Abstract
Amyloidosis is heterogeneous group of disorder characterized by extracellular deposition of misfolded insoluble proteinaceous material with cross beta pleated sheet structure leading to organ dysfunction. This disease is rare and indeed heterogeneous, as it may be hereditary (familial amyloidosis), secondary to spectrum of inflammatory conditions (AA amyloidosis) or member of plasma cell neoplasm family (AL amyloidosis). AL amyloidosis is the most common type of amyloid, however, is rarely accompanied by multiple myeloma or other lymphoproliferative disorder. This disparity in its origin and presentation needs to be addressed by exhaustive battery of investigation tools, to arrive at right diagnosis with correct typing. This is of utmost importance in guiding the treating physicians to choose appropriate therapeutic options. This review deals with diagnostic approach to amyloidosis and its various subtypes.
Keywords: Amyloidosis, Proteinaceous, Cross beta pleated, Multiple myeloma, AL amyloid, AA amyloid
Amyloidosis has been a mysterious disease historically. Along with evolution of knowledge about nature of this amorphous substance, armamentarium of diagnostic tools for amyloid has also evolved [1].
Before we embark on diagnostic work up of amyloidosis, a brief discussion about basic terminologies is essential.
What is Amyloidosis?
Amyloidosis is a generic term that refers to the extracellular tissue deposition of fibrils composed of low molecular weight subunits of a variety of serum proteins. It is characterized by
Antiparallel ß-pleated sheet configuration- noted on x-ray diffraction
Ability to bind Congo red -leading to apple green birefringence under polarized light
Classification of Amyloidosis and Terminology
Depending on type of protein deposited more than 28 type of amyloidosis has been described so far. Recent nomenclature is based on abbreviation of amyloidogenic protein in that entity. Example:
Immunoglobulin light chain (AL) amyloidosis- Earlier known asPrimary amyloidosis
Deposition of unmutated transthyretin (ATTR) in heart- Age related senile amyloidosis
Deposition of mutated forms of transthyretin (TTR), the alpha chain of fibrinogen A (Afib), apolipoprotein AI and AII(AApo AI andAApo AII), lysozyme(Alys), and gelsolin comes under umbrella of hereditary amyloidosis
AA amyloidosis (Earlier known as secondary Amyloidosis)-Fragments of the acute phase reactant (serum amyloid A) are deposited secondary to inflammation
Recently added entity to this list is ALECT2 (leukocyte cell derived chemotaxin-2) [2]
Systemic and localized is another terminology used in reference of amyloidosis. Localized amyloidosis means deposition of amyloid is restricted to a particular site. They usually have indolent clinical course. Common sites of localized amyloidosis are skin, larynx, and bladder. Localized form rarely progress to systemic forms, however, are notorious to have local recurrence. Systemic amyloidosis is characterized by deposition of amyloid at multiple sites and visceral organ leading to organ dysfunction. It may be restricted to one organ e.g. cardiac involvement in age related senile amyloidosis or diffuse involvement of multiple system like AL amyloidosis [2, 3].
Detailed etio-pathogenesis of amyloidosis is beyond the scope of this review article and we intend to focus on diagnostic approach of this entity.
Historical Perspective
Diagnostic techniques for amyloidosis have evolved over time. Term amyloid is derived from Latin word “amylum” for starch. It was actually coined by German botanist Matthias Schleiden to describe starch like substance in plants that reacts with iodine-sulphuric acid to turn from brown to blue [4]. In 1854 Rudolf Virchowfirst used this term for substance with similar chemical properties in brain- “corpora amylacea”. Following this three scientist independently described utility of metachromatic stain- methyl violet in detection of amyloid. In 1922 German chemist Herman Bennhold discovered the capacity of Congo red to bind to amyloid. This test for amyloid stood the test of time and still is pathognomic feature of same. With advances in technology, immunohistochemistry, electron microscopy, X ray diffraction and proteomic studies assisted in understanding actual structure and chemical nature of amyloid. Depending on the availability of resources various combinations of these tools are used by diagnostician to make the diagnosis of amyloidosis with correct typing [1].
First and key step in making correct diagnosis is suspecting this rare disease in correct clinical setting. Following table describes clinical scenario to initiate the work up for amyloidosis [5].
Detailed history and clinical features are first and essential step in amyloidosis work up. Inflammatory condition like tuberculosis, autoimmune disorders should raise suspicion of secondary (AA) amyloidosis. Positive family history will suggests the possibility of hereditary forms of disease. Predominant system involved also gives a clue about possible types of amyloidosis. Example: Predominant cardiac involvement is more common with AL, ATTR amyloid and rather uncommon with AA amyloid. In a case of multiple myeloma, above mentioned clinical features should alert the physician to suspect coexisting amyloidosis (Table 1).
Table 1.
Alarming signs of amyloidosis
| 1. Nephrotic syndrome |
| 2. Unexplained cardiac dysfunction |
| 3. Neuropathy |
| 4. Easy brusiability-“raccoon eyes” |
| 5. Monoclonal gammopathy with (For AL amyloid) |
| a. Autonomic or sensory neuropathy |
| b. unexplained fatigue |
| c. Edema |
| d. Unintentional weight loss |
Battery of investigations required in work up of amyloidosis is summarized in Table 2. Lab work up of amyloidosis is extensive and should aim in answering specific questions.
Table 2.
List of investigations required for work up of amyloidosis
| For establishing diagnosis and typing of amyloid |
| Target organ or surrogate site (abdominal fat pad) biopsy |
| H & E and Congo red stain |
| IHC/Immunoelectron microscopy/proteomic study for amyloid typing |
| Serum protein electrophoresis (SPEP),Immunofixation electrophoresis (IFE), Serum free light chain assay (sFLC) for evidence of monoclonal plasma cell proliferation |
| Bone marrow aspirate and biopsy for clonal plasma cells |
| Mutation studies for various hereditary amyloidosis |
| Work up for rheumatoid arthritis, tuberculosis and other inflammatory conditions associated with AA amyloidosis |
| For confirming systemic involvement, assess distribution and complications |
| Renal function test |
| 24 h urine protein/albumin |
| MRI/ECHO for cardiac involvement |
| Pro NT-beta natriuretic peptide |
| ECG |
| USG/CT scan for liver span |
| Alkaline phosphatase |
| X ray or CT scan of lung |
| GIT biopsy with evidence of amyloid |
| SAP scintigraphy/technetium scintigraphy |
| Coagulation screen |
Morphological Evidence of Amyloid
First step is to demonstrate amyloid deposition in the tissue. It can be achieved by either involved organ (liver, kidney) or surrogate site biopsy. Abdominal fat pad or rectal biopsy is positive for amyloid in 80% cases and bone marrow detects it in 56% cases. 15% cases of amyloidosis may be missed when only surrogate sites are biopsied [6]. Advantages of surrogate site biopsy are easy accessibility, minimal invasion, and it can be repeated during follow-up if necessary. Target organ biopsy increases the diagnostic yield; however, risk of bleeding should be weighed before considering liver and kidney biopsy.
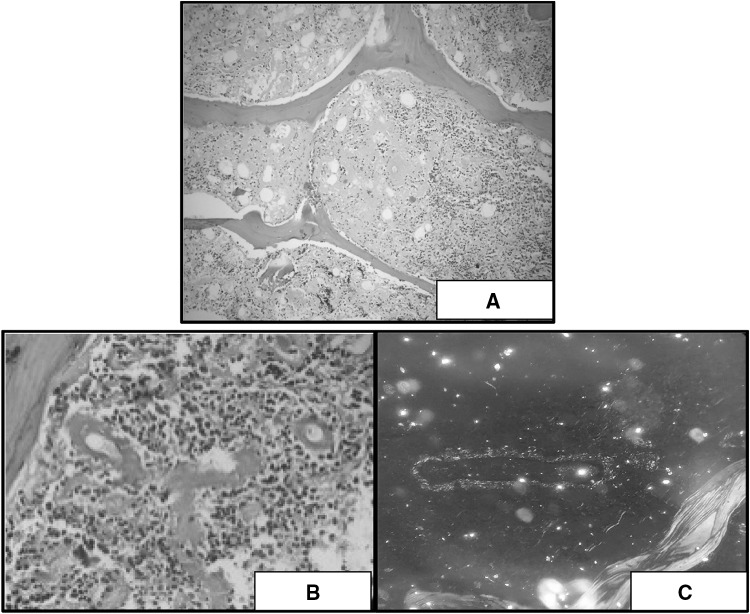
On hematoxylin and eosin stain amyloid appears as amorphous eosinophilic acellular deposits (Fig. 1a) [7]. These morphological features are shared byother substances like collagen, fibrin, plasma, light chain deposits, heavy chain deposits and presents the diagnostic challenge to pathologist. To address this issue presence of congophilic material in biopsy is only considered equivalent to amyloid deposition.
Fig. 1.
a H & E staining showing amorphous, extracellular, eosinophilic material (×100). b Congo red stain showing deep pink to red colored perivascular amyloid (×400). c Amyloid deposits showing apple green birefringence under polarized light (×400)
In 1922 German chemist Herman Bennhold discovered the capacity of Congo red to bind to amyloid. Selective congo red staining (congophilia) by amyloid is attributed to formation of non-ionic hydrogen bond between amyloid and dye imparting it deep pink to red color (Fig. 1b). It is enhanced by alkaline PH and depends on thickness of section. 6–10 µ thick sections are required for optimal staining. This congo red positive amyloid produces characteristic apple green birefringence when viewed by polarized light (Fig. 1c) due to alignment of dye molecules on the linearly arranged amyloid fibrils [8]. This finding should be interpreted carefully and yellow green birefringence of collagen should be excluded. These peculiar features of amyloid help to differentiate it from other deposited materials and ascertain the diagnosis. Hence presence of congo red positive amyloid material is one of the essential diagnostic criteria [9] for primary AL amyloidosis as well.
On electron microscopy amyloid is comprised of haphazardly distributed, non-branching solid fibrils with a mean diameter of 10 nm (range 8–12 nm). It is characteristic, but not specific feature to amyloid as fibril deposition may be seen in fiibrillary glomerulonephritis, immunotactoid glomerulonephritis, glomerular sclerosis, diabetic fibrillosis, fibronectin glomerulopathy and collagenofibrotic glomerulopathy. One can differentiate these entities by character of fibrils on electron microscopy, light microscopy findings & negative congo red staining [3] (Fig. 2).
Fig. 2.
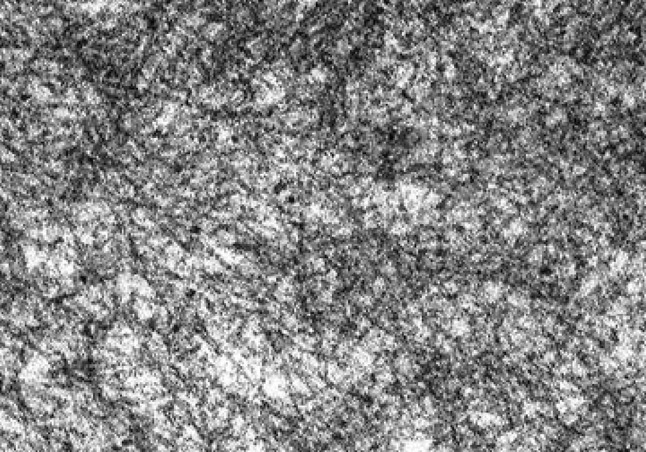
Electron microscopy picture of randomly-oriented, non-branching amyloid fibrils, 7.5–10 nm in diameter, of indeterminate length
Typing of Amyloid
Once the diagnosis of amyloidosis is established, next important step is the correct typing of amyloid which enables clinicians to select appropriate therapy. This step is very crucial, as therapy for amyloid is type specific and varies from liver transplant to chemotherapy and HSCT [2]. Various lab tools available for amyloid typing are:
Immunofluorescence
Immunohistochemistry
Immunoelectron microscopy
Proteomic study
Immunofluorescence for Amyloid Typing
This technique is widely used in case of renal biopsy, due to availability of frozen sections. Flurochrome labelled monoclonal antibodies directed against immunoglobulin components are treated with tissue and viewed under fluorescent microscope. However, in other paraffin embedded tissues IHC is preferred technique for amyloid typing. [2]
Immunohistochemistry (IHC) for Amyloid Typing
Basic principle of IHC remains the same. Primary antibodies against various components of amyloid (antigen) are used. This antigen–antibody complex is detected and visualized by various methods. Use of antibody directed against the component common to different types of amyloid (e.g. amyloid P component), suggestsmerely the presence of amyloid. However, IHC can be used as important tool in typing of amyloidalso by using extended panel of antibodies against specific components. Antibodies directed against AA amyloid stains only AA (secondary amyloidosis) cases. IHC for (TTR, apoAI, fibrinogen etc.) is used for familial amyloidosis and is characterized by a strong and even immunostaining of the entire amyloid deposits with the antibodies directed against apoAI and TTR. Spotty staining with these antibodies may be seen in other types as well. Strong and even immunostaining of the entire amyloid deposit by 1 non-anti-AL antibody is categorized as proof of the non-AL-fibril protein with exception of Afib amyloidosis. Afib is diagnosed with characteristic morphology and spotty immunostaining [10].
With regards to light chain amyloidosis, IHC haspoor specificity (< 50%).IHC as a tool for amyloid typing has certain limitations. There is difficulty in detecting light chain because of conformational differences between native versus tissue-fixed light chains.The amyloid protein(s) are derived from the variable region of lambda light chain (more commonly) or kappa light chain. Each patient with AL amyloid has a unique amyloid protein, reducing the chance that a single antibody will ever be able to stain all ‘‘different types’’ of AL amyloid. Antigen masking, light chain fragmentation during amyloid fibril formation and variation in tissue processing are other reasons for suboptimal staining of AL amyloid by IHC.
Coexistence and co-deposition of 2 or more amyloid fibril proteins in the same patient and even in the same amyloid deposit further complicate the immunohistochemical classification of amyloid. More so, absence of immunostaining with antibodies directed against immunoglobulin-derived λ-light chain and κ-light chain does not exclude AL amyloidosis.
It’s equally important to be aware of the fact that IHC positivity for light chain is not equivalent to amyloidosis. Possibility of other monoclonal protein deposition diseases like light chain and heavy chain deposition disease needs to be excluded. Especially in cases with isolated renal involvement. Absence of congo red staining, absence of immunostaining for SAP, predilection for kappa chain involvement and presence of more restricted organ involvement helps to diagnose LCDD and HCDD correctly [3].
Despite all these limitations, in resource limited setting IHC is extensively used tool for amyloid subtyping. With availability of extensive panel of antibodies, higher sensitivity and specificity can be obtained [11]. However, to overcome these shortcomings of IHC and to type amyloid with more certainty other techniques are used.
Immunoelectron Microscopy for Amyloid Typing
Immunoelectron microscopy combines immunohistochemistry with electron microscopy. Ultra-thin sections of specimen are prepared and fixed in Karnovsky solution and post-fixed in osmium tetroxide. Primary antibody (anti k, anti TTR, anti L etc.) is allowed to bind with their epitopes in amyloid. Following this section is treated with secondary antibody (anti-rabbit or anti-mouse immunoglobulin G) bound to colloidal gold and stained with uranyl acetate and lead citrate. Gold-labeled secondary antibodies can localize the protein within amyloid fibrils and greatly reduce background staining which is the most common cause of reduced specificity in immunohistochemistry.
Larrea et al. showed that IEM correctly identified the specific form of amyloid in > 99% of the cases [12]. Because of focal distribution of amyloid IEM has good positive predictive value but low negative predictive value. In contrast to kappa cases AL lambda cases are easily detected by IEM. Hence possibility of false Negative IEM should be considered when kappa AL amyloidosis is suspected [13].
Proteomic Study for Amyloid Typing
Proteomic study is basically complete identification and quantification of the proteome. It’s superior to all other techniques of amyloid typing as it’s not dependent on availability of antibodies against amyloid antigen. Each type of amyloid has peculiar proteomic signature which is decoded by single test. Different techniques can be used to analyze proteomic structure of amyloid deposits and to type them correctly. Proteomic study by mass spectrometry is now considered standard method for amyloid typing. It offers following advantages:
Unequivocal identification of the amyloid protein
Independent of availability of specific antibodies
Can be done profitably when centralized
Even performed on paraffin embedded sample
Modifications like laser microdissection with tandem mass spectrometry based proteomic analysis is shown to type amyloid with high sensitivity and specificity even on paraffin embedded biopsy specimen. Sections are stained with congo red and laser microdissection helps to sample part of the section with amyloid deposits. Tandem mass spectrometry based proteomic analysis of these amyloid deposits helps to type it accurately [14]. With advances in technology multidimensional Protein Identification Technology (MudPIT) is proposed as an automated, high-throughput proteomic approach for amyloid typing in fat pad aspirate samples [15].
Immunoelectron microscopy and spectrometry enables amyloid typing with sensitivity and specificity reaching almost 100%. However, these techniques are available in very few centers and this limits their utility for amyloid typing. Hence IHC still remains important tool for amyloid typing especially in resource limited setting. Information obtained by IHC should be correlated with clinical details and other ancillary investigations to arrive at correct diagnosis.
Tests to Assess Distribution of Amyloid
Once diagnosis of amyloidosis is established with correct typing, imaging techniques can be used to assess distribution of amyloid in patient. Serum amyloid P component (SAP) is normal circulating protein deposited in amyloid fibrils. Radiolabeled SAP i.e.I123 labelled SAP is injected intravenously and its uptake in scintigraphy images indicate amyloid deposition. It may be positive even if biopsy is negative for amyloid. SAP can be used for diagnosing, locating, and monitoring the extent of systemic amyloidosis [16]. This method’s utilization is limited because of its high cost, limited availability and being less helpful in detecting cardiac amyloid. Alternative options like Technetium scintigraphy (e.g. technetium99mTc) and magnetic resonance imaging (MRI) may allow identification of cardiac amyloid.
Special Tests for Hereditary and AA Amyloidosis
Inflammatory markers like serum amyloid associated protein level (SAA) and C reactive protein are elevated in AA amyloidosis and can be directly measured. SAA is found to be more sensitive acute phase response marker than CRP in systemic amyloidosis particularly AA type [17]. However one cannot rely on these markers to differentiate AA versus AL amyloidosis. Elevated SAA with IHC evidence of AA amyloid can be taken as supportive evidence of AA amyloidosis [3].
Hereditary amyloidosis usually has autosomal dominant inheritance but family history might be negative in some cases due to incomplete penetrance. Diagnosis can be made by direct identification of the target protein by tandem mass spectrometry or mutation testing can be done in appropriate clinical setting. (E.g. Test for fibrinogen mutations in isolated renal amyloidosis)
Serological tests for AL—Amyloid
AL amyloidosis is the most common form of amyloidosis and needs to be screened in suspected cases. Many cases are indeed diagnosed as one of the screening tests for monoclonal protein comes positive in appropriate clinical setting followed by histopathological evidence of amyloid deposition.
SPEP and IFE attains sensitivity of 70% for monoclonal protein detection in amyloidosis as it usually involves only light chain. Addition of sFLC to SPEP, UPEP& IFE increase the sensitivity by 10–15% [2]. In one of the series sFLC increased monoclonal protein detection up to 90–99% [12]. The monoclonal protein in light chain AL amyloidosis is lambda light chain in 70% cases, kappa in 25% cases and biclonal in remaining 5% [18]. It needs to be clarified that mere presence of monoclonal protein in case of amyloidosis is not sufficient for diagnosis of AL amyloidosis. Possibility of monoclonal gammopathy of undetermined significance (MGUS) with coexisting AA or hereditary amyloidosis needs to be excluded [19]. Hence correct typing of amyloid to be light chain related by IEM, mass spectrometry or IHC is equally important.
Documentation of monoclonal protein by above mentioned techniques serves as evidence of monoclonal plasma cell proliferative disorder in patient having biopsy proven light chain amyloidosis. It is one of the essential diagnostic criteria for AL amyloidosis (Table 3).
Table 3.
International Myeloma Working group diagnostic criteria for AL amyloidosis [20]
| Presence of an amyloid-related systemic syndrome |
| (e.g., renal, liver, heart, gastrointestinal tract or peripheral nerve involvement- The organ damage must be felt to be related to amyloid deposition and not to another common disease, such as diabetes or hypertension) |
| Positive amyloid staining by Congo red in any tissue (e.g., fat aspirate, bone marrow or organ biopsy) |
| Evidence that the amyloid is light chain-related established by direct examination of the amyloid using spectrometry-based proteomic analysis or immunoelectron microscopy |
| Evidence of a monoclonal plasma cell proliferative disorder (e.g., presence of a serum or urine M protein, abnormal serum free light chain ratio, or clonal plasma cells in the bone marrow) |
Bone Marrow Biopsy in Amyloidosis
Bone marrow examination can be done for two reasons in amyloidosis. It’s a very good surrogate site for histopathological evidence of amyloid. Second reason is demonstration of monoclonal plasma cells in marrow is considered as evidence of underlying monoclonal plasma cell proliferative disorder.
AL Amyloidosis Versus Multiple Myeloma
Diagnosis of MM associated AL amyloidosis requires fulfillment of diagnostic criteria for both the conditions- namely AL amyloidosis and multiple myeloma. Mere presence of bone marrow clonal plasmacytosis not sufficient as 18% of AL amyloidosis patients may show > 20% clonal plasma cells in bone marrow. In only 10–15% of the patients with MM concurrent diagnosis of AL amyloidosis is made at presentation or sometime during the course of the myeloma. On the contrary, 30% of myeloma patients are found to have subclinical amyloid deposits. Occult amyloidosis appears to have no impact on the toxicity and outcome of MM patients; however the presence of symptomatic amyloidosis clearly worsens their prognosis and alerts treating physician for modification in therapy. Amyloidosis work up should be initiated in MM patients with nephrotic range proteinuria, infiltrative cardiomyopathy, autonomic neuropathy, hepatomegaly and symptoms of partial bowel obstruction [21].
Very few cases of AL Amyloidosis (0.4%) show delayed (> 6 months) progression to full blown MM. This subset mainly includes cases without cardiac and hepatic involvement [22]. In primary case of AL amyloidosis 10% is the significant plasma cell cut off in marrow as it helps to choose the therapy and predict the outcome.
Investigation to Demonstrate Amyloid Related Systemic Syndrome
Systemic amyloidosis and specifically AL amyloidosis has well defined criteria for organ involvement [23]. In clinically suspected case of amyloidosis battery of investigations need to be done to establish amyloid related organ dysfunction. Investigation work up should assist to attribute the organ dysfunction to amyloid and rule out other aetiology for same. Following Table 4 summarises criteria for systemic involvement in AL amyloidosis with tests required to demonstrate the same.
Table 4.
Criteria for systemic involvement in AL amyloidosis with appropriate investigations
| System involved | Criteria Biopsy of affected or alternate site AND |
Tests required to demonstrate systemic involvement |
|---|---|---|
| Renal | Proteinuria of > 0.5 g/24 h (mainly albumin) | 24 h urine protein |
| Cardiac involvement | Lab or clinical evidence of involvement Echocardiography showing more than 12 mm wall thickness in absence of other causes |
Echo Troponin I N terminal pro brain natriuretic peptide ECG showing low voltage (< 5 mm) in all 12 leads |
| Liver |
Liver span > 15 cm in absence of heart failure Alkaline phosphatase > 1.5 times the normal range of lab |
CT scan or radionuclide imaging Alkaline phosphatase |
| Lung | Radiographic evidence of diffuse interstitial lung disease is taken as evidence of amyloidosis | CT scan/X ray |
| Gastro intestinal tract | Biopsy evidence with amyloid |
Summary
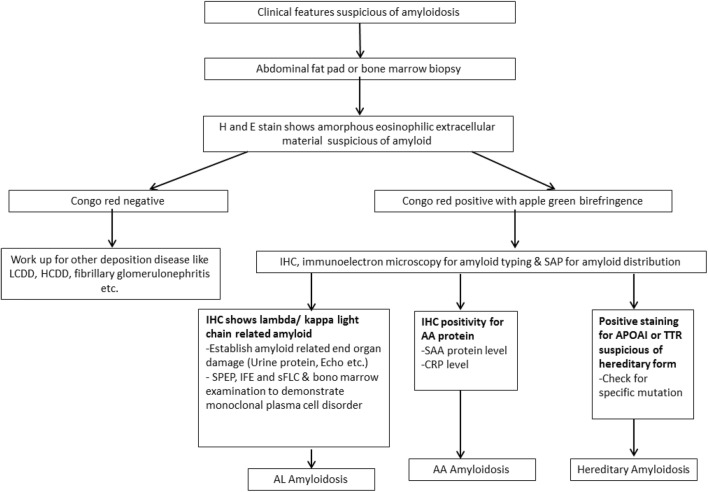
There has been paradigm shift in understanding of pathophysiology of amyloidosis. This led to significant improvement in diagnostic tools available for the same. Figure 3 summarizes the sequential stepwise approach to diagnose and type amyloid deposit. Diagnosis of amyloidosis can be challenging, especially in cases with single organ involvement. High index of suspicion and extensive lab/radiological work up helps to make the correct diagnosis. Congophilic property of amyloid was landmark discovery and still remains the essential first step to label any deposit as amyloid. Correct typing of amyloid is of paramount importance in making correct therapeutic decision. As per IMWG 2014 criteria for AL amyloidosis, immune electron microscopy and mass spectrometry based proteomic studies are techniques recommended for correct typing of amyloid. However, IHC with extended panel of antibodies also serves as helpful tool for the same. Evidence of monoclonal plasma cells in marrow, abnormal SPEP/UPEP/IFE/sFLC, SAA, CRP, mutation studies for hereditary amyloidosis etc. are other important investigations required to arrive at the correct diagnosis. With availability of above mentioned techniques in our diagnostic armamentarium correct diagnosis of amyloidosis can be made possible in majority of cases.
Fig. 3.
Diagnostic approach to amyloidosis. *Test for amyloid typing should be chosen depending on availability of tissue and technique
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Richa Juneja, Email: drrichajuneja@gmail.com.
H. P. Pati, Email: harappati@yahoo.co.in
References
- 1.Sipe JD, Cohen AS. Review: history of the amyloid fibril. J Struct Biol. 2000;130(2):88–98. doi: 10.1006/jsbi.2000.4221. [DOI] [PubMed] [Google Scholar]
- 2.Leung N, Nasr SH, Sethi S. How I treat amyloidosis: the importance of accurate diagnosis and amyloid typing. Blood. 2012;120(16):3206–3213. doi: 10.1182/blood-2012-03-413682. [DOI] [PubMed] [Google Scholar]
- 3.Mollee P, Renaut P, Gottlieb D, Goodman H. How to diagnose amyloidosis. Intern Med J. 2014;44(1):7–17. doi: 10.1111/imj.12288. [DOI] [PubMed] [Google Scholar]
- 4.Tanskanen M. “Amyloid”—Historical aspects. Rijeka: InTech; 2013. [Google Scholar]
- 5.Rosenzweig M, Landau H. Light chain (AL) amyloidosis: update on diagnosis and management. J Hematol OncolJ Hematol Oncol. 2011;18(4):47. doi: 10.1186/1756-8722-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gertz MA. Immunoglobulin light chain amyloidosis: 2016 update on diagnosis, prognosis, and treatment. Am J Hematol. 2016;91(9):947–956. doi: 10.1002/ajh.24433. [DOI] [PubMed] [Google Scholar]
- 7.Kumar V, Abbas AK, Aster JC, Perkins JA. Robbins basic pathology. Philadelphia, PA: Elsevier/Saunders; 2013. [Google Scholar]
- 8.Puchtler H, Sweat F, Levine M. On the binding of Congo red by amyloid. J Histochem Cytochem. 1962;10(3):355–364. doi: 10.1177/10.3.355. [DOI] [Google Scholar]
- 9.Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification and management. Am J Hematol. 2016;91(7):719. doi: 10.1002/ajh.24402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kebbel A, Röcken C. Immunohistochemical classification of amyloid in surgical pathology revisited. Am J Surg Pathol. 2006;30(6):673–683. doi: 10.1097/00000478-200606000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Schönland SO, Hegenbart U, Bochtler T, Mangatter A, Hansberg M, Ho AD, et al. Immunohistochemistry in the classification of systemic forms of amyloidosis: a systematic investigation of 117 patients. Blood. 2012;119(2):488–493. doi: 10.1182/blood-2011-06-358507. [DOI] [PubMed] [Google Scholar]
- 12.Fernández de Larrea C, Verga L, Morbini P, Klersy C, Lavatelli F, Foli A, et al. A practical approach to the diagnosis of systemic amyloidoses. Blood. 2015;125(14):2239–2244. doi: 10.1182/blood-2014-11-609883. [DOI] [PubMed] [Google Scholar]
- 13.Arbustini E, Verga L, Concardi M, Palladini G, Obici L, Merlini G. Electron and immuno-electron microscopy of abdominal fat identifies and characterizes amyloid fibrils in suspected cardiac amyloidosis. Amyloid Int J Exp Clin Investig Off J Int Soc Amyloid. 2002;9(2):108–114. [PubMed] [Google Scholar]
- 14.Vrana JA, Theis JD, Dasari S, Mereuta OM, Dispenzieri A, Zeldenrust SR, et al. Clinical diagnosis and typing of systemic amyloidosis in subcutaneous fat aspirates by mass spectrometry-based proteomics. Haematologica. 2014;99(7):1239–1247. doi: 10.3324/haematol.2013.102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brambilla F, Lavatelli F, Di Silvestre D, Valentini V, Rossi R, Palladini G, et al. Reliable typing of systemic amyloidoses through proteomic analysis of subcutaneous adipose tissue. Blood. 2012;119(8):1844–1847. doi: 10.1182/blood-2011-07-365510. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins PN, Lavender JP, Pepys MB. Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N Engl J Med. 1990;323(8):508–513. doi: 10.1056/NEJM199008233230803. [DOI] [PubMed] [Google Scholar]
- 17.Gertz MA, Skinner M, Sipe JD, Cohen AS, Kyle RA. Serum amyloid A protein and C-reactive protein in systemic amyloidosis. Clin Exp Rheumatol. 1985;3(4):317–320. [PubMed] [Google Scholar]
- 18.Kyle RA, Gertz MA, Greipp PR, Witzig TE, Lust JA, Lacy MQ, et al. A trial of three regimens for primary amyloidosis: colchicine alone, melphalan and prednisone, and melphalan, prednisone, and colchicine. N Engl J Med. 1997;336(17):1202–1207. doi: 10.1056/NEJM199704243361702. [DOI] [PubMed] [Google Scholar]
- 19.Lachmann HJ, Booth DR, Booth SE, Bybee A, Gilbertson JA, Gillmore JD, et al. Misdiagnosis of hereditary amyloidosis as AL (primary) amyloidosis. N Engl J Med. 2002;346(23):1786–1791. doi: 10.1056/NEJMoa013354. [DOI] [PubMed] [Google Scholar]
- 20.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M-V, et al. International Myeloma Working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 21.Bahlis NJ, Lazarus HM. Multiple myeloma-associated AL amyloidosis: is a distinctive therapeutic approach warranted? Bone Marrow Transpl. 2006;38(1):7–15. doi: 10.1038/sj.bmt.1705395. [DOI] [PubMed] [Google Scholar]
- 22.Rajkumar SV, Gertz MA, Kyle RA. Primary systemic amyloidosis with delayed progression to multiple myeloma. Cancer. 1998;82(8):1501–1505. doi: 10.1002/(SICI)1097-0142(19980415)82:8<1501::AID-CNCR11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th international symposium on amyloid and amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol. 2005;79(4):319–328. doi: 10.1002/ajh.20381. [DOI] [PubMed] [Google Scholar]