Abstract
The evolution of the structure of amorphous carbon (a-C) films during deposition and thermal annealing is of significant interest from both the materials science and application perspectives. However, despite the voluminous literature of studies dealing with the deposition and physical properties of a-C films, basic understanding of the structure evolution due to phase change during film growth and heating is fairly sparse and empirical, presumably due to the lack of high-resolution instruments that can probe structural changes at the atomic and molecular levels in real time. Molecular dynamics (MD) is a powerful computational method for studying atomic/molecular-scale movement and interactions. Thus, the objective of this study was to perform MD simulations that provide insight into changes in the structure of ultrathin a-C films during deposition and annealing. Simulation results reveal a multi-layer film structure, even for a-C films as thin as ~20 Å, the existence of a deposition energy that yields a-C films with the highest sp3 content, the transient and steady-state stages of the structure evolution during annealing at different temperatures, and the changes in the hybridization state (mainly in the bulk layer) encountered during annealing at elevated temperatures. The MD results of this study are of particular importance to applications where the deposition conditions and operation temperature affect the structure and, in turn, the physical properties of ultrathin a-C films used as protective overcoats.
Subject terms: Materials science, Physics
Introduction
Amorphous carbon (a-C) is a solid consisting of multiple types of bonding configurations that lack long-range ordered structures and may exhibit deviations in interatomic distances and bond angles due to the presence of a high concentration of dangling bonds1. In principle, carbon atoms can form three different bonding configurations, i.e., sp3, sp2, and sp1 atomic carbon hybridizations2. Thus, most a-C films contain graphite-like (sp2) and diamond-like (sp3) carbon crystals. Various studies have shown that the physical properties of a-C films, such as hardness, friction, and thermal stability, are mainly determined by the fraction of sp3 bonding3–10.
The development of thin-film deposition techniques during the late 20th century accelerated the use of carbon films in applications requiring protective coatings with excellent tribomechanical properties and coatings with exceptional optical and electrical properties11,12. For example, in contemporary hard-disk drives (HDDs) an ultrathin (~20 Å thick) a-C film is used to protect the magnetic head and the hard disk against corrosion and surface damage caused by intermittent asperity contact that may lead to mechanical wear and seizure. In addition to outstanding tribomechanical properties, the a-C films may be required to exhibit good thermal stability if the device operates at an elevated temperature. An example is heat-assisted magnetic recording (HAMR), a contemporary data storage technology that promises to provide extremely high areal storage densities, in which intensive laser heating is applied to both the head and disk media to enable data to be written in the magnetic medium of the hard disk; thus, the thermal stability of the protective a-C film is vital to the functionality and longevity of these HDDs that operate under intense thermal conditions13,14. Earlier studies were focused on the investigation of the structure of a-C films deposited under various process conditions (e.g., incidence angle, substrate bias voltage and duty cycle, and deposition time) using high-resolution transmission electron microscopy and electron energy loss spectroscopy15–19. These studies have revealed a strong dependence of deposition parameters on a-C film structure and the deposition conditions for synthesizing a-C films with a high sp3 content. The thermal stability of ultrathin a-C films deposited on various substrates has also been assessed in the light of annealing experiments6,20.
Despite important insight into the growth and thermal stability of ultrathin a-C films derived from aforementioned investigations, knowledge of the structure evolution of these ultrathin films during deposition and thermal annealing is mostly empirical, presumably due to the lack of high-resolution instruments for real-time probing of structural changes at the atomic and molecular levels. Molecular dynamics (MD) is a powerful computational method that promises to bridge the knowledge gap of atomic-scale structural changes instigated in nanometer-thick films during deposition and heating. MD simulations of carbon deposition on diamond performed with the Tersoff or adaptive intermolecular reactive empirical bond-order potential have elucidated the effect of deposition parameters on the a-C film structure21–23. Graphitization of a-C films due to thermal annealing or ion bombardment has also been investigated in MD studies that used the modified Brenner and Tersoff potentials to model atomic interaction24–26. The former studies have provided important information about the growth and graphitization of ultrathin a-C films, which is difficult, if not impossible, to obtain experimentally. The multi-layer structure of a-C films synthesized by energetic deposition processes, such as filtered cathodic vacuum arc17, makes the experimental study of structural changes in these films even more challenging, especially for nanometer-thick films.
The evolution of the structure of a-C films during thermal annealing strongly influences the continuity, permeability, and nanomechanical/tribological properties of these films. However, the majority of previous MD studies only considered spatial changes in the multi-layer structure of ultrathin a-C films before and after annealing at different temperatures, lacking critical time-dependent information about spatial changes in film structure. Knowledge of time-dependent changes in a-C film structure during deposition and thermal annealing is critical to the fundamental understanding of the film-growth process and the development of thermally-induced damaging processes, such as graphitization and oxidation. Consequently, the objective of this investigation was to perform a comprehensive MD analysis of ultrathin a-C film deposition on silicon followed by thermal annealing, using the Tersoff potential to model carbon-carbon, silicon-silicon, and carbon-silicon atomic interactions. The analysis comprised two simulation phases. First, carbon atom deposition on a silicon substrate was simulated and the evolution of the a-C film structure under deposition conditions of various atom carbon kinetic energies was investigated. Second, thermal annealing of a-C films with the highest sp3 content was simulated at various temperatures to illuminate transient changes in the film structure. The MD simulations of this study confirm the multi-layer structure of ultrathin a-C films and reveal spatiotemporal variations in the film structure at different annealing temperatures, providing insight into the structural stability of a-C films under application conditions where thermal effects are significant.
Analytical Methodology
Interatomic potential function
In all the MD analyses of multi-atom systems where interatomic forces are described by a potential energy function, atomic movement is determined by solving a set of differential equations of motion. Commonly used interatomic potential energy functions, such as the one-, two-, and three-body potentials, provide a description of the atom positions. However, these interatomic potentials may fail to describe the different bonding states of atoms, such as carbon and silicon. This inadequacy can be remedied by a bond-order potential that couples a two-body potential with a multi-atom correlation27. Using the so-called Tersoff potential, the total energy E of an atomic system can be expressed as
| 1 |
| 2 |
where Ei is the potential energy of atom i, Vij is the bonding energy between atoms i and j, rij is the distance between atoms i and j, fC is a smooth cutoff function used to generate a gradual energy decrease to zero with increasing atomic distance, fR and fA are repulsive and attractive two-body potentials, respectively, and bij is termed the bond order and represents the bond strength in the local environment, i.e., the bond strength of atoms having more neighboring atoms is characterized by a lower potential energy. The potential parameters are obtained by theoretical and experimental fits of the cohesive energy of element configurations. The first two elements to be fit with the Tersoff potential are carbon and silicon27,28. More details about the Tersoff potential can be found elsewhere29,30.
Simulation procedure
As mentioned earlier, the MD simulations of the present study comprise two phases: (1) a-C film growth on a Si(100) substrate for various carbon atom kinetic energies and (2) thermal annealing at various temperatures. Film growth was simulated on a 23 × 23 × 59.7 Å silicon substrate consisting of 1620 atoms (Fig. 1). To model an infinitely large deposition area, periodic boundary conditions were applied to the lateral sides of the substrate. The silicon substrate atoms are classified in three categories, shown by different colors in Fig. 1. To simulate a semi-infinite substrate, the two lattice layers at the bottom of the MD model consisting of yellow atoms were fixed. The hollow prism made of red atoms is a Berendsen heat bath that controls the temperature during the simulation process31. The green atoms inside the hollow prism of red silicon atoms are free to interact with the incident carbon atoms. This model dissipates the internal energy rise caused by incident carbon atoms and maintains the substrate temperature at the desired level.
Figure 1.
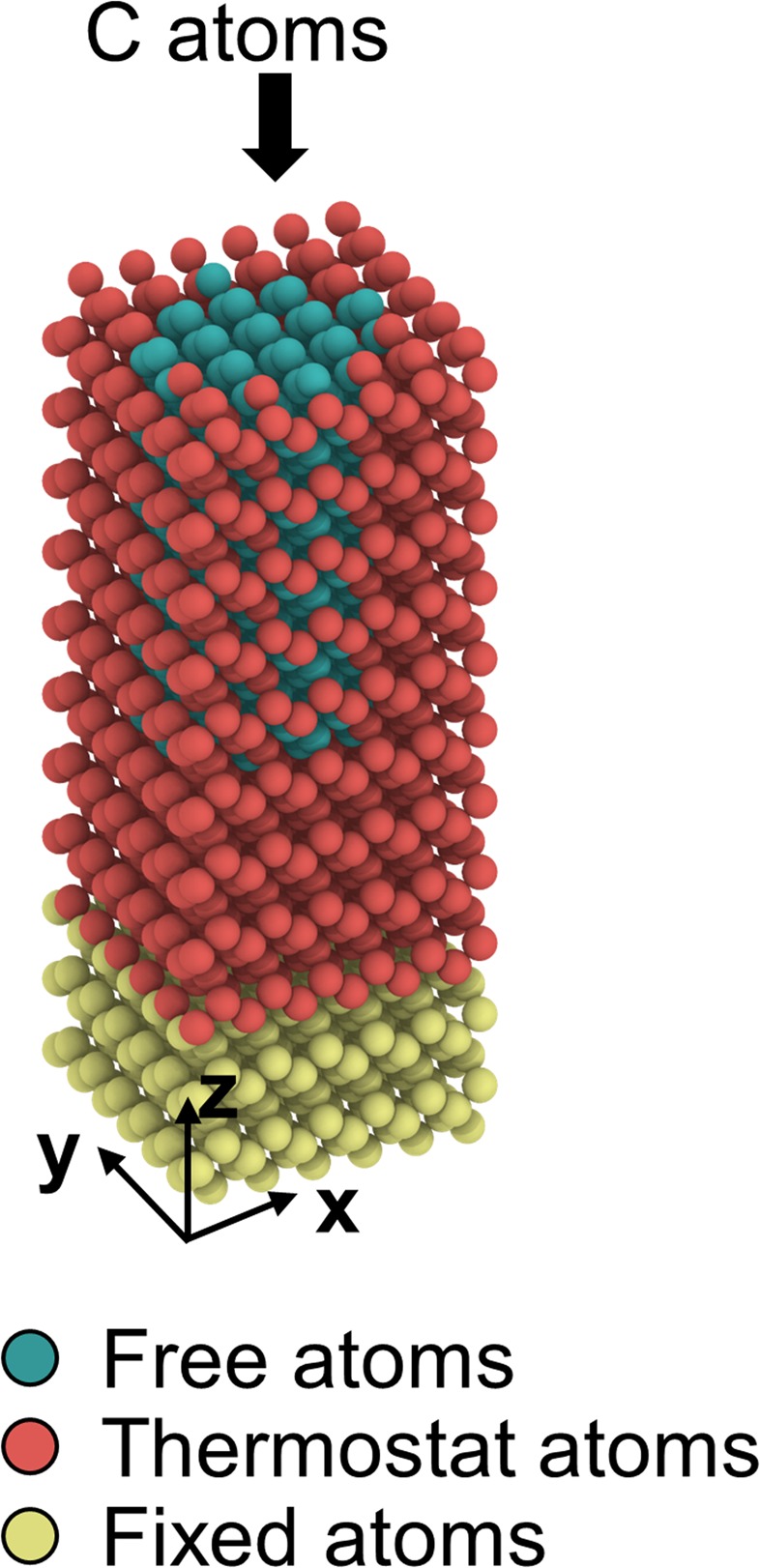
The MD model of the crystalline Si(100) substrate. The yellow atoms at the bottom of the model are fixed to simulate a semi-infinite bulk substrate. The red atoms comprise a Berendsen heat bath that is used to control the temperature of the whole model. The green atoms that are fully surrounded by the thermostat atoms can freely interact with incoming carbon atoms.
Before initiating carbon atom deposition, the silicon substrate was allowed to relax so that to minimize both the energy and the internal stress in the system. This was accomplished by increasing the temperature from 300 to 800 K, maintaining the temperature at 800 K for 5 ps, and finally cooling to 300 K. Thermal relaxation prevented the development of high internal stresses due to the effect of surface tension. After the relaxation of the silicon substrate, 2000 carbon atoms were randomly introduced one at a time from a height of 57 Å above the substrate surface and with velocity normal to the substrate surface. Using a time interval of 2 ps between sequential carbon atom injections allowed the system to relax and reach equilibrium via the Berendsen heat bath using a damping constant of 100 fs. Considering that the thermal spikes generated by bombarding energetic particles of kinetic energy less than 100 eV typically last for less than 0.5 ps32, using a time interval of 2 ps was adequate for simulating film growth for a particle kinetic energy of less than 120 eV. In all the deposition simulations, the time step was fixed at 0.5 fs and the heat bath temperature was set at 300 K.
In the thermal annealing simulations, the a-C film formed in the deposition phase of the simulation was kept at a constant temperate for 50 ns. The temperature was controlled by the Berendsen heat bath using a damping constant of 100 fs. In all the annealing simulations, the time step was fixed at 0.1 fs. All the MD simulations were performed with the Large-scale Atomic/Molecular Massively Parallel Simulator (LAMMPS) using a node with a 20-core Intel Xeon E5-2670 CPU. To validate the MD model, several simulations were performed to determine the time step and model dimensions that did not affect the results. Representative results from these simulations are shown in Fig. S1 of the Supplementary Information (SI).
Results and Discussion
Film growth
Figure 2 shows a-C film growth on a silicon substrate for a carbon atom kinetic energy in the range of 1–120 eV. Silicon and carbon atoms are shown in green and yellow colors, respectively. The bombardment of carbon atoms changes the crystalline structure of the silicon substrate, leading to the formation of an amorphous layer at the surface, hereafter referred to as the intermixing layer. The atoms in the intermixing layer comprise various silicon components bonded to neighboring silicon or carbon atoms. The intermixing layer acts as an adhesion layer that bonds the a-C film to the silicon substrate. The thicknesses of the intermixing layer and the a-C film strongly depend on the kinetic energy of the incident carbon atoms. The carbon atoms with a high kinetic energy can penetrate deeper into the substrate, producing a thicker intermixing layer. The intense bombardment of high energy (i.e., ≥80 eV) carbon atoms also causes a small fraction of silicon atoms to migrate into the a-C film.
Figure 2.
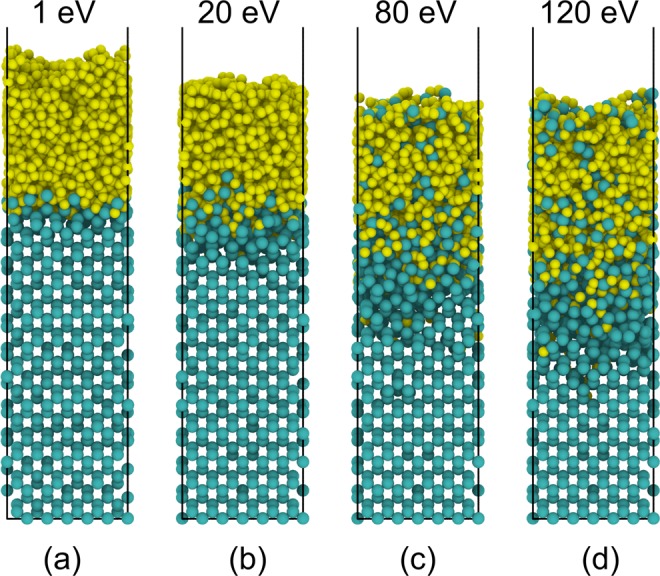
Simulated a-C film growth on a crystalline Si(100) substrate for carbon atom deposition energy equal to (a) 1, (b) 20, (c) 80, and (d) 120 eV. Silicon and carbon atoms are shown in green and yellow colors, respectively.
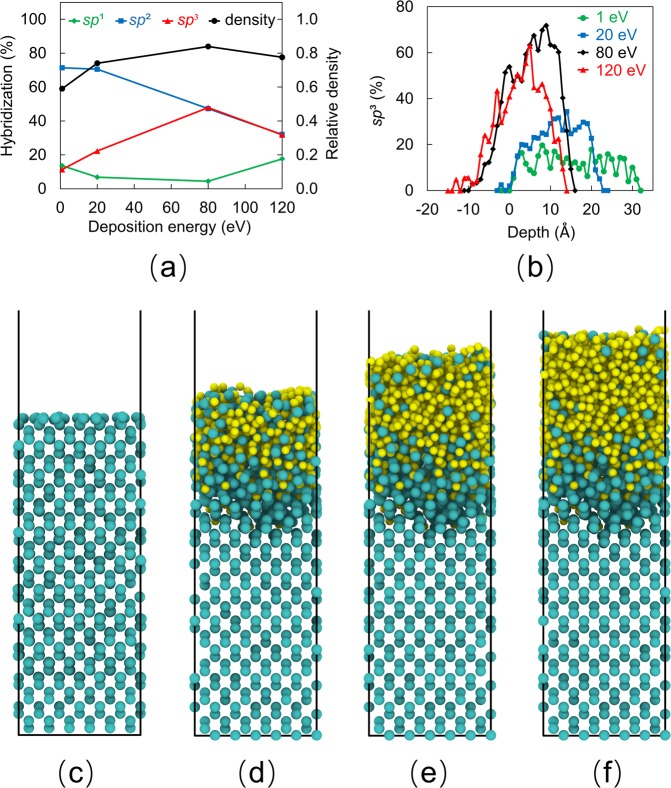
The coordination number of carbon atoms affects the structure and properties of the a-C film significantly. This number is defined as the number of nearest neighbor atoms and is indicative of the hybridization state, i.e., a coordination number equal to 4, 3, and 2 implies sp3 (tetrahedral, diamond-like), sp2 (trigonal, graphite-like), and sp1 (linear) hybridization, respectively. The coordination number of an atom depends on the cutoff distance of the nearest neighbor atoms. The position of the minimum between the first peak (r1) and the second peak (r2) of the radial distribution function is a plausible choice for the cutoff distance. Typical values of r1 and r2 for C-C bonds are 1.45 and 2.55 Å, whereas for C-Si bonds r1 and r2 are equal to 1.85 and 2.85 Å, respectively. In the present study, the cutoff distance for C-C bonds was set at 1.9 Å, while that for C-Si bonds was set at 2.0 Å, which is close to the 2.1 Å cutoff distance used in previous works33,34. Because carbon atoms can form bonds with both carbon and silicon atoms, the sp2 and sp3 contents reported here include both C-C and C-Si bonds. Although over- and under-coordinated carbon atoms may co-exist, they represent a very small fraction. Specifically, very few (0.7%) under-coordinated carbon atoms (coordination number = 1) were found at the film surface, which are attributed to the limited atomic bombardment of the outermost atomic plane and the effect of surface relaxation. A very small amount (3.9%) of over-coordinated carbon atoms (coordination number = 5) was found in the as-deposited film structure. Within a very short time from the inception of thermal annealing, all the over-coordinated atoms attained a more stable state corresponding to a coordination number of 2, 3, or 4. Consequently, the effect of under- and over-coordinated carbon atoms on the film structure was secondary. Figure 3(a) shows the sp3, sp2, and sp1 hybridization contents and the relative density of the a-C film (defined as the ratio of the film density to that of diamond) versus the deposition energy of carbon atoms. Low deposition energy (e.g., 1 eV) yields a low density and mostly sp2-hybridized a-C film forming by carbon atom physisorption. A graphite-like film structure (~72% sp2) is produced because the low kinetic energy of impinging carbon atoms is not effective in breaking the thermodynamically stable sp2 and sp1 carbon-carbon bonds. The increase of the carbon atom energy enhances carbon-silicon intermixing and sp3 hybridization in the a-C film, resulting in a thicker intermixing layer (Fig. 2) and a thinner, denser, and more sp3-hybridized film structure (Fig. 3(a)). Energetic incident carbon atoms can break existing carbon-carbon bonds (mainly the relatively weaker sp1 and sp2 bonds) and cause resputtering and/or recoil implantation, increasing the film density and promoting sp3 hybridization. The highest sp3 content (~48%) and relative density (~0.84) correspond to carbon atom deposition energy equal to 80 eV. Increasing further the deposition energy (e.g., 120 eV) decreases both the sp3 fraction and the relative density of the film. This is attributed to localized relaxation prompted by thermal spikes and the greater prospect of sp3 bond breakage and resputtering of deposited carbon material resulting from the intense atomic bombardment. All of the foregoing effects are conducive to graphitization. The similar variations of the sp3 content and the relative density of the a-C film with the increase of deposition energy (Fig. 3(a)) reveal a close dependence of film density on sp3 content.
Figure 3.
(a) Variation of sp1, sp2, and sp3 contents and relative density (normalized by the density of diamond) of a-C films with the carbon atom deposition energy, (b) sp3 depth profiles of a-C films for carbon atom deposition energy equal to 1, 20, 80, and 120 eV (negative depth values correspond to the silicon substrate), (c) silicon substrate after relaxation, (d) carbon-silicon intermixing during the initial deposition phase resulting in the formation of an intermixing layer, (e) main deposition phase involving the growth of a bulk layer, and (f) formation of a surface layer during the end of the deposition process. Silicon and carbon atoms are shown in green and yellow colors, respectively.
It is well-known that a-C films synthesized by energetic particle bombardment exhibit a multi-layer structure consisting of intermixing, bulk, and surface layers characterized by significantly different hybridizations17,35,36. The bulk layer possesses a much higher sp3 content than the other two layers. The sp3 content of the intermixing layer increases gradually from the substrate toward the interface with the bulk layer, while that of the surface layer decreases sharply from the interface with the bulk layer toward the film surface. To examine whether the MD model can simulate the multi-layer structure of a-C films, sp3 depth profiles were computed by averaging the sp3 content of 1-Å-thick slices through the film thickness. Figure 3(b) shows the effect of carbon deposition energy on the sp3 depth profile of simulated a-C films. All cases reveal a sharply increasing sp3 content at the film’s interface with the silicon substrate (intermixing layer), a high sp3 content in the middle region (bulk layer), and a sharply decreasing sp3 content in the region adjacent to the film surface (surface layer). For very low deposition energy of 1 eV, the sp3 depth profile is fairly flat, the sp3 content is below 20%, and the intermixing layer is very thin, indicating the formation of a graphite-like a-C film that is slightly integrated with the substrate. Increasing the deposition energy to 80 eV not only enhances carbon-silicon intermixing, which is critical to the bond strength of the film to the substrate, but also decreases the film thickness and increases significantly the sp3 content in the bulk layer, which controls the overall properties of the a-C film. However, the sp3 content in the bulk layer decreases with the increase of the deposition energy to 120 eV, indicating the existence of an energy threshold for achieving the highest sp3 content, in agreement with the experimental evidence19. Interestingly, all simulation cases show the formation of a surface layer with sp3 content sharply decreasing toward the film surface, also in agreement with the results of a previous experimental study15. The formation of a graphite-like surface layer is attributed to less atomic bombardment than the bulk layer (because it forms at the end of the deposition) and surface relaxation resulting in a minimum energy state. During surface relaxation, the carbon atoms adjacent to the film surface migrate to lower energy sites where it is easier for them to become sp2 hybridized and the decrease of the intrinsic compressive stress promotes sp3→sp2 rehybridization.
The above simulation results and analysis suggest a film-growth process characterized by the sequential main phases depicted in Fig. 3(c–f). An animation of the film-growth process can be seen in Video A of the SI. During the initial deposition phase, carbon atom physisorption and/or implantation are the dominant physical mechanisms, depending on the kinetic energy of the carbon atoms, which control the formation of an ultrathin carbon layer on top of the intermixing layer. Newly arriving carbon atoms collide with previously deposited carbon atoms, knocking them deeper into the substrate (recoil implantation) and contributing to the formation of the intermixing layer (Fig. 3(d)). An animation of recoil implantation where a carbon atom at the surface of the silicon substrate is knocked to a deeper location by an impinging energetic carbon atom can be seen in Video B of the SI. This deposition phase ends when the substrate becomes fully saturated with carbon atoms and carbon-silicon atom interaction diminishes. Next, the incident carbon atoms impinging onto the formed carbon atom planes lead to the growth of the bulk layer (Fig. 3(e)). The densification of the bulk layer and the evolution of high compressive stresses that are conducive to sp3 hybridization and sp2→sp3 rehybridization are encountered under deposition conditions of energetic atom bombardment (e.g., deposition energy of 80 eV). In the final deposition phase, the resputtering of weakly bonded carbon atoms decreases and the development of high compressive stresses for sp3 hybridization diminishes due to less atomic bombardment of the topmost carbon planes. These effects together with surface relaxation are responsible for the formation of a surface layer rich in sp2 hybridization (Fig. 3(f)).
Table 1 shows the effect of the deposition energy of carbon atoms on the thickness of the intermixing, bulk, and surface layers. The boundaries of these layers were determined by computing the number of carbon atoms Nc in each 1-Å-thick slice. The Nc of the surface layer showed a sharp decrease, whereas that of the bulk layer remained fairly constant. The thickness of each layer was consequently determined from its known boundaries with other layers. For low deposition energy (e.g., ≤20 eV), penetration of the crystalline silicon structure by impinging carbon atoms is minimal; thus, the thickness of the intermixing layer is very small (2–3 Å). The increase of deposition energy (e.g., ≥80 eV) enhances carbon atom penetration, direct implantation, and recoil implantation in the silicon substrate, producing a significantly thicker intermixing layer. Indeed, for deposition energy equal to 80 and 120 eV, the thickness of the intermixing layer is 12 and 15 Å, respectively, corresponding to about 2.2 and 2.8 times the silicon lattice constant (5.431 Å). In contrast to the intermixing layer thickness, the bulk layer thickness decreases with increasing deposition energy because carbon atoms with a higher kinetic energy are implanted into the silicon substrate, contributing to the formation of a thicker intermixing layer than the growth of a thicker bulk layer. Nevertheless, the effect of carbon atom deposition energy on the surface layer thickness is secondary. This is attributed to less pronounced atomic bombardment of the surface layer in the last deposition phase and the effect of surface relaxation, as explained above.
Table 1.
Thickness of intermixing, bulk, and surface layers comprising the multi-layer structure of a-C films versus carbon atom deposition energy.
| Deposition energy (eV) | Thickness (Å) | ||
|---|---|---|---|
| Intermixing layer | Bulk layer | Surface layer | |
| 1 | 2 | 33 | 6 |
| 20 | 3 | 22 | 3 |
| 80 | 12 | 13 | 4 |
| 120 | 15 | 11 | 3 |
Thermal annealing
MD simulations of thermal annealing were performed for the a-C film with the highest (48%) sp3 content, corresponding to carbon atom deposition energy of 80 eV (Fig. 3(b)). To examine the thermal stability of this film, simulations of thermal annealing were performed for a temperature between 150 and 450 °C. As stated earlier, the thermal stability of ultrathin a-C films is critical to the performance of devices operating at elevated temperatures, such as the HDDs designed for HAMR. Thus, the selected range of annealing temperatures is typical of the temperature range of a-C films used as protective overcoats of HAMR media.
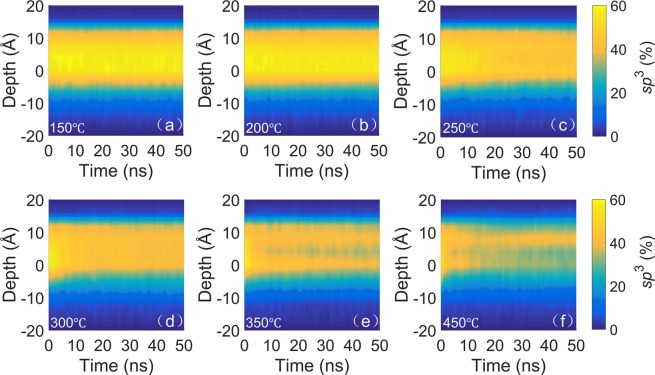
Figure 4 shows the overall sp3 film content (computed as the average of the sp3 contents of the intermixing, bulk, and surface layers) versus annealing time in the temperature range of 150–450 °C. The film’s structural stability is determined by the minimum temperature that causes the sp3 fraction to decrease sharply, resulting in sp3→sp2 rehybridization. Annealing at 150 and 200 °C decreases the sp3 fraction by only ~2%, whereas annealing at 250, 300, 350, and 450 °C decreases significantly the sp3 fraction by 9%, 11%, 15%, and 17%, respectively. These predictions are in agreement with experimental results showing that annealing at 350 °C causes a decrease of the sp3 content of a-C films deposited on various substrates by 4–16%20. For annealing at 300, 350, and 450 °C, the sp3 content reaches a steady state in a very short time, indicating that rehybridization is a very rapid process. The increase of the annealing temperature decreases the time for the sp3 fraction to reach a steady state. Table 2 shows the effect of annealing temperature on the thickness of the intermixing, bulk, and surface layers. The thickness of the surface layer increases with the temperature at the expense of the bulk layer thickness, while the thickness of the intermixing layer remains constant. The latter is in agreement with electron energy loss spectroscopy results of a previous study20 showing that annealing at 350 °C yields minor changes of the intermixing layer thickness of a-C films deposited on Au, SiN, NiCr, TaOx, and FeCo substrates.
Figure 4.
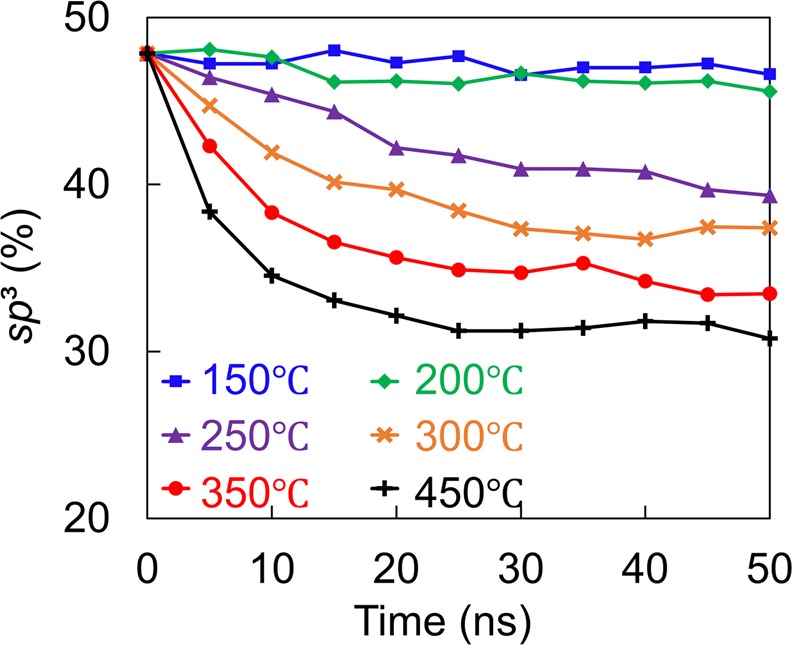
Variation of the overall sp3 content of a-C film with 48% sp3 content versus annealing time and temperature in the range of 150–450 °C.
Table 2.
Thickness of intermixing, bulk, and surface layers comprising the multi-layer structure of a-C film with 48% sp3 overall content obtained after annealing versus annealing temperature.
| Annealing temperature (°C) | Thickness (Å) | ||
|---|---|---|---|
| Intermixing layer | Bulk layer | Surface layer | |
| 150 | 12 | 13 | 4 |
| 200 | 12 | 13 | 4 |
| 250 | 12 | 13 | 5 |
| 300 | 12 | 12 | 5 |
| 350 | 12 | 12 | 6 |
| 450 | 12 | 12 | 6 |
Although Fig. 4 shows the evolution of the a-C film structure due to heating, i.e., sp3→sp2 rehybridization, it does not provide insight into spatiotemporal changes of the film structure. Thus, to investigate localized changes in the film structure in the course of annealing, the variation of the sp3 fraction through the film thickness with the annealing time and temperature was examined. As shown in Fig. 5(a,b), the effect of annealing at 150 and 200 °C on the spatial and temporal variation of the sp3 fraction is practically insignificant, consistent with the invariance of the overall sp3 content at these temperatures (Fig. 4). However, annealing at 250 °C causes a discernible decrease in the sp3 content of the bulk layer (Fig. 5(c)), with the effect intensifying as the temperature is further increased (Fig. 5(d–f)). Different from the profound heating effect on the structure of the bulk layer, the effect of annealing on the structure of the intermixing and surface layers is much less pronounced, although a similar decreasing trend of the sp3 content of these layers is also observed with increasing temperature. For instance, annealing at 250 °C causes the overall sp3 content to decrease by 9%, with a 12% decrease in sp3 content sustained by the bulk layer, while the change of the sp3 content of the intermixing and surface layers is relatively insignificant. Annealing at a temperature above 250 °C intensified the rehybridization process in the bulk layer, which controls the overall sp3 content of the a-C film. Specifically, annealing at 300, 350, and 450 °C resulted in the decrease of the overall sp3 film content and the bulk layer sp3 content by 11% and 17%, 15% and 20%, and 17% and 25%, respectively. As with the overall sp3 content, the sp3 content at each depth also reached a steady state within a very short annealing time.
Figure 5.
Depth profiles of sp³ fraction of a-C film with 48% sp3 content versus annealing time and temperature equal to (a) 150 °C, (b) 200 °C, (c) 250 °C, (d) 300 °C, (e) 350 °C, and (f) 450 °C. Negative depth values correspond to the silicon substrate.
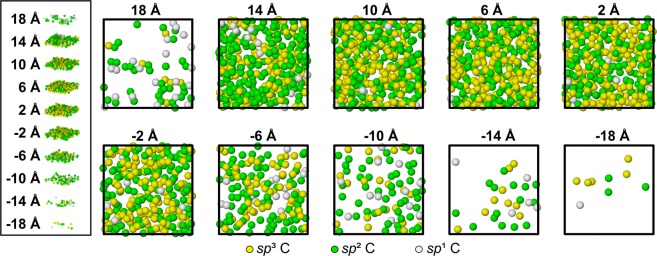
Additional insight into the evolution of the film structure was obtained by examining the spatial distribution of carbon atoms in sp1, sp2, and sp3 hybridizations. This was accomplished by slicing the a-C film with 48% overall sp3 content (Table 1) into ten 4-Å-thick horizontal (x-y) slices, as shown in Fig. 6. The spatial distributions of carbon atoms with different hybridizations reveal a multi-layer film structure. The first slice (z = 18 Å), representing the surface layer of the a-C film, contains a few carbon atoms that are mostly sp2 hybridized. Thus, it may be inferred that the surface layer of the a-C film possesses a graphite-like porous structure of relatively low density. The slices corresponding to the bulk layer (2 Å ≤ z ≤ 14 Å) contain roughly the same number of carbon atoms, which is significantly higher than that of the surface layer. These slices show a gradual increase in sp3 hybridization with increasing depth in the range 6 Å ≤ z ≤ 14 Å, followed by a slight decrease in sp3 hybridization at a depth z = 2 Å, which is adjacent to the interface of the bulk layer with the intermixing layer. The slices assigned to the intermixing layer (z < 0) show a sharp decrease in sp3 hybridization and carbon atom density with increasing depth. The foregoing variation of the sp3 content of the surface, bulk, and intermixing layers is consistent with the sp3 depth profile corresponding to a carbon atom deposition energy equal to 80 eV (Fig. 3(b)) and in qualitative agreement with experimental findings19.
Figure 6.
Carbon atoms of different hybridizations in 4-Å-thick horizontal (x-y) slices of a-C film with 48% sp3 content. The exploded view on the left shows the depth of each slice. Negative depth values correspond to the silicon substrate. Silicon atoms are not shown for clarity.
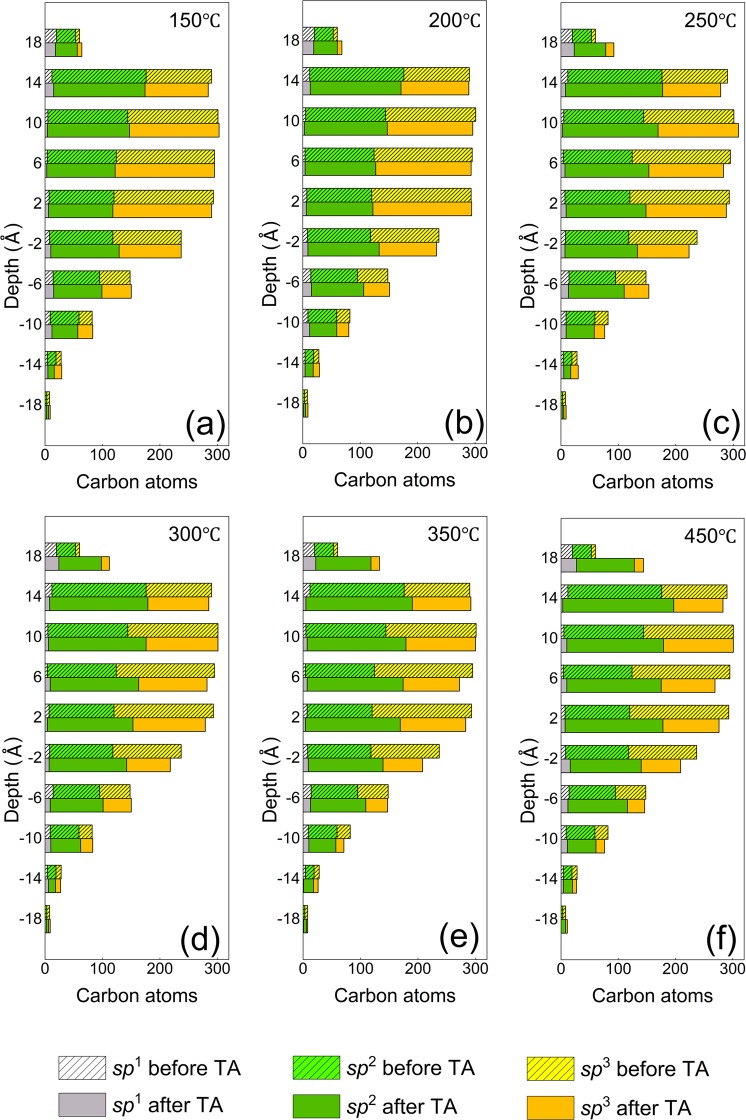
The data shown in Fig. 6 provide a reference for the annealing simulations. Figure 7 shows the number of carbon atoms in different hybridizations at various depths through the film thickness before and after thermal annealing in the temperature range of 150–450 °C. Figures 7(a,b) show that annealing at 150 and 200 °C, respectively, does not change significantly the carbon atom density and the hybridization state. However, as evidenced from Fig. 7(c–f), annealing at a temperature above 200 °C changes significantly the number of carbon atoms in each slice and also triggers sp3→sp2 rehybridization, more notably in the bulk layer (0 ≤ z ≤ 13 Å). For instance, while annealing at 250 °C increases the number of carbon atoms in the surface layer (13 Å ≤ z ≤ 18 Å) by 29 (46%) and decreases those in the bulk layer (0 Å ≤ z ≤ 13 Å) by 32 (2.3%), annealing at 450 °C causes the carbon atoms in the surface layer to sharply increase by 84 (140%) and those in the bulk layer to decrease by 78 (5.5%). Carbon atom rehybridization induced by annealing decreases the sp3 content of the bulk layer by 9% at 250 °C and 16.7% at 450 °C. Compared to the bulk and surface layers, the intermixing layer exhibits insignificant changes in carbon atom number at all temperatures. The carbon atom changes in the surface layer and the intermixing layer indicate atom migration during annealing, which is attributed to stress relaxation of the bulk layer. The increase of carbon atoms and insignificant hybridization changes in the surface layer indicate the development of a thicker surface layer as a result of annealing at an elevated temperature (i.e., >250 °C). The decrease of the number of carbon atoms and the sp3 content of the bulk layer due to annealing in the temperature range 250–450 °C suggests the development of a thinner, lower density, and partially graphitized bulk layer. The numbers of sp2- and sp3-hybridized carbon atoms in each slice versus annealing temperature shown in Fig. S2 (SI) provide further evidence for the instigation of sp3→sp2 rehybridization mainly in the bulk layer.
Figure 7.
Number of carbon atoms of different hybridizations in 4-Å-thick horizontal (x-y) slices of a-C film with 48% sp3 content before and after thermal annealing (TA) at a temperature of (a) 150 oC, (b) 200 oC, (c) 250 oC, (d) 300 oC, (e) 350 oC, and (f) 450 oC.
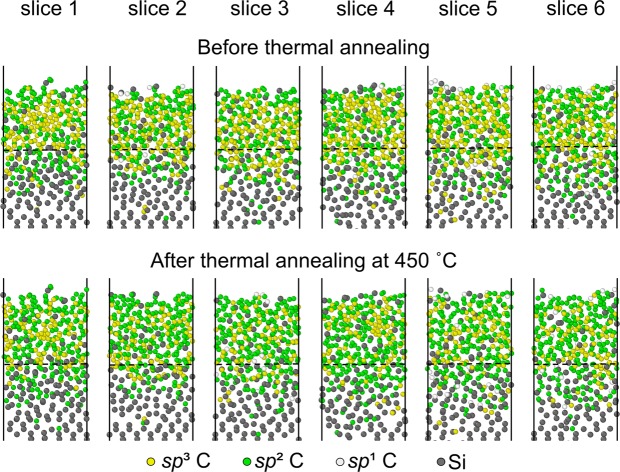
In addition to the distributions and hybridizations of carbon atoms in horizontal slices through the film thickness, carbon atom hybridization in 4-Å-thick vertical (y-z) slices was also examined to identify any changes in the as-deposited film structure due to annealing at planes perpendicular to the film surface. Figure 8 shows sp1, sp2, and sp3 hybridized carbon and silicon atoms in vertical slices before and after annealing at 450 °C. The simulations reveal two important results. First, only changes in hybridization can be observed after thermal annealing, whereas differences in the number of carbon atoms in each vertical slice are insignificant. In the light of this result and the periodic boundary conditions, it may be inferred that the film possesses a fairly uniform structure. Second, a comparison of carbon atom hybridizations in each slice before and after annealing shows a uniform trend for sp3→sp2 rehybridization throughout the film structure, especially in the bulk layer.
Figure 8.
Carbon atoms of different hybridizations in adjacent 4-Å-thick vertical (y-z) slices of a-C film with 48% sp3 content before and after annealing at 450 °C. The region below each dashed line is the silicon substrate. The few under-coordinated carbon atoms are shown with the same color as the sp1 atoms.
In some elevated-temperature applications, the decrease of the sp3 content of the protective a-C film as a result of heating may enhance its chemical reactivity, especially in an oxidizing atmosphere. The evolution of the a-C structure in a hot, oxidizing atmosphere could be more complex than that simulated for inert thermal annealing conditions. Thermal annealing in an oxidizing atmosphere may accelerate oxidation and material loss at the film surface, with the thermally excited oxocarbon molecules entering the oxidizing gaseous environment. The reaction of an oxidizing gas with the film in conjunction with the development of mechanical traction due to surface contact may promote or even accelerate carbon oxidation at elevated temperatures, thus degrading the structural integrity of the a-C film and, in turn, its protective effectiveness. The detrimental effect of oxidizing gas diffusion on the film’s structural stability may be more pronounced than that of sp3→sp2 rehybridization propelled by thermal effects. An MD analysis of ultrathin a-C films rich in sp3 hybridization exposed to an elevated temperature in an oxidizing atmosphere is the objective of a forthcoming publication.
Conclusions
A comprehensive MD analysis of energetic carbon atom deposition on silicon was performed to elucidate the growth process of ultrathin a-C films and identify the structural changes induced by thermal annealing. The MD simulations confirmed the multi-layer structure of a-C films synthesized by energetic particle deposition methods. The study of the effect of carbon deposition energy on the film growth characteristics revealed a deposition energy threshold for synthesizing a-C films of maximum density and sp3 hybridization. By increasing the deposition energy toward the deposition energy threshold, the intensifying carbon atom bombardment promoted direct and recoil implantation and the development of high compressive stresses, which are conducive to film/substrate intermixing, preferential resputtering of weakly bonded atoms, sp3 hybridization, and sp2→sp3 rehybridization. However, for deposition energy above the deposition energy threshold, the intense bombardment of the growing film promoted resputtering and, presumably, the development of thermal spikes, which reduced the film density and high compressive stresses, favoring sp2 hybridization and sp3→sp2 rehybridization.
The MD simulations of thermal annealing of the a-C film with the highest sp3 content (48%) in this study demonstrated insignificant structural changes for temperatures up to 200 °C, with progressively intensifying carbon atom migration in the intermixing and surface layers and sp3→sp2 rehybridization in the bulk layer with the increase of temperature from 250 to 450 °C. While annealing increased the thickness of the surface layer, it decreased the thickness of the intermixing layer and the density of the bulk layer. An important finding is that sp3→sp2 rehybridization at an elevated temperature is a very rapid process. Annealing at a relatively high temperature (e.g., 450 °C) may also have a negative effect on the film’s permeability because it may create potential oxidizing gas diffusion sites.
The results of this study provide new insight into the dependence of the growth and structure of a-C films on deposition process conditions and annealing at various temperatures. The knowledge derived from the present MD simulations has direct implications on the physical properties of ultrathin a-C films used as protective overcoats of devices operating at elevated temperatures. The good agreement of the simulation trends and results of this study with experimental findings of earlier investigations validate the present MD model and employed analytical methodology. This work provides impetus for using the present MD modeling approach to guide the selection of appropriate film deposition and annealing conditions and to perform parametric studies aimed at tuning the multi-layer structure of a-C films according to specific application requirements.
Supplementary information
Acknowledgements
This research was supported by Western Digital Technologies, Inc. The videos and Figs. 1, 2, 3(c–f), 6 and 8 were generated by the authors with the open-source software OVITO 2.9.0, which can be accessed from https://www.ovito.org/. This research used the Savio computational cluster resource provided by the Berkeley Research Computing program at the University of California, Berkeley, supported by the UC Berkeley Chancellor, Vice Chancellor for Research and Chief Information Officer.
Author contributions
S.W. performed all the MD simulations and wrote the first draft of the paper. K.K. defined the scope of the entire work, supervised the research of S.W., examined the simulation results, and wrote the final manuscript of the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-64625-w.
References
- 1.Robertson J. Diamond-like amorphous carbon. Mater. Sci. Eng. R: Rep. 2002;37:129–281. doi: 10.1016/S0927-796X(02)00005-0. [DOI] [Google Scholar]
- 2.Robertson J. Amorphous carbon. Adv. Phys. 1986;35:317–374. doi: 10.1080/00018738600101911. [DOI] [Google Scholar]
- 3.Robertson, J. Deposition of diamond-like carbon. In Thin Film Diamond (eds. Lettington, A. & Steeds, J. W.), 107–116 (Springer, 1994).
- 4.Taki Y, Kitagawa T, Takai O. Preparation of diamond-like carbon thin films by shielded arc ion. J. Mater. Sci. Lett. 1997;16:553–556. doi: 10.1023/A:1018513804905. [DOI] [Google Scholar]
- 5.Xie J, Komvopoulos K. Friction, nanostructure, and residual stress of single-layer and multi-layer amorphous carbon films deposited by radio-frequency sputtering. J. Mater. Res. 2016;31:1857–1864. doi: 10.1557/jmr.2015.404. [DOI] [Google Scholar]
- 6.Xie J, Komvopoulos K. Thermal stability of ultrathin amorphous carbon films synthesized by plasma-enhanced chemical vapor deposition and filtered cathodic vacuum arc. Philos. Mag. 2017;97:820–832. doi: 10.1080/14786435.2017.1282180. [DOI] [Google Scholar]
- 7.Matlak J, Komvopoulos K. Friction properties of amorphous carbon ultrathin films deposited by filtered cathodic vacuum arc and radio-frequency sputtering. Thin Solid Films. 2015;579:167–173. doi: 10.1016/j.tsf.2014.12.043. [DOI] [Google Scholar]
- 8.Xie J, Komvopoulos K. Hybridization and tribomechanical properties of ultrathin amorphous carbon films synthesized by radio-frequency low-pressure plasma discharges. Surf. Coat. Technol. 2015;262:15–20. doi: 10.1016/j.surfcoat.2014.11.052. [DOI] [Google Scholar]
- 9.Zhang Q, et al. Deposition of hydrogenated diamond-like carbon films under the impact of energetic hydrocarbon ions. J. Appl. Phys. 1998;84:5538–5542. doi: 10.1063/1.368599. [DOI] [Google Scholar]
- 10.Weiler M, et al. Highly tetrahedral, diamond‐like amorphous hydrogenated carbon prepared from a plasma beam source. Appl. Phys. Lett. 1994;64:2797–2799. doi: 10.1063/1.111428. [DOI] [Google Scholar]
- 11.Dalouji V, Asareh N, Hashemizadeh SA, Solaymani S. Carbon films embedded by nickel nanoparticles: The effect of deposition time on Berthelot-type hopping conduction parameters. Eur. Phys. J. Plus. 2016;131:442. doi: 10.1140/epjp/i2016-16442-6. [DOI] [Google Scholar]
- 12.Dalouji V, Elahi SM, Alecasir MS. Study of electric susceptibility, electrical resistivity and energy loss functions of carbon–nickel composite films at different annealing temperatures. Phys. Scr. 2015;90:115802. doi: 10.1088/0031-8949/90/11/115802. [DOI] [Google Scholar]
- 13.Wang S, Komvopoulos K. Electromagnetic and thermomechanical analysis of near-field heat transfer in heat-assisted magnetic recording heads. IEEE Trans. Magn. 2018;54:3000906. [Google Scholar]
- 14.Wang S, Komvopoulos K. Effect of material optical properties on thermo-plasmonics of heat-assisted magnetic recording devices. J. Appl. Phys. 2018;124:185109. doi: 10.1063/1.5046099. [DOI] [Google Scholar]
- 15.Xie J, Komvopoulos K. The effect of Argon ion irradiation on the thickness and structure of ultrathin amorphous carbon films. J. Appl. Phys. 2016;119:095304. doi: 10.1063/1.4942440. [DOI] [Google Scholar]
- 16.Xie J, Komvopoulos K. The role of duty cycle of substrate pulse biasing in filtered cathodic vacuum arc deposition of amorphous carbon films. IEEE Trans. Magn. 2015;51:3302009. [Google Scholar]
- 17.Wang N, Komvopoulos K. The multilayered structure of ultrathin amorphous carbon films synthesized by filtered cathodic vacuum arc deposition. J. Mater. Res. 2013;28:2124–2131. doi: 10.1557/jmr.2013.206. [DOI] [Google Scholar]
- 18.Wang N, Komvopoulos K. Incidence angle effect of energetic carbon ions on deposition rate, topography, and structure of ultrathin amorphous carbon films deposited by filtered cathodic vacuum arc. IEEE Trans. Magn. 2012;48:2220–2227. doi: 10.1109/TMAG.2012.2190295. [DOI] [Google Scholar]
- 19.Matlak J, Komvopoulos K. Ultrathin amorphous carbon films synthesized by filtered cathodic vacuum arc used as protective overcoats of heat-assisted magnetic recording heads. Sci. Rep. 2018;8:9647. doi: 10.1038/s41598-018-27528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matlak J, Rismaniyazdi E, Komvopoulos K. Nanostructure, structural stability, and diffusion characteristics of layered coatings for heat-assisted magnetic recording head media. Sci. Rep. 2018;8:9807. doi: 10.1038/s41598-018-27688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang N, Komvopoulos K. The effect of deposition energy of energetic atoms on the growth and structure of ultrathin amorphous carbon films studied by molecular dynamics simulations. J. Phys. D: Appl. Phys. 2014;47:245303. doi: 10.1088/0022-3727/47/24/245303. [DOI] [Google Scholar]
- 22.Li X, Ke P, Lee K-R, Wang A. Molecular dynamics simulation for the influence of incident angles of energetic carbon atoms on the structure and properties of diamond-like carbon films. Thin Solid Films. 2014;552:136–140. doi: 10.1016/j.tsf.2013.12.012. [DOI] [Google Scholar]
- 23.Li X, Ke P, Zheng H, Wang A. Structural properties and growth evolution of diamond-like carbon films with different incident energies: A molecular dynamics study. Appl. Surf. Sci. 2013;273:670–675. doi: 10.1016/j.apsusc.2013.02.108. [DOI] [Google Scholar]
- 24.Petersen A, Gillette V. High-temperature annealing of graphite: A molecular dynamics study. J. Nucl. Mater. 2018;503:157–163. doi: 10.1016/j.jnucmat.2018.03.011. [DOI] [Google Scholar]
- 25.Kupka K, et al. Graphitization of amorphous carbon by swift heavy ion impacts: Molecular dynamics simulation. Diamond Relat. Mater. 2018;83:134–140. doi: 10.1016/j.diamond.2018.01.015. [DOI] [Google Scholar]
- 26.Saada D, Adler J, Kalish R. Computer simulation of damage in diamond due to ion impact and its annealing. Phys. Rev. B. 1999;59:6650–6660. doi: 10.1103/PhysRevB.59.6650. [DOI] [Google Scholar]
- 27.Tersoff J. New empirical approach for the structure and energy of covalent systems. Phys. Rev. B. 1988;37:6991–7000. doi: 10.1103/PhysRevB.37.6991. [DOI] [PubMed] [Google Scholar]
- 28.Tersoff J. Empirical interatomic potential for carbon, with applications to amorphous carbon. Phys. Rev. Lett. 1988;61:2879–2882. doi: 10.1103/PhysRevLett.61.2879. [DOI] [PubMed] [Google Scholar]
- 29.Tersoff J. Modeling solid-state chemistry: Interatomic potentials for multicomponent systems. Phys. Rev. B. 1989;39:5566–5568. doi: 10.1103/PhysRevB.39.5566. [DOI] [PubMed] [Google Scholar]
- 30.Tersoff J. Erratum: Modeling solid-state chemistry: Interatomic potentials for multicomponent systems. Phys. Rev. B. 1990;41:3248. doi: 10.1103/PhysRevB.41.3248.2. [DOI] [PubMed] [Google Scholar]
- 31.Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984;81:3684–3690. doi: 10.1063/1.448118. [DOI] [Google Scholar]
- 32.Marks NA. Evidence for subpicosecond thermal spikes in the formation of tetrahedral amorphous carbon. Phys. Rev. B. 1997;56:2441–2446. doi: 10.1103/PhysRevB.56.2441. [DOI] [Google Scholar]
- 33.Caro MA, Zoubkoff R, Lopez-Acevedo O, Laurila T. Atomic and electronic structure of tetrahedral amorphous carbon surfaces from density functional theory: Properties and simulation strategies. Carbon. 2014;77:1168–1182. doi: 10.1016/j.carbon.2014.06.060. [DOI] [Google Scholar]
- 34.Huang D, Pu J, Lu Z, Xue Q. Microstructure and surface roughness of graphite‐like carbon films deposited on silicon substrate by molecular dynamic simulation. Surf. Interf. Anal. 2012;44:837–843. doi: 10.1002/sia.4898. [DOI] [Google Scholar]
- 35.Davis CA, Amaratunga GAJ, Knowles KM. Growth mechanism and cross-sectional structure of tetrahedral amorphous carbon thin films. Phys. Rev. Lett. 1998;80:3280–3283. doi: 10.1103/PhysRevLett.80.3280. [DOI] [Google Scholar]
- 36.Ferrari AC, et al. Density, sp3 fraction, and cross-sectional structure of amorphous carbon films determined by x-ray reflectivity and electron energy-loss spectroscopy. Phys. Rev. B. 2000;62:11089–11103. doi: 10.1103/PhysRevB.62.11089. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.