Abstract
Transfusion-dependent E-Beta (EB) thalassemia is one of the major causes of hereditary hemoglobinopathies in India. Hydroxyurea has been tried for HbF induction and amelioration of the transfusion frequency in EB thalassemia. The primary objective of this retrospective study, conducted between January 2017 and December 2018, was to determine the efficacy of thalidomide in reducing transfusion frequency in patients with EB thalassemia who have failed a reasonable trial of hydroxyurea. Of the 21 patients studied, 15 (71.4%) attained transfusion independence (complete responders) and 1 (4.7%) attained partial response (50% decrease in transfusion requirement) while 5 (23.9%) were non-responders. 12 patients attained their response within 1 month, 2 patients achieved within 1–3 months, and 1 patient beyond 3 months. Median time to transfusion independence in complete responders was 1 month. The median time on thalidomide for the complete responders and partial responders was 16.48 months. No major grade 3/4 toxicities were documented. This approach needs larger randomised controlled studies. Thalidomide is a safe and effective strategy at reducing or abrogating transfusion requirement in patients with EB thalassemia. This approach requires further testing in systematic clinical trials.
Keywords: E-Beta thalassemia, Thalidomide, Hemoglobinopathy, Transfusion-independence
Introduction
Thalassemia is the commonest monogenic disorder of the world [1]. In India and a major part of south-east Asia, E-Beta (EB) thalassemia accounts for a vast majority of the patients affected with thalassemia [2]. EB thalassemia can be quite diverse in its phenotype, ranging from transfusion-dependent to non-transfusion dependent patients. In those with transfusion dependent EB thalassemia, strategies that aim at HbF induction continue to be explored.
Among the drugs targeting HbF induction, hydroxyurea [Hydroxycarbamide] has the largest body of evidence [3]. Other drugs that have been explored include beta-hydroxybutyrate, and decitabine. Thalidomide has been shown to induce stress erythropoiesis and HbF induction. It has also been studied in pediatric population for a variety of indications and has been found to be safe and well tolerated [4]. Available evidence of the effect of thalidomide in patients with beta-thalassemia has been mostly anecdotal, in the form of case reports and few case series [5, 6].
Our study aimed at assessing the effect of thalidomide in patients visiting our service, who had transfusion-dependent EB thalassemia and had failed hydroxyurea therapy.
The primary aim of this retrospective study was to determine the efficacy of thalidomide in reducing transfusion frequency in patients with EB thalassemia who have failed a reasonable trial of hydroxyurea (at least 6 months at optimum dosing). The secondary objectives were to study the spectrum of significant adverse drug reaction with thalidomide (gastro-intestinal, somnolence, thrombosis), as well to document any change in serum ferritin and spleen size with thalidomide therapy.
Materials and Methods
This is a retrospective, single-center observational study, between January 2017 and December 2018. For logistic issues, we used convenience sampling and studied consecutive patients with a diagnosis of EB thalassemia based on hemoglobin electrophoresis/hemoglobin high-pressure liquid chromatography, who attended the haematology out-patient clinic of our hospital. The patients were required to be transfusion-dependent (at least 1unit packed RBC transfusion per month), with a reasonable trial of Hydroxyurea therapy (10–20 mg/kg body weight for at least 6 months) and naïve to other HbF-induction therapies.
The starting dose of thalidomide was 50 mg once daily in patients less than 12 years age and 100 mg in patients more than 12 years age. Dose escalation to 100 mg was allowed in patients < 12 years age with an inadequate response to 50 mg as evidenced by a failure to achieve at least 50% reduction in transfusion requirement. The patients were followed up 3 monthly for assessment of clinical parameters (symptoms, transfusion requirement, spleen size, any evidence of adverse effects due to drug specially any evidence of excessive somnolence, peripheral neuropathy, constipation, any clinical evidence of venous thrombosis) and laboratory parameters (complete blood count, ferritin, liver function test, renal function test and thyroid function tests). Thalidomide was discontinued if the patients did not attain at least ‘partial response’ after a trial of 3 months on the drug. Standard iron chelation strategies were employed.
Thalidomide dose-modification was allowed based on presence of Grade 2 or more adverse drug reaction (neuropathy, somnolence, constipation, or cytopenia). Females of child bearing potential were informed about the teratogenic potential of thalidomide and counselled to avoid getting pregnant during each clinic visit. They were specifically asked to use contraceptive measures. Urine pregnancy tests were done monthly and documented.
Since there is no standard definition of clinical/laboratory response to Hb-F induction strategies in patients with thalassemia, we devised an institutional response criterion for characterization of response to thalidomide in our cohort of patients. Transfusion threshold was kept at 7 gm/dl throughout the period of study. Complete response was defined as transfusion independence (100% reduction in transfusion requirement). However, allowance for transfusion during periods of physiological stress (infection, fever, surgery, etc.) was made and this was not considered while calculating the transfusion requirement for the patient. Partial response was defined as a 50–99% reduction in transfusion requirement. Anything less than partial response was categorized as ‘no response’.
Statistical Analysis
Patient demographics were collected and tabulated (Table 1). The parameters of interest (mean hemoglobin, transfusion frequency, spleen size, and serum ferritin) pre- and post-thalidomide therapy were compared using the Wilcoxon signed-rank test and dependent samples t test, as applicable, as applicable. Statistical analysis was done using SPSS software (SPSS Ver 20.0, IBM Inc, Chicago, IL, USA).
Table 1.
Baseline characteristics among overall patient population and among complete responders and non-responders
| Overall | Complete responders (n = 15) | Non-responders (n = 5) | |
|---|---|---|---|
| Age (years, median) | 20 | 20.5 | 18 |
| 0–10 (n) | 1 | 1 | 0 |
| 10–20 (n) | 9 | 5 | 4 |
| 20–30 (n) | 9 | 7 | 1 |
| 30–40 (n) | 1 | 1 | 0 |
| > 40 (n) | 1 | 1 | 0 |
| Sex (n, %) | |||
| Male | 7 (33) | 6 (40) | 1 (20) |
| Female | 14 (67) | 9 (60) | 3 (80) |
| Splenectomised (n, %) | 3 (14) | 3 (20) | 0 |
| Thalidomide starting dose (n, %) | |||
| 50 mg OD | 7 (33) | 7 | 0 |
| 100 mg OD | 14 (67) | 8 | 4 |
| Thalidomide final dose (n, %) | |||
| 50 mg OD | 4 (19) | 2 | 1 |
| 100 mg OD | 17 (81) | 13 | 3 |
| Spleen Size, Baseline, (cms) (median, range) | 4 (0–18) | 7 (0–18) | 5.5 (0–10) |
| Ferritin, Baseline (ng/ml), (median, range) | 2405.67 (131–10,250) | 1919 (131–6649) | 3931(862–10,250) |
Results
21 consecutive patients with a diagnosis of EB thalassemia and those who met the inclusion criteria were included in the study. Their baseline characteristics are summarized in Table 1.
Of the 21 patients enrolled, 15 (71.4%) attained transfusion independence (complete responders) and 1 (4.7%) attained partial response (50% decrease in transfusion requirement) while 5 (23.9%) were non-responders. 12 patients attained their response within 1 month, 2 patients achieved within 1–3 months, and 1 patient beyond 3 months). Median time to transfusion independence in complete responders was 1 month.
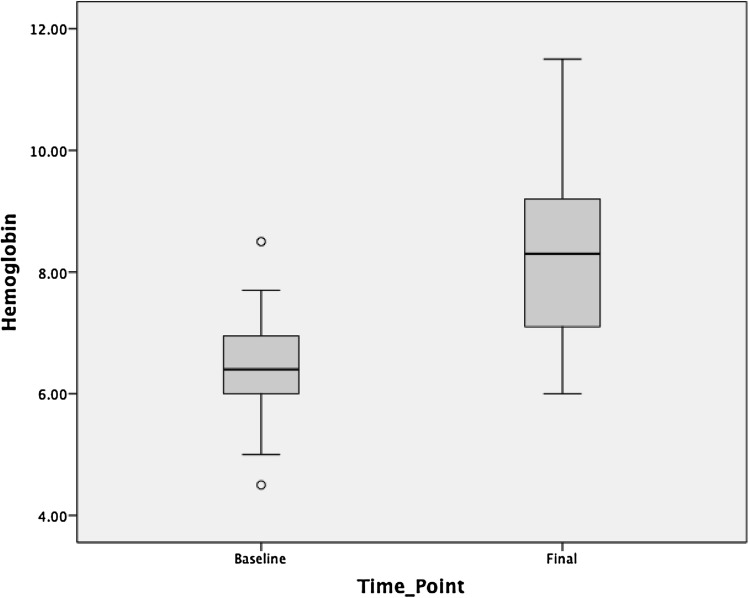
Median values of hemoglobin increased from 6.5 (± 1.08) gm/dl at baseline to 7.2 (± 1.74) gm/dl after Thalidomide therapy. Among complete responders, the median hemoglobin increased from 6.4 (± 1.0) gm/dl to 8.3 (± 1.5) gm/dl (Fig. 1). The median serum ferritin decreased from 1919 ng/ml at baseline to 1404 ng/ml after thalidomide therapy. Interestingly median ferritin in non-responders also decreased from 3931 ng/ml to 2610 ng/ml. While comparing the size of the spleen at the baseline and after 3 months of thalidomide therapy, the difference was not statistically significant (7.22 ± 1.37 cms, 7.56 ± 1.14 cms, p: 0.574).
Fig. 1.
Median Hemoglobin values pre- and post-thalidomide in responders
The major adverse effect documented was constipation (n = 10, 47%). This could be managed with bulk laxatives. No other major toxicities were documented. There was no drug discontinuation due to drug-related toxicities. The median time on thalidomide for the complete responders and partial responders was 16.48 months. Those that did not achieve a response had their drug discontinued after 3 months.
Discussion
E beta thalassemia contributes to a major burden of hereditary hemoglobinopathies in India. A significant proportion of EB thalassemia patients are transfusion dependent. HbF induction therapies have been tried at ameliorating the transfusion requirement in patients with EB thalassemia [3, 7, 8]. Approximately 30–40% patients show response with hydroxyurea in terms of reduction of transfusion requirements [8]. This study aimed to analyse the effect of thalidomide in mitigating the transfusion requirements in patients with EB thalassemia who were refractory to hydroxyurea.
Thalidomide has been tried previously in transfusion-dependent beta thalassemia as well as beta-thalassemia intermedia patients [5, 6]. However there has not been any published reports on the use of thalidomide monotherapy in patients with transfusion-dependent EB thalassemia, to the best of our knowledge. We found a 76.1% overall response rate (71.4% complete response and 4.7% partial response) with thalidomide which is more than the effect of any HbF induction strategy reported so far in EB thalassemia [1, 7, 8]. In patients who showed a complete transfusion independence, there was a 2.6 g/dl increment in median hemoglobin levels. The median ferritin levels also showed a decrease in both responders and non-responders (difference in median ferritin levels of the study population: 505 ng/dl). While there was no statistically significant difference noted in the splenic size in the entire cohort or within any of the subsets (responders, non-responders), the small sample size and the comparatively short follow up would probably confound any tests of significance. A prospective study looking systematically at the effect of thalidomide in EB thalassemia patients is mandated to have a more meaningful conclusion, especially given the fact that the drug holds promise in ameliorating transfusion requirements with minimal toxicities.
Conclusions
Thalidomide is a safe and effective strategy at reducing or abrogating transfusion requirement in patients with EB thalassemia. Initial results with the use of thalidomide monotherapy at our center have been encouraging. We are considering a larger, systematic clinical trial looking into the effect of thalidomide in thalassemia and the pathobiology of the drug response.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Olivieri NF, Pakbaz Z, Vichinsky E. Hb E/beta-thalassaemia: a common & clinically diverse disorder. Indian J Med Res. 2011;134:522–531. [PMC free article] [PubMed] [Google Scholar]
- 2.Weatherall DJ, Clegg JB. Inherited haemoglobin disorders: an increasing global health problem. Bull World Health Organ. 2001;79:704–712. [PMC free article] [PubMed] [Google Scholar]
- 3.Makala LH, Torres CM, Clay EL, Neunert C, Betty SP. Fetal hemoglobin induction to treat b-hemoglobinopathies: from bench to bedside. J Hematol Transfus. 2014;2:1–12. [Google Scholar]
- 4.Yang CS, Kim C, Antaya RJ. Review of thalidomide use in the pediatric population. J Am Acad Dermatol. 2015;72:703–711. doi: 10.1016/j.jaad.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Ren Q, Zhou Y-L, Wang L, Chen Y-S, Ma Y-N, Li P-P, Yin X-L. Clinical trial on the effects of thalidomide on hemoglobin synthesis in patients with moderate thalassemia intermedia. Ann Hematol. 2018;97:1933–1939. doi: 10.1007/s00277-018-3395-5. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Zhu W, Cai N, Bu S, Li J, Huang L. Thalidomide induces haematologic responses in patients with β-thalassaemia. Eur J Haematol. 2017;99:437–441. doi: 10.1111/ejh.12955. [DOI] [PubMed] [Google Scholar]
- 7.Singer ST, Kuypers FA, Olivieri NF, Weatherall DJ, Mignacca R, Coates TD, Davies S, Sweeters N, Vichinsky EP, E/beta Thalassaemia Study Group Fetal haemoglobin augmentation in E/beta0 thalassaemia: clinical and haematological outcome. Br J Haematol. 2005;131:378–388. doi: 10.1111/j.1365-2141.2005.05768.x. [DOI] [PubMed] [Google Scholar]
- 8.Biswas S, Nag A, Ghosh K, Ray R, Roy K, Bandyopadhyay A, Bhattacharyya M. Genetic determinants related to pharmacological induction of foetal haemoglobin in transfusion-dependent HbE-β thalassaemia. Ann Hematol. 2019 doi: 10.1007/s00277-018-3536-x. [DOI] [PubMed] [Google Scholar]