With the stringently laid down WHO criteria and hallmark molecular signatures, the diagnosis, and sub-classification of myeloproliferative neoplasms (MPN) in the current era is usually straightforward [1]. We document here a rather unusual case of primary myelofibrosis in a young male, splenectomized in his childhood due to unknown reasons. The peripheral blood examination revealed numerous circulating micromegakaryocytes leading to a diagnostic dilemma.
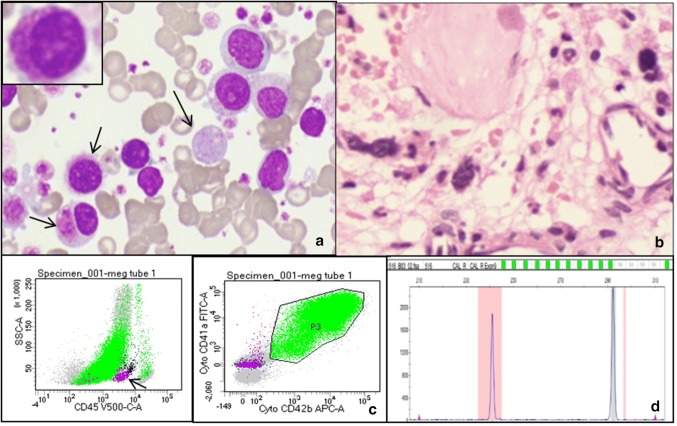
30 years old male presented to the Hematology OPD with weakness and mild grade fever of 2 months duration. Examination revealed mild hepatomegaly and non tender, mobile, right lateral chest wall swelling measuring 4 × 3.5 × 2 cm. He had undergone splenectomy 20 years back, the records of which were unavailable. CBC showed a haemoglobin of 9.2 g/dl, TLC—98.6 × 109/l and platelet count of 781 × 109/l. A differential leucocyte count revealed approximately 7% blasts, 4% basophils, granulocytes, and their precursors and 50% circulating micromegakaryocytes (Fig. 1a, b). Additionally, there were numerous naked nuclei and nRBC (~ 10/100WBC). The corrected leucocyte count was thus observed to be 69.0 × 109/l. Peripheral smear examination showed numerous giant and large platelets, megakaryocyte cytoplasmic fragments, outnumbering the analyzer count. Bone marrow aspirate smears were aparticulate yet cellular, showing numerous micromegakaryocytes entrapped in large platelet clumps, along with few myeloblasts and myeloid precursors. Bone marrow biopsy had a ‘spent out’ appearance with thickened irregular bony trabeculae, myelofibrosis, dilated sinusoids and scattered dyspoietic megakaryocytes. FNA from the swelling showed a few scattered mature myeloid cells and occasional micromegakaryocytes. Flow cytometry on the peripheral blood revealed approximately 68% cells expressing cytoplasmic CD41, CD42, CD36 and CD9 and negative for CD45, CD34 and HLA-DR (Fig. 1c). Additionally, a distinct population (1%) of myeloid blasts and basophils (CD9 positive) was also observed. Genetic work-up revealed a normal karyotype, and absence of BCR-ABL1 translocation, and JAK2 V617F mutation. However, the sample tested positive by fragment analysis for type I Calreticulin (CALR) mutation (52 bp deletion) (Fig. 1d). Hence, a final diagnosis of primary myelofibrosis, with CALR mutation was proposed. The patient was administered ruxolitinib at a dose of 20 mg twice daily and ecospirin at a dose of 75 mg. During his follow up (4 months duration), the patient expressed well being; there was a reduction in the chest wall swelling as well as the blood counts.
Fig. 1.
Panel of photomicrographs depicting, a, May Grunwald Giemsa stained peripheral blood smears showing presence of numerous micormegakaryocytes (inset) and giant platelets, b, Hand E stained bone marrow biopsy smears showing spent out marrow with scattered dyspoietic megakaryocytes, c, dot plots depicting a large population negative for CD45 expression and positive for Cyto CD41/CD42. A distinct population of CD34 positive myeloid blasts (pink) was also appreciated, d, fragment analysis for CALR (52 bp deletion) mutation
The most intriguing aspect of the case is the presence of circulating micromegakaryocytes, accounting for two-thirds of the cellular component. They may be seen in high numbers in myelodysplastic syndrome and myeloproliferative neoplasms particularly chronic myeloid leukemia (CML), where its presence may herald a disease progression [2, 3]. These cells may be confused with lymphocytes/lymphoblasts because of their size, configuration, and scanty cytoplasm. Phenotypically similar yet micromegakaryocytes may be differentiated from megakaryoblasts based on the presence of high nucleo-cytoplasmic ratio, scant basophilic cytoplasm with blebbing in the latter [4].
Leucocytosis and thrombocytosis favored the diagnosis of MPN with the possibility of megakaryoblastic crisis. However, immunophenotyping and presence of exhausted fibrotic bone marrow with dysplastic megakaryocytes in the absence of immature cell aggregates eliminated a megakaryoblastic blast crisis in MPN. Micromegakaryocytes may also be seen in cases of AML-M7 where they represent the mature form of megakaryocytes [5]. Presence of a long clinical history, absence of ‘true megakaryoblasts’ and diagnostic molecular profile, negated the possibility of AML-M7 and acute myelofibrosis with panmyelosis in this case. Myelodysplastic syndrome, another close differential, was also not considered due to the absence of cytopenias, a normal karyotype, and CALR mutation.
Sale et al. [6] also reported similar findings in a splenectomized JAK2 V617F-positive essential thrombocythemia case progressing to myelofibrosis. It is thus plausible that absence of spleen leads to an extensive spillover of the micromegakaryocytes in the peripheral blood which would otherwise have been filtered.
In conclusion, the presence of circulating micromegakaryocytes is not restricted to CML and an elaborate molecular work-up for all the driver mutations is mandated in such cases for an appropriate management.
Author Contribution
RG designed the research and analyzed data. DC, KR and PS assisted in drafting the manuscript. AS assisted in carrying out the molecular work-up. AG and SN were actively involved in the management of the patient.
Funding
None.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study has been approved by the Institutional Ethics Committee.
Informed Consent
Informed consent has been obtained.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 2.Erber WN, Jacobs A, Oscier DG, O’Hea AM, Mason DY. Circulating micromegakaryocytes in myelodysplasia. J Clin Pathol. 1987;40(11):1349–1352. doi: 10.1136/jcp.40.11.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Udomratn T, Steinberg MH, Dreiling BJ, Lockhard V. Circulating micromegakaryocytes signaling blast transformation of chronic myeloid leukaemia. Scand J Haematol. 1976;16(5):394–400. doi: 10.1111/j.1600-0609.1976.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 4.Wiesneth M, Pflieger H, Kubanek B, Heimpel H. Micromegakaryocytes in human bone marrow Acta Haematol. 1980;64:65–71. doi: 10.1159/000207213. [DOI] [PubMed] [Google Scholar]
- 5.Koike T. Megakaryoblastic leukemia: the characterization and identification of megakaryoblasts. Blood. 1984;64(3):683–692. doi: 10.1182/blood.V64.3.683.683. [DOI] [PubMed] [Google Scholar]
- 6.Sale S, Rocco V, Pancione Y, Carri D, Fumi M, Bain B. A confusing “white cell count”: circulating micromegakaryocytes in post-thrombocythemia myelofibrosis. Am J Hematol. 2018;94:617–618. doi: 10.1002/ajh.25377. [DOI] [PubMed] [Google Scholar]