Manag Care Pharm. 2013;19:53. doi: 10.18553/jmcp.2013.19.s1.S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller DH, Leary SM. Primary-progressive multiple sclerosis. Lancet Neurol. 2007;6:903–912. doi: 10.1016/S1474-4422(07)70243-0. [DOI] [PubMed] [Google Scholar]
- 29.McKay KA, Kwan V, Duggan T, Tremlett H. Risk factors associated with the onset of relapsing-remitting and primary progressive multiple sclerosis: a systematic review. Biomed Res Int. 2015;2015:817238. doi: 10.1155/2015/817238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koch M, Kingwell E, Rieckmann P, Tremlett H. The natural history of primary progressive multiple sclerosis. Neurology. 2009;73:1996–2002. doi: 10.1212/WNL.0b013e3181c5b47f. [DOI] [PubMed] [Google Scholar]
- 31.Tremlett H, Zhao Y, Devonshire V. Natural history comparisons of primary and secondary progressive multiple sclerosis reveals differences and similarities. J Neurol. 2009;256:374–381. doi: 10.1007/s00415-009-0039-7. [DOI] [PubMed] [Google Scholar]
- 32.Gross HJ, Watson C. Characteristics, burden of illness, and physical functioning of patients with relapsing-remitting and secondary progressive multiple sclerosis: a cross-sectional US survey. Neuropsychiatr Dis Treat. 2017;13:1349–1357. doi: 10.2147/NDT.S132079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen K, Schüssel K, Kieble M, et al. Adherence to disease modifying drugs among patients with multiple sclerosis in germany: a retrospective cohort study. PLoS One. 2015;10:e0133279. doi: 10.1371/journal.pone.0133279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gyllensten H, Wiberg M, Alexanderson K, Friberg E, Hillert J, Tinghög P. Comparing costs of illness of multiple sclerosis in three different years: a population-based study. Mult Scler. 2018;24:520–528. doi: 10.1177/1352458517702549. [DOI] [PubMed] [Google Scholar]
- 35.Casado V, Martínez-Yélamos S, Martínez-Yélamos A, et al. Direct and indirect costs of multiple sclerosis in Baix Llobregat (Catalonia, Spain), according to disability. BMC Health Serv Res. 2006;6:143. doi: 10.1186/1472-6963-6-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruno D, Marc D, Ouarda P, et al. Economic burden of multiple sclerosis in France estimated from a regional medical registry and national sick fund claims. Mult Scler Relat Disord. 2019;36:101396. doi: 10.1016/j.msard.2019.101396. [DOI] [PubMed] [Google Scholar]
- 37.Flachenecker P, Kobelt G, Berg J, Capsa D, Gannedahl M. New insights into the burden and costs of multiple sclerosis in Europe: results for Germany. Mult Scler. 2017;23:78–90. doi: 10.1177/1352458517708141. [DOI] [PubMed] [Google Scholar]
- 38.Bergvall N, Petrilla AA, Karkare SU, et al. Persistence with and adherence to fingolimod compared with other disease-modifying therapies for the treatment of multiple sclerosis: a retrospective US claims database analysis. J Med Econ. 2014;17:696–707. doi: 10.3111/13696998.2014.940422. [DOI] [PubMed] [Google Scholar]
- 39.Bansback N, Chiu JA, Carruthers R, et al. Development and usability testing of a patient decision aid for newly diagnosed relapsing multiple sclerosis patients. BMC Neurol. 2019;19:173. doi: 10.1186/s12883-019-1382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noyes K, Weinstock-Guttman B. Impact of diagnosis and early treatment on the course of multiple sclerosis. Am J Manag Care. 2013;19:s321–s331. [PubMed] [Google Scholar]
- 41.Gansen FM. Health economic evaluations based on routine data in Germany: a systematic review. BMC Health Serv Res. 2018;18:268. doi: 10.1186/s12913-018-3080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreis K, Neubauer S, Klora M, Lange A, Zeidler J. Status and perspectives of claims data analyses in Germany—a systematic review. Health Policy. 2016;120:213–226. doi: 10.1016/j.healthpol.2016.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
German data protection law (SGB X) does not allow us to distribute the analyzed dataset.