Abstract
Multiple sclerosis (MS) is a common autoimmune disease of the central nervous system, causing neurological disability in young adults. A growing understanding of its immunopathogenesis has led to an expanding array of therapies. Notable new advances in disease-modifying therapies for relapsing forms of multiple sclerosis that are based on anti-inflammatory activity have recently been developed. Management of progressive MS is still challenging. Data published in 2014 suggested that daily high doses of biotin, a vitamin involved in myelin synthesis, might have a beneficial impact on disability and progression in progressive MS. However, some patients worsened while on biotin without any clear explanation for this effect. We report the case of a 41-year-old patient suffering from primary progressive (PP) MS who presented after 16 months of treatment with high doses of biotin (QIZENDAY) with worsening of his Expanding Disability Status Scale (EDSS) score and the appearance of a symptomatic new T2 pseudo-tumoural lesion on brain magnetic resonance imaging (MRI), suggestive of tardive inflammatory reactivation possibly due to the biotin. The newer and more effective therapies for MS are, however, associated with risks that necessitate an active management strategy and continuous vigilance. Physicians should be aware of iatrogenic neurological complications and the possible paradoxical effects of biotin. Future treatment approaches to progressive MS must include identification of a biomarker of disease activity. The study of neurofilaments in the cerebrospinal fluid (CSF) and the serum could be of interest when determining the optimal treatment strategy.
Keywords: Adverse drug reaction, Biotin, MRI, Multiple sclerosis, Pseudo-tumoral lesion
Key Summary Points
| Multiple sclerosis (MS) is the most common autoimmune disease of the central nervous system |
| High doses of biotin might have a beneficial impact on disability and progression in progressive MS |
| A patient with high doses of biotin presented with worsening of his EDSS score and the appearance of a symptomatic new T2 pseudo-tumoural lesion on brain MRI |
| These data are suggestive of tardive inflammatory reactivation probably due to the biotin |
| Future treatment approaches to progressive MS must include identification of a biomarker of disease activity |
Introduction
New advances in disease-modifying therapies for relapsing and progressive forms of multiple sclerosis (MS) have recently emerged that are based on anti-inflammatory mechanisms. Treatment of relapsing multiple sclerosis (MS) has been revolutionized by novel drugs like natalizumab and fingolimod. Ocrelizumab, a B cell-targeted anti-CD20 therapy, and cladribine have also been approved recently for relapsing MS [1]. However, while management of progressive MS is still challenging, neuroprotective therapy has been developed. Published data have suggested that high doses of biotin (100 mg, three times a day) are beneficial for patients with progressive MS [2]. Biotin is a vitamin acting as a coenzyme for carboxylases involved in several key steps of energy metabolism and fatty acid synthesis that activate acetyl-CoA carboxylase, a potentially rate-limiting enzyme involved in myelin synthesis [3]. However, some patients inexplicably worsened while on biotin [4]. We report the case of a 43-year-old patient suffering from primary progressive (PP) MS who presented after 16 months of treatment with high doses of biotin (QIZENDAY) with worsening of his Expanding Disability Status Scale (EDSS) score and relapse with the appearance of a symptomatic new T2 pseudo-tumoural lesion on brain magnetic resonance imaging (MRI), suggestive of tardive inflammatory reactivation possibly due to the biotin. Informed consent was obtained from the patient for being included in the study. We have received ethics board approval (Comité d’éthique du Groupe Hospitalier du Havre). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Case Report
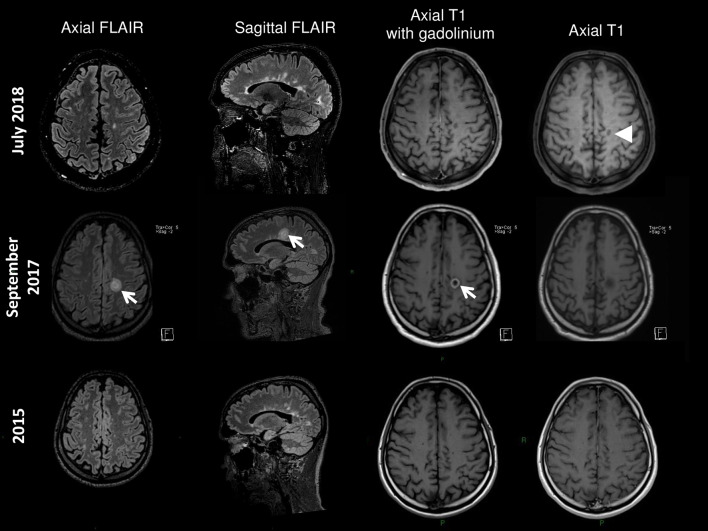
A 43-year-old man suffering from PP MS was hospitalized for subacute right hemiparesis. His initial symptoms occurred at age 34 years (progressive spastic paraparesis in 2008, EDSS 3.5). His first MRI revealed multiple cerebral and spinal cord lesions without gadolinium enhancement. Betaferon was prescribed from 2009 until December 2015. Progressive worsening occurred (EDSS 4.5 in March 2012 to EDSS 6 in May 2015) with tetraparesis, ataxia, bilateral internuclear ophthalmoplegia and cognitive dysfunction. Neither relapses nor radiological progression was noted. Betaferon was stopped and a monthly bolus of corticosteroids was administered (1 g intravenous per month) from December 2015 to April 2016. High doses of biotin (300 mg daily) were introduced in May 2016 (EDSS 6). Then, a rapid and unusual clinical worsening occurred: severe worsening of walking impairment, spasticity and ataxia leading to rollator use, and asthenia (EDSS 7.5 in May 2017). In September 2017, he was hospitalized for subacute right hemiparesis (EDSS 8.5). Cerebral MRI revealed a pseudo-tumoural inflammatory lesion with gadolinium enhancement (Fig. 1). Spinal cord MRI was unchanged. Blood tests were normal. The biotin was discontinued. Intravenous corticosteroids (1 g per day for 3 days) were initiated. His EDSS progressively improved within 3 weeks. Rituximab therapy was commenced and given for two infusions in February 2018; 10 months after withdrawal of biotin, his EDSS improved (6) and the pseudo-tumoural lesion had decreased, albeit with the appearance of a persistent black hole (Fig. 1).
Fig. 1.
MRI follow-up. The cerebral MRI before biotin (2015) revealed multiple cerebral lesions without gadolinium enhancement. Sixteen months after initiation of biotin (September 2017) a pseudo-tumoural inflammatory lesion with gadolinium enhancement appeared. Ten months after withdrawal of biotin, intra-venous corticosteroids and Rituximab therapy, the pseudo-tumoural lesion had decreased, albeit with the appearance of a persistant persistent black hole
Informed consent was obtained from the patient for being included in the study. We have received ethics board approval (Comité d’éthique du Groupe Hospitalier du Havre).
Discussion
This case showed clear worsening of disability in a patient suffering from PP MS with radiological progression 16 months after initiation of biotin. In a cohort of 43 patients with progressive MS treated with biotin for 1 year, one-third worsened, with two patients worsening sufficiently to increase their EDSS scores by 0.5 [5]. Several worsened patients improved after stopping the biotin. Their increase in disability was attributed to the neurodegenerative component of the disease, or the inability of the injured central nervous systems to respond to the increased metabolic demands induced by biotin. Labauge and colleagues [4] previously reported the case of a 40-year-old patient with symptoms of solitary sclerosis treated for more than 2 years with high doses of biotin who presented with the appearance of asymptomatic new T2 white matter lesions on brain MRI. A latent inflammatory condition revealed by biotin was suspected. A possible hypothesis could be that high-dose biotin competes with the metabolism or transport of other B vitamins like riboflavin. B vitamins are a group of eight water-soluble vitamins acting as coenzymes that perform essential, closely interrelated roles in cellular functioning (catabolic and anabolic enzymatic reactions). Alternative potential explanations for this dramatic clinical and radiologic worsening of this PP MS after 1 year of taking high-dose biotin can be discussed. This could be related to a tardive effect of betaferon and discontinuation of monthly steroids. Another explanation could be the aggressive course of the disease in a patient with aggressive relatively young onset MS, with superimposed severe relapses and MRI evidence of disease activity as increasingly documented in progressive MS. In our case, no clinical relapses or new lesions were noted before biotin introduction. The increase in the patient’s EDSS score was unusually rapid (an increase of 2.5 points over 1 year). Furthermore the radiological progression was severe, with a new pseudo-tumoural lesion. This clinical and MR severe evolution occurred 1 year after initiating biotin therapy. The patient improved after biotin discontinuation and anti-inflammatory therapy (corticosteroids and rituximab). These findings strongly suggest an inflammatory activation induced by biotin in a patient with PP MS.
Conclusion
On the basis of our case and others, biotin may well cause paradoxical effects and worsen some specific patients with progressive MS, activating a latent inflammatory condition. The newer and more effective therapies are, however, also associated with risks that necessitate an active management strategy and continuous vigilance. Future treatment approaches to progressive MS must include the identification of a biomarker of disease activity. Neurofilaments in the cerebrospinal fluid (CSF) and the serum could be interesting to evaluate to see if they can assist in determining the optimal strategy.
Acknowledgements
We thank all the participants of the study.
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Alexis Demas, Jean-Philippe Cochin, Clémence Hardy, Yvan Vaschalde, Bertrand Bourre and Pierre Labauge have nothing to disclose.
Compliance with Ethics Guidelines
Informed consent was obtained from the patient for being included in the study. We have received ethics board approval (Comité d’éthique du Groupe Hospitalier du Havre). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Enhanced digital features
To view enhanced digital features for this article go to 10.6084/m9.figshare.11342420.
References
- 1.Deisenhammer F, Auer M, Hegen H. Ocrelizumab in primary progressive and relapsing multiple sclerosis. N Engl J Med. 2017;376(17):1693–1694. doi: 10.1056/NEJMc1702076. [DOI] [PubMed] [Google Scholar]
- 2.Birnbaum G, Stulc J. High dose biotin as treatment for progressive multiple sclerosis. Mult Scler Relat Disord. 2017;18:141–143. doi: 10.1016/j.msard.2017.09.030. [DOI] [PubMed] [Google Scholar]
- 3.McCarty MF, DiNicolantonio JJ. Neuroprotective potential of high-dose biotin. Med Hypotheses. 2017;109:145–149. doi: 10.1016/j.mehy.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Lebrun C, Cohen M, Mondot L, Ayrignac X, Labauge P. A case report of solitary sclerosis: this is really multiple sclerosis. Neurol Ther. 2017;6(2):259–263. doi: 10.1007/s40120-017-0082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peyro Saint Paul L, Debruyne D, Bernard D, Mock DM, Defer GL. Pharmacokinetics and pharmacodynamics of MD1003 (high-dose biotin) in the treatment of progressive multiple sclerosis. Expert Opin Drug Metab Toxicol. 2016;12(3):327–344. doi: 10.1517/17425255.2016.1136288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.