See the article by Liu and Sun et al in this issue, pp. 625–638.
In most tumors, including medulloblastoma, it is becoming increasingly clear that nonneoplastic elements of the tumor microenvironment can be key drivers of tumor growth and metastasis. In this issue, Liu et al describe a novel molecular circuit in which necroptotic astrocytes reacting to therapeutic stress release the cytokine C-C ligand 2 (CCL2), which promotes a more aggressive stem cell phenotype in recurrent or disseminated medulloblastoma.1
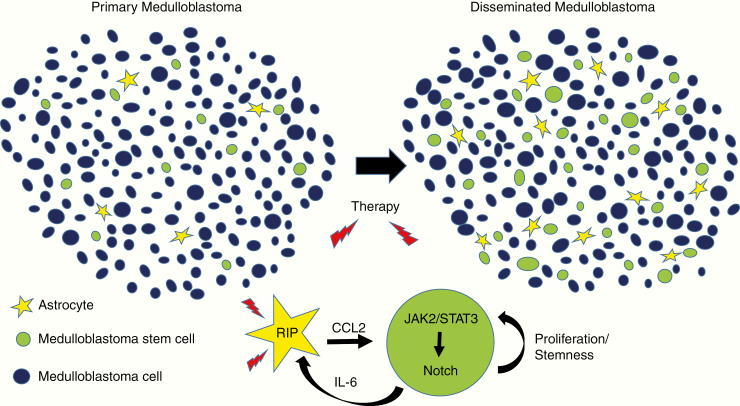
The work takes advantage of the large cohort of medulloblastoma cases and tissue specimens available to the group, and builds on their prior report showing that astrocytes can play a key role in medulloblastoma by releasing sonic hedgehog ligand.2 In the current study, they initially examined differences between primary medulloblastoma and tumors disseminating after therapy. The percentage of cells expressing stem cell markers was increased in disseminated tumors compared with primary ones (Figure 1). Interestingly, these stemlike medulloblastoma cells were rapidly proliferating, a finding at odds with some models in which cancer stem cells are thought to cycle slowly. Astrocytic cells were also significantly more common in posttreatment disseminated medulloblastoma. The recruitment of astrocytes was due at least in part to enhanced migration in response to interleukin (IL)-6 ligand released by stemlike medulloblastoma cells, and knockdown of the IL-6 receptor on astrocytes could inhibit the process.
Fig. 1.
CCL2 released by treated necroptotic astrocytes promotes proliferation and stemness in medulloblastoma cells.
In other contexts, astrocytes are known to be potent sources of cytokines. CCL2 was the most abundant cytokine detected by the group in the serum of patients with disseminated medulloblastoma, and was also elevated in surgical specimens. Higher levels of CCL2 were also present in the media of cultured astrocytes isolated from disseminated medulloblastoma compared with primary ones. Elevated CCL2 levels were associated with large cell/anaplastic histology, molecular group 3 tumors, and shorter survival. The addition of CCL2 to medulloblastoma cultures lacking growth factors was able to stimulate the proliferation of cells, the expression of stem cell markers such as sex-determining region Y‒box 2 and cluster of differentiation (CD)133/CD15, and clonogenic growth as spheres. In contrast, loss of function studies including the use of short hairpin RNA to reduce expression of CCL2 in astrocytes impaired the induction of stemness markers in co-cultured tumor cells, as well as the proliferation of tumor in organotypic models and subcutaneous xenografts.
Liu et al also examined the mechanisms by which CCL2 promoted stemness in medulloblastoma cells, focusing on the possibility that Notch played an important role. Notch activity is critical in a wide range of cancer stem cells, including those in medulloblastoma.3 They confirmed that Notch receptors and effectors, including Notch3 and Hes1, were highly expressed in disseminated tumors, and showed that CCL2 could stimulate transcription of canonical Notch pathway reporters. Pharmacological blockade of Notch reduced the expression of stem cell markers. Links to Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling were also investigated, and pharmacologic inhibition of C-C receptor 2 suppressed JAK2/Notch3 interactions and the phosphorylation of STAT3. Finally, it was suggested that Notch could promote secretion of IL-1 from medulloblastoma stemlike cells.
One strength of the study was the focus on clinically problematic recurrent tumors, and how treatment changed the local microenvironment. In recurrent or disseminated medulloblastoma which had previously been treated using chemotherapy and radiation, an increase in TUNEL*-positive cells was found. The investigators examined potential mechanisms contributing to the death of astrocytes and noted increased levels of necroptosis markers including receptor-interacting protein (RIP)3, but no increase in the apoptotic marker cleaved caspase-3. Necroptosis is a form of cell death morphologically similar to necrosis regulated in a caspase-independent manner by RIP kinases, and has been shown to promote cytokine release in other systems.4 In medulloblastoma cultures, the induction of CCL2 secretion following chemotherapy could be suppressed by pretreatment with necroptosis inhibitors or the addition of a short hairpin targeting RIP3. These observations were therapeutically relevant, as pretreatment of murine orthotopic medulloblastoma xenografts with a necroptosis inhibitor prior to chemotherapy decreased CCL2 levels, tumor size, and metastatic dissemination, as well as prolonging survival.
While necroptosis is increasingly being implicated in inflammatory brain diseases,5 relatively little is known about how it modulates the biology of central nervous system tumors. This aspect of the work is thus conceptually quite novel, particularly for medulloblastoma, and supports the growing interest in how the inflammatory microenvironment alters brain tumor growth and therapeutic response. It also suggests that necroptosis inhibitors may represent novel therapeutic agents for medulloblastoma.
The study complements other recent publications highlighting the importance of astrocytes and CCL2 in medulloblastoma. Garzia et al recently documented the role of CCL2 in promoting medulloblastoma metastasis, but reported secretion of this cytokine from tumor cells rather than from tumor-associated astrocytes.6 However, they focused on tumors prior to treatment, and in the model proposed by Liu et al the induction of necroptosis by therapeutic stress is critical for CCL2 release from astrocytes.
Another interesting issue is whether the astrocytes undergoing necroptosis are truly entrapped nonneoplastic cells. It has recently been shown by Yao et al that medulloblastoma cells which differentiate into astrocytes can play a key role in supporting long-term tumor growth via IL-4 secretion.7 Intriguingly, the astrocytes in the latter study were microscopically bland and generally did not have an overtly malignant appearance. This raises the question of whether tumor-associated astrocytes in the current study are all normal cells attracted to the tumor mass, or if a degree of trans-differentiation may also be occurring. Another recent link between medulloblastoma and astrocytes comes from a study by Tao et al showing that Group 3 medulloblastoma can arise from astrocyte progenitors in response to increased levels of MYC.8 Astrocytes are truly rising stars of the medulloblastoma show.
Footnotes
Terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling.
References
- 1. Liu H, Sun Y, O’Brien JA, et al. . Necroptotic astrocytes contribute to maintaining stemness of disseminated medulloblastoma through CCL2 secretion. Neuro Oncol. 2019. doi: 10.1093/neuonc/noz214. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu Y, Yuelling LW, Wang Y, et al. . Astrocytes promote medulloblastoma progression through hedgehog secretion. Cancer Res. 2017;77(23):6692–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fan X, Matsui W, Khaki L, et al. . Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66(15):7445–7452. [DOI] [PubMed] [Google Scholar]
- 4. Place DE, Kanneganti TD. Cell death-mediated cytokine release and its therapeutic implications. J Exp Med. 2019;216(7):1474–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yuan J, Amin P, Ofengeim D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev Neurosci. 2019;20(1): 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garzia L, Kijima N, Morrissy AS, et al. . A hematogenous route for medulloblastoma leptomeningeal metastases. Cell. 2018;172(5):1050–1062.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yao M, Ventura PB, Jiang Y, et al. . Astrocytic trans-differentiation completes a multicellular paracrine feedback loop required for medulloblastoma tumor growth. Cell. 2020;180(3):502–520.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tao R, Murad N, Xu Z, et al. . MYC drives group 3 medulloblastoma through transformation of Sox2+ astrocyte progenitor cells. Cancer Res. 2019;79(8):1967–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]