Abstract
Background
Improvements in detection and molecular characterization of leptomeningeal metastasis from lung cancer (LC-LM) coupled with cerebrospinal fluid (CSF)-penetrating targeted therapies have altered disease management. A barrier to formal study of these therapies in LM is quantification of disease burden. Also, outcomes of patients with targetable mutations in LC-LM are not well defined. This study employs molecular and radiographic measures of LM disease burden and correlates these with outcome.
Methods
We reviewed charts of 171 patients with LC-LM treated at Memorial Sloan Kettering. A subset had MRI and CSF studies available. Radiographic involvement (n = 76) was scored by number of gadolinium-enhancing sites in 8 locations. CSF studies included cytopathology, circulating tumor cell (CTC) quantification (n = 16), and cell-free DNA (cfDNA) analysis (n = 21). Clinical outcomes were compared with Kaplan–Meier log-rank test and Cox proportional hazards methodologies.
Results
Median overall survival was 4.2 months (95% CI: 3.6–4.9); 84 patients (49%) harbored targetable mutations. Among bevacizumab-naïve patients with MRI and CSF cytology at time of LC-LM diagnosis, extent of radiographic involvement correlated with risk of death (hazard ratio [HR]: 1.16; 95% CI: 1.02–1.33; P = 0.03), as did CSF CTC (HR: 3.39, 95% CI: 1.01–11.37; P = 0.048) and CSF cfDNA concentration (HR: 2.58; 95% CI: 0.94–7.05; P = 0.06). Those without a targetable mutation were almost 50% more likely to die (HR: 1.49; 95% CI: 1.06–2.11; P = 0.02).
Conclusions
Extent of radiographic involvement and quantification of CSF CTC and cfDNA show promise as prognostic indicators. These findings support molecular characterization and staging for clinical management, prognostication, and clinical trial stratification of LC-LM.
Keywords: leptomeningeal metastases, lung cancer, circulating tumor cells, cell-free DNA
Key Points.
1. The number of radiographic sites of leptomeningeal metastases is associated with prognosis.
2. CSF CTCs and cfDNA show promise as prognostic indicators in leptomeningeal metastasis from non-small-cell lung cancer (NSCLC).
3. Clinically actionable mutations predict longer survival in NSCLC leptomeningeal metastases.
Importance of the Study
The purpose of this study was to assess whether a novel approach to objective radiographic disease burden assessment and modern CSF analysis can be employed to predict survival in patients with LC-LM. We also evaluated the role of clinically actionable mutations in outcome of this disease. We found that the number of radiographic sites of LC-LM, quantification of CSF CTCs, and quantification of CSF cfDNA concentration from the time of LC-LM diagnosis can predict patient survival. Our results show that an objective scoring of MRI sites of disease and quantification of CSF CTCs and cfDNA may be used in conjunction with tumor mutational profile to predict outcome among patients with LC-LM. A prospective study is warranted to validate these objective radiographic and CSF metrics as tools to more accurately predict prognosis.
Leptomeningeal metastasis (LM) is a devastating complication of non-small-cell lung cancer and portends a grim prognosis. LM develops in 3–8% of all patients with non-small-cell lung cancer (NSCLC), and is the second most common cause of LM in the United States.1,2 Average survival after diagnosis of LM from lung cancer (LC-LM) is around 3–4 months, although some patients may survive years after diagnosis.3,4 While the presence of a clinically actionable mutation, such as epidermal growth factor receptor (EGFR), has altered disease management and improved prognosis, many patients with LC-LM and EGFR mutations do not survive beyond 3–4 months.3,5,6
In an effort to improve treatment response predictions, a Response Assessment in Neuro-Oncology (RANO) working group with expertise in LM created standardized assessment metrics to evaluate patients being treated for LM.7,8 The RANO LM criteria uses 3 elements of treatment response: the neurological exam, cerebrospinal fluid (CSF) cytology or flow cytometry, and central nervous system (CNS) imaging. While the community awaits validation of the RANO LM assessment tool on a large scale in patients with LM, there remains a need to better predict prognosis for patients with LM at time of diagnosis.
Classically, larger tumors and more extensive disease involvement portend a worse prognosis; the larger the tumor, the more widespread the metastases, the more elevated the tumor markers, the poorer the outcome.9–11 Inhabiting a compartment with complex anatomy, and existing both in suspension and in radiographically apparent plaques of disease, LM presents a unique measurement challenge. Traditional diagnostic tools, such as radiographic reports, are vulnerable to inter-examiner variability. The inability to accurately measure burden of disease and predict outcome poses a major obstacle in clinical practice when evaluating response, counseling patients, discussing treatment options, and limits clinical trial risk stratification and interpretation. We therefore evaluated the utility of molecular characteristics, radiographic extent of disease, and CSF studies as predictors of prognosis among patients with LC-LM.
Methods
Patients
We retrospectively reviewed charts of 171 patients with diagnoses of LC-LM between June 1, 2009 and June 30, 2017 at Memorial Sloan Kettering Cancer Center (MSKCC). This study was approved by the MSKCC institutional review board with waiver of written informed consent. Patients were identified through an MSKCC database search. Patients 18 years of age or older with NSCLC with either positive cytology and/or unequivocal radiographic evidence of LM met criteria for LC-LM. Patients who did not meet these criteria were excluded. Date of LC-LM diagnosis was defined as date of first CSF cytology revealing malignant cells or date of first MRI (brain or spine) demonstrating LM. If both MRI and CSF were positive for LC-LM, the date of the first test demonstrating LC-LM was used as the date of LC-LM diagnosis. Patients who underwent CSF cytology examination, MRI brain with gadolinium contrast enhancement, and MRI spine with gadolinium contrast enhancement within 30 days of LC-LM diagnosis comprised our “complete LC-LM staging” cohort. If a patient had multiple mutational analyses performed at different times in the course of his/her illness, the analysis from the timepoint closest to LC-LM diagnosis was used for analysis in this study. If both CNS and systemic tumor molecular testing results were performed and available, the CNS mutation analysis was used; this occurred for 8 patients.
MRI Scoring
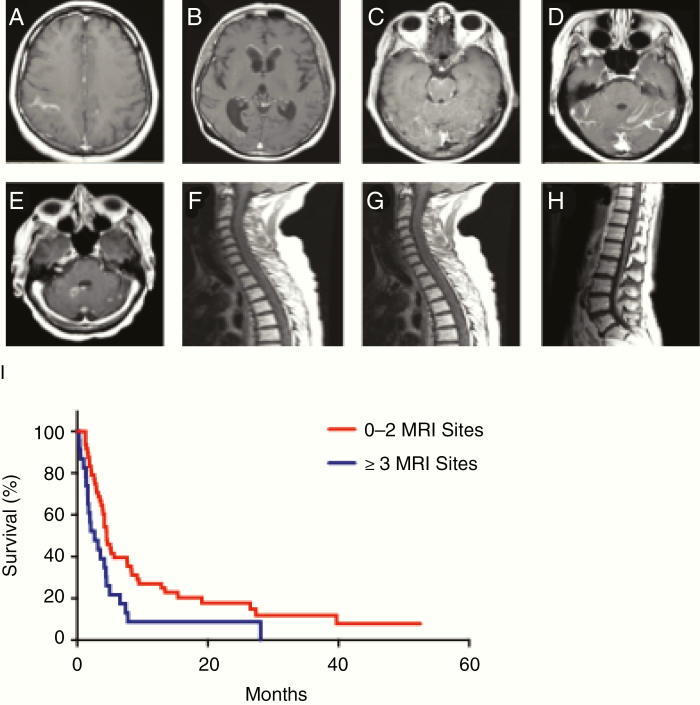
Radiographic involvement was scored by number of gadolinium-enhancing sites in 8 predetermined locations (Fig. 1A–H). Patients received one point per location of radiographically evident LM; for example, a patient with 4 discontinuous LM nodules in the cerebellum, and otherwise normal MRI of the brain and spine, was awarded one point. MRIs from the time of LC-LM diagnosis in the complete staging cohort were scored. MRIs were interpreted by a neuro-radiologist at the time of imaging. Number of MRI sites of disease for each patient was determined based on the original radiology report and confirmed by independent MRI review by the authors K.S.N., E.P., or A.B.; if there was a disagreement between the radiology report and one of the study author’s interpretation of the imaging, a consensus on number of radiographic sites of LC-LM was reached by K.S.N., E.P., and A.B.
Fig. 1.
Eight defined sites of radiographic involvement of LC-LM. (A) Cerebrum. (B) Ventricle. (C) Brainstem (pons and medulla included in this category). (D) Cerebellum. (E) Cranial nerves. (F) Cervical spinal cord. (G) Thoracic spinal cord. (H) Lumbosacral spinal cord. (I) Among the 76 patients with complete staging of LC-LM who did not receive bevacizumab within 30 days prior to MRI, there was a significantly increased risk of death with 3+ radiographic sites of disease versus 0–2 sites of disease (HR for 3+ sites of disease: 1.95; 95% CI: 1.16–3.30; P = 0.01).
CSF Circulating Tumor Cells and Cell-Free DNA Evaluation
CSF data including number of circulating tumor cells (CTCs), glucose, protein, cytology, white blood cell count, and opening pressure were recorded. In the case of multiple CSF samples, CSF collected nearest to the time of LC-LM diagnosis was employed for analyses.
CSF CTC enumeration was performed via epithelial cell adhesion molecule–based rare cell capture technology immunomagnetic platform, which is a New York Clinical Laboratory Improvement Amendments certified test at MSKCC, and is described in detail in prior studies.12 Results are reported as CSF-CTC/mL of CSF. Prior study has shown that ≥1 CSF-CTC/mL or ≥3 CSF-CTC/3 mL was optimal for diagnosis of LM. CSF CTC analysis was performed the same day as CSF collection.12 CSF CTC molecular sequencing was not performed in our study.
At the time of the study, we identified a cohort of 30 patients with LC-LM who had CSF available in the MSKCC Brain Tumor Center CSF bank. Of the 30 patients, 21 had CSF banked within 30 days of LC-LM diagnosis. CSF from these 21 patients underwent cell-free DNA (cfDNA) extraction sequencing (by targeted exome sequencing MSKCC IMPACT) for the quantification of CSF cfDNA.
Statistical Analysis
Descriptive statistics, including frequency distributions, medians, means, and ranges, were calculated for variables of interest. Overall survival was calculated from LC-LM diagnosis until death (event) or last follow-up (censored). The Kaplan–Meier method was used to estimate and graphically present overall survival. Survival curves were compared with the log-rank test. Models associating variables of interest and overall survival were constructed using Cox proportional hazards methodology. In an extended Cox model, we utilized time-dependent statistical methodology to examine the effect of first targeted therapy on or after LC-LM diagnosis and its relationship with overall survival. Statistical analyses were performed using SAS version 9.4. Figures were constructed with GraphPad Prism.
Results
Patient Characteristics
We identified 171 patients with LM evaluated at MSKCC between June 2009 and June 2017 (Table 1). There was no difference in survival between the subset of patients with complete staging within 30 days of LC-LM diagnosis (n = 93) and the remaining patients in the cohort (n = 78; log-rank P = 0.13). Approximately half of the patients had a targetable mutation identified from primary or metastatic malignant pathology specimens.
Table 1.
Patient characteristics (n = 171)
| Median age,* y (range) | 63 (30–86) |
| Sex, women N (%) | 97 (57) |
| Complete staging within 30 days (%) | 93 (54) |
| CSF cytology,* N (%) | |
| Positive | 141 (83) |
| Negative | 28 (16) |
| Not performed | 2 (1) |
| MRI brain,* N (%) | |
| Positive | 108 (63) |
| Negative | 60 (35) |
| Not performed | 3 (2) |
| MRI spine,* N (%) | |
| Positive | 58 (34) |
| Negative | 83 (48) |
| Not performed | 30 (18) |
| Targetable mutation, + N (%) | |
| Yes | 84 (49) |
| Specific mutation,+N (%) | |
| EGFR^ | 63 (75) |
| ALK^ | 8 (10) |
| HER2^ | 7 (8) |
| BRAF^ | 3 (4) |
| ROS1^ | 2 (2) |
| MET^ | 1 (1) |
| No | 69 (40) |
| Not tested | 18 (11) |
*At diagnosis of leptomeningeal metastases.
+If molecular testing was performed in the same patient at several different timepoints, the results closest in proximity to time of LC-LM diagnosis were used. If CNS and systemic tumor molecular testing results were available, the CNS mutation analysis results were used.
^EGFR = epidermal growth factor receptor; ALK = anaplastic lymphoma kinase; HER2 = human epidermal growth factor receptor 2; BRAF = B-Raf murine sarcoma gene; ROS1 = ROS proto-oncogene 1; MET = MET proto-oncogene.
The overall survival of the entire cohort (171 patients) was 4.18 months (95% CI: 3.61‒4.93 mo); 19 patients were still alive at time of data analysis (Supplementary Fig. 1A). Age 60 or older at time of LC-LM diagnosis was associated with an increased risk of death (hazard ratio [HR] for age ≥60: 1.54; 95% CI: 1.10–2.16; P = 0.01; Supplementary Fig. 1B). Karnofsky performance scale (KPS) scores from time of LC-LM diagnosis ranged 30–100, with a median of 70. Patients with a KPS of 70 or greater lived longer (HR for KPS ≥70: 0.47; 95% CI: 0.33–0.69; P < 0.0001; Supplementary Fig. 1C). The median time from LC diagnosis to LC-LM diagnosis was 578 days (range: −6 to 6985 days, one patient was diagnosed with LM 6 days prior to confirmation of LC as etiology). Using the median cutpoint, there was no association with time from primary cancer diagnosis to LC-LM and survival (HR for 578 days or more between cancer diagnosis and LC-LM diagnosis: 1.19; 95% CI: 0.86–1.63; P = 0.30; Supplementary Fig. 1D). Adding targetable mutation (yes/no) to the model did not alter these results.
Radiographic Burden of Disease
Extent of radiographic involvement at LC-LM diagnosis ranged from 0 to 8 sites involved; median was 1 site and mean was 1.86 sites. In the entire complete staging cohort (n = 93), there was no association between number of sites involved (continuous) and overall survival (HR: 1.08; 95% CI: 0.95–1.22; P = 0.26).
Of the 93 patients with complete staging, radiographic interpretation was complicated in 17 who had received bevacizumab within 30 days prior to LC-LM diagnosis. Among the 76 bevacizumab-naïve patients with complete staging, extent of radiographic involvement ranged from 0 to 8, with a median of 2 and mean of 1.99. There was a statistically significant association between number of sites involved (continuous) and risk of death (HR: 1.16; 95% CI: 1.02–1.33; P = 0.03). There was also a statistically significant association using 3+ sites versus 0–2 (HR for 3 sites of disease or more: 1.95; 95% CI: 1.16–3.30; P = 0.01; Fig. 1I). There was no association between anatomic location of LM and survival (ie, ventricular location was not significantly associated with a different prognosis than the other locations). Although LM is widely believed to begin cranially and gradually seed the neuroaxis caudally, we did not see evidence of this pattern in our dataset: sites of metastasis were rather evenly distributed throughout the neuroaxis (Supplementary Fig. 2A). In addition, we observed a substantial portion of patients with radiographic disease only apparent in the spine (Supplementary Fig. 2B).
CSF Burden of Disease
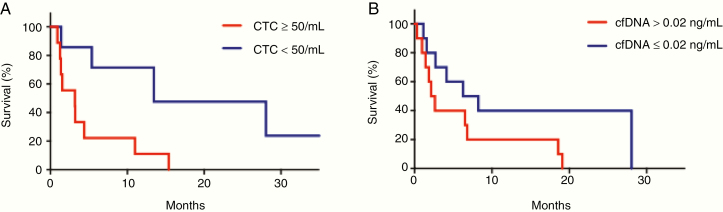
CTCs were analyzed in a subset of 16 patients (9%) who had CSF analyzed at the time of LC-LM diagnosis. The median CTC value was 145 CTC/3 mL and the mean was 139.7 CTC/mL (range 0–605). Using the median cutpoint, there was a marginally significantly increased risk of death associated with the above median value CTCs (HR: 3.03; 95% CI: 0.97–9.45; P = 0.056). Due to the novelty of using CSF CTCs for prognostication and thus lack of a priori knowledge regarding significant CSF CTC cutoff values, we arbitrarily chose a cutoff value of 50 CTC/3 mL to evaluate for significance (reasoned as values ranged 0–605, with most between 35 and 225). Patients with 50 CTC/3 mL or greater CTCs were more than 3 times more likely to die than those with fewer than 50 CTC/3 mL (HR: 3.39, 95% CI: 1.01–11.37; P = 0.048; Fig. 2A).
Fig. 2.
Survival in patients with LC-LM using quantification of cell-free DNA and circulating tumor cells. (A) Patients with 50 or greater CTCs were more than 3 times more likely to die than those with fewer than 50 CTCs (HR for patients with 50 CTCs3: 3.39, 95% CI: 1.01–11.37; P = 0.048). (B) An increased cfDNA concentration was associated with increased risk of death; using the median cutpoint, there was an increased risk of death among those patients with cfDNA concentration equal to or above the median value (HR for patients with cfDNA concentration3 0.02 ng/mL: 2.58; 95% CI: 0.94–7.05; P = 0.06).
Cell-free DNA was extracted from a subset of 21 (12%) patients who had CSF collected at time of LC-LM diagnosis. Cell-free DNA concentration ranged from 0.00393 pg/µL to 0.562 ng/µL, with a median of 0.022 ng/µL and mean of 0.08 ng/µL. Downstream analyses were complicated by small sample size; however, cfDNA correlated with outcome such that increasing values of cfDNA concentration (continuous) were associated with an increased risk of death (HR: 16.33; 95% CI: 0.69–384; P = 0.08). Using the median cutpoint, there was also an increased risk of death associated with the above median value of cfDNA concentration (HR for cfDNA concentration above the median cutpoint: 2.58; 95% CI: 0.94–7.05; P = 0.06; Fig. 2B). Though neither the continuous concentration nor the median cutpoint concentration modeling met statistical significance (P > 0.05), both were likely impacted by small sample size and had a trend toward statistical significance.
CSF protein levels from time of LC-LM diagnosis were available in 115 patients; a CSF protein level above the upper limit of normal was not significantly associated with survival (P = 0.20; Supplementary Fig. 3A). However, an elevated CSF white blood cell count and abnormally low CSF glucose from time of LC-LM diagnosis were both associated with an increased risk of death (P = 0.04 and P < 0.0001, respectively; Supplementary Fig. 3B, C). CSF cytology results were available in 131 patients from time of LC-LM diagnosis. We used the definition of positive CSF cytology as cytology that definitively demonstrated malignant cells or cells suspicious for malignancy in a patient with appropriate clinical signs and symptoms; negative cytology consisted of either negative or atypical cells. A positive CSF cytology at time of diagnosis in this cohort was not associated with increased risk of death (P = 0.1015; Supplementary Fig. 3D).
Presence of a Targetable Mutation
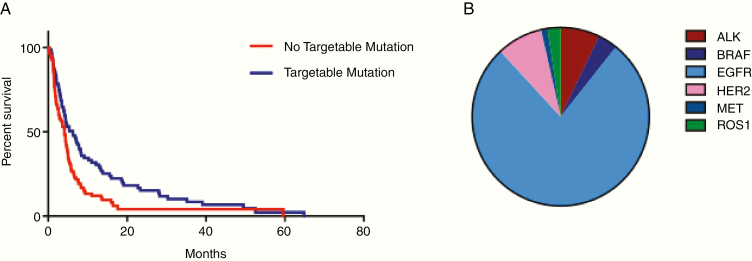
Eighty-four (49%) patients harbored a targetable mutation, which included mutations in EGFR, anaplastic lymphocyte kinase, B-raf murine sarcoma gene, human epidermal growth factor receptor 2, ROS proto-oncogene 1, and MET proto-oncogene. Those without a clinically actionable mutation were almost 50% more likely to die (HR: 1.49; 95% CI: 1.06–2.11; P = 0.02; Fig. 3). At one year, 29.8% (95% CI: 19.8–39.9%) of patients with a targetable mutation were alive versus 15.5% (95% CI: 6.8–24.3%) of those without. Due to the limited number of patients with each specific targetable mutation (other than EGFR mutations, which dominated the cohort), the potential prognostic importance of each individual mutation was unable to be determined.
Fig. 3.
Patients harboring LM from primary tumors with targetable mutations demonstrate improved overall survival. (A) Eighty-four of 171 patients (49%) harbored a targetable mutation. Patients with a targetable mutation had a significantly improved survival compared with those without a targetable mutation (log-rank P = 0.02). (B) Breakdown of specific mutation types among the 84 patients in this study with targetable mutations.
Patients who were treated with targeted therapy for LC-LM had a statistically significantly reduced hazard of death in an extended Cox model (HR: 0.68; 95% CI: 0.49–0.95; P = 0.02). In a subset of 153 patients who had been treated with some type of cancer-directed therapy after LC-LM diagnosis (whole brain and/or spine radiation, systemic chemotherapy with CNS penetrance, immunotherapy, ventriculoperitoneal shunt, targeted therapy), there was a marginally statistically significant reduced hazard of death with targeted therapy (HR for targeted therapy after LC-LM diagnosis: 0.70; 95% CI: 0.49–1.00; P = 0.0502). A subset of patients with targetable mutations were not started on targeted therapy until after LM diagnosis. This was due to a variety of factors (not standard of care at the time, targetable mutation not tested/identified in systemic tumor until after LM disease diagnosis, etc). Patients harboring a targeted mutation who received targeted therapy prior to LM disease diagnosis were much more likely to die than those who had a targeted mutation and were naïve to targeted therapy prior to LM disease diagnosis (HR: 2.57; 95% CI: 1.49–4.45; P = 0.0007; data not shown).
Discussion
In one of the largest retrospective studies of patients with LC-LM, we quantitatively assessed patients’ burden of LM using a radiographic scoring system and CSF liquid biopsy from the time of LC-LM diagnosis (Fig. 4). In bevacizumab-naïve patients, an increasing number of sites of radiographic leptomeningeal disease at time of LC-LM diagnosis correlated with progressively shorter survival times. Among patients for whom CSF CTCs or cfDNA was analyzed, a CSF CTC value of 50 CTC/mL or greater or a cfDNA concentration at or above the median cutpoint of 0.022 ng/mL was associated with increased risk of death. These data support the use of these methods as tools for quantification of disease burden in LC-LM.
Fig. 4.
Proposed methods of quantification of burden of disease in patients with LM. Radiographic assessment, CTC quantification, and cfDNA concentration can be used as quantitative measures of disease burden at the time of LM diagnosis. Increasing burden of disease (more radiographic sites of disease, elevated CTCs, and higher CSF cfDNA concentration) are associated with shorter survival times in patients with LC-LM.
Determining the burden of disease radiographically is challenging in LM, as most lesions are nonmeasurable, serpiginous, or otherwise amorphous. In the past, dichotomization of MRI findings (positive vs negative for radiographic evidence of LM) was associated with outcome, without compelling results.13 There is also evidence that where the LM is radiographically visible in the CNS may be associated with outcome, as demonstrated in a cohort of breast cancer patients with LM.14 This finding has not been studied on a larger scale, and in our study there was no difference in survival between the 2 groups; median overall survival: 89 days (95% CI: 51–125) in patients with radiographic leptomeningeal disease in the spine versus no radiographic leptomeningeal disease in the spine: 116 days (95% CI: 79–139, P = 0.27), see also Supplementary Fig. 2B.
Radiographically “bulky” LM intuitively associates with a worse outcome than smooth-linear appearing plaques of disease. This categorization clearly carries the risk of variable interpretation between evaluators; this was included in the 2019 RANO LM assessment tool,8 with a considerable degree of interreader variability. Indeed, the somewhat cumbersome RANO LM assessment tool was found to have poor interrater reliability and is no longer deemed a feasible approach for quantification of LM.15
In an effort to simplify radiographic interpretation, we created an easily reproducible method of measuring radiographic extent of disease by counting the number of MRI-evident disease sites (up to 8 sites) along the CNS in each patient.16 Previously employed in a diverse set of solid tumor patients harboring LM at various timepoints in clinical care, this tool is used by us to assess radiographic burden in lung cancer patients at the time of LM diagnosis. Among patients who were bevacizumab naïve, the number of MRI-evident sites of disease significantly correlated with outcome, though with a small HR (1.16). This same finding did not hold true for all patients with complete staging (including the patients who had received bevacizumab 10 mg/kg or more 30 days or less before imaging), perhaps because bevacizumab is known to reduce visibility of enhancing tumor, thus falsely lowering the number of MRI sites of disease in the patients who received bevacizumab. A prospective analysis of interreader variability with this tool is warranted.
We also evaluated the quantification of CSF CTCs and cfDNA concentration from the time of LC-LM diagnosis as tools for direct measurement of LM burden. Peripheral CTCs and cfDNA in systemic cancers are used as tools to diagnose, prognosticate, and create individualized treatment plans.17–19 CTC burden in the periphery serves as an independent predictor of survival in patients with systemic involvement of solid tumor malignancies, including breast, colorectal, and prostate.20–22 Similarly, serum cfDNA concentrations are predictive of prognosis in patients with systemic NSCLC.23 The role of liquid biopsy in patients with CNS metastases, however, is still being defined, though a few small studies have shown that CSF CTC enumeration and CSF cfDNA concentration may be associated with LM disease activity in patients with breast cancer and melanoma, respectively.24,25 Our study shows that CSF CTC enumeration and cfDNA are potentially prognostic for survival in patients with LM. Though limited by small sample size, our study shows that patients with 50 CTCs/3 mL or more in the CSF at time of LC-LM diagnosis demonstrated a shorter median overall survival.
In addition to correlating outcome with measurements of disease burden, certain classical patient characteristics have been found to influence patient survival. For example, KPS and age have been explored as predictors of survival; higher KPS typically leads to better prognosis, but age has produced variable results.3,13,26,27 In our study population, younger age and higher KPS were associated with longer survival times, as might be expected.
Since the discovery of molecular targets in NSCLC a little over a decade ago,28 research and drug development of targeted therapies for these tumors have increased exponentially.29–31 Reflective of these efforts, patients with LC-LM and clinically actionable mutations survive longer than patients without targetable mutations, and those who are treated with targeted therapy survive even longer.3,6 In our study, patients whose tumors harbored a clinically actionable mutation demonstrated improved overall survival; treatment with drugs targeted at that mutation improved survival. Despite this improved survival, among the 84 patients with clinically actionable mutations in our study, 65 had documented follow-up within the month prior to death and all 65 had progression of leptomeningeal disease (either clinical, radiographic, or CSF). Provocatively, we also found that the timing of targeted therapy appears to impact survival. In our study cohort, patients who received targeted therapy prior to LC-LM diagnosis displayed reduced overall survival. This is consistent with a model whereby prior exposure to targeted drugs generates resistance, with more limited options for treatment than a comparable patient harboring targeted treatment–naïve LC-LM.
Our study has several limitations. In addition to being retrospective, we restricted our inclusion criteria to patients with NSCLC. It is unclear if these results are also applicable to patients with LM from other solid tumor malignancies, such as breast cancer and melanoma. Due to the retrospective nature of our study, the dataset comprised a heterogeneous population of patients in regard to number and types of treatments received prior to LC-LM diagnosis, sites of systemic disease, and treatments received after LC-LM diagnosis. Most patients received some sort of cancer-directed therapy after diagnosis of LC-LM (153 of 171 patients). However, due to the retrospective nature of the study and differences between treatment types, treatment combinations, and timing, we were unable to control or evaluate for possible impact of treatment regimen on outcome. We were similarly unable to identify which, if any, specific targeted therapies had an impact on survival among patients with targetable mutations.
In our cohort, almost 50% of patients with LC-LM had a targetable mutation identified, which is likely higher than the general LC-LM population. While one study found that patients with EGFR mutations are much more likely to develop LM than those without, it is unclear in the general population how many patients with LC-LM harbor clinically actionable mutations.32 The high proportion of patients with targetable mutations in our study may be a result of referral bias to a quaternary cancer center; patients who are suspected to have a better chance for longer survival (like those with targetable mutations) are most likely to be referred to a cancer center for specialized care and consideration of clinical trials, versus patients with suspected poorer prognosis who perhaps are more likely to be treated locally or transition to hospice care. In addition, due to the relatively small number of each specific targetable mutation, we were unable to identify if individual molecular alterations are more impactful than others on survival.
Though one of the largest cohorts of patients with LC-LM is described in this study, complete CNS axis imaging, CTC enumeration, and cfDNA analysis were performed on only a subset of these patients. In particular, the limited number of patients who underwent CSF CTC enumeration and cfDNA analysis make it difficult to draw firm conclusions about prognostic importance of these evaluations. Though the analyses on survival outcome based on CSF cfDNA concentration had a trend toward significance, it statistically did not reach level of significance, which may be due to the small number of patients who had cfDNA testing performed. However, our data demonstrate potential prognostic value of these emerging studies, which if validated on a larger scale would have important implications for clinical decision making and therapeutic trials.
These findings support molecular characterization and CNS staging (using MRI sites of disease and CSF liquid biopsy quantification) for clinical management and prognostication of patients with LC-LM. Our findings are in agreement with the recent RANO recommendations for liquid biopsy in metastasis.33 Future prospective, multicenter trials of LM should employ these assessments in patients with LM. We also recommend that therapeutic trials for patients with LM use CSF biomarkers and radiographic assessments to risk stratify patients and quantify response.
Funding
This research was supported by grants from the National Institutes of Health (P30 CA008748), The Damon Runyon Clinical Investigator Award, and the Memorial Sloan Kettering Brain Tumor Center.
Supplementary Material
Acknowledgments
Part of this study was presented at the American Society of Clinical Oncology meeting in Chicago, 2017, and the Society of Neuro-Oncology meeting in New Orleans, 2018.
Conflict of interest statement. A.B. has consulted for Arix Bioscience and is on the Scientific Advisory Board for Evren Biosciences. She owns United States Patent #10413522, granted September 17, 2019. Patent pending application no. 62/258,044, November 20, 2015.
Authorship statement. KN designed the research study, performed the research, analyzed the data, and wrote the paper. ND, AS, XL, SO performed the research and wrote the paper. AR analyzed the data and wrote the paper. EP designed the research study, performed the research, analyzed the data, and wrote the paper. AB conceptualized and designed the research study, performed the research, analyzed the data, and wrote the paper.
References
- 1. Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology. 2010; 74(18):1449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49(4):759–772. [DOI] [PubMed] [Google Scholar]
- 3. Kuiper JL, Hendriks LE, van der Wekken AJ, et al. Treatment and survival of patients with EGFR-mutated non-small cell lung cancer and leptomeningeal metastasis: a retrospective cohort analysis. Lung Cancer. 2015;89(3):255–261. [DOI] [PubMed] [Google Scholar]
- 4. Park JH, Kim YJ, Lee JO, et al. Clinical outcomes of leptomeningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer. 2012;76(3):387–392. [DOI] [PubMed] [Google Scholar]
- 5. Grommes C, Oxnard GR, Kris MG, et al. Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011; 13(12):1364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol. 2016;11(11):1962–1969. [DOI] [PubMed] [Google Scholar]
- 7. Chamberlain M, Junck L, Brandsma D, et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol. 2017;19(4):484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Le Rhun E, Devos P, Boulanger T., et al. The RANO Leptomeningeal Metastasis Group proposal to assess response to treatment: lack of feasibility and clinical utility and a revised proposal. Neuro Oncol. 2019; 21(5):648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rami-Porta R, Bolejack V, Crowley J, et al. ; IASLC Staging and Prognostic Factors Committee, Advisory Boards and Participating Institutions The IASLC lung cancer staging project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10(7):990–1003. [DOI] [PubMed] [Google Scholar]
- 10. Konishi T, Shimada Y, Hsu M, et al. Association of preoperative and postoperative serum carcinoembryonic antigen and colon cancer outcome. JAMA Oncol. 2018;4(3):309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amin MB, Edge S, Greene F, et al. AJCC (American Joint Committee on Cancer) Cancer Staging Manual. 8th edition, 3rd printing. Chicago: Springer; 2018. [Google Scholar]
- 12. Lin X, Fleisher M, Rosenblum M, et al. Cerebrospinal fluid circulating tumor cells: a novel tool to diagnose leptomeningeal metastases from epithelial tumors. Neuro Oncol. 2017;19(9):1248–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herrlinger U, Förschler H, Küker W, et al. Leptomeningeal metastasis: survival and prognostic factors in 155 patients. J Neurol Sci. 2004;223(2):167–178. [DOI] [PubMed] [Google Scholar]
- 14. Morikawa A, Jordan L, Rozner R, et al. Characteristics and outcomes of patients with breast cancer with leptomeningeal metastasis. Clin Breast Cancer. 2017;17(1):23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le Rhun E, Devos P, Boulanger T, et al. ; European Organisation for Research and Treatment of Cancer (EORTC) Brain Tumor Group (BTG) Central Nervous System (CNS) Metastases Committee and the EORTC BTG Imaging Committee The RANO Leptomeningeal Metastasis Group proposal to assess response to treatment: lack of feasibility and clinical utility and a revised proposal. Neuro Oncol. 2019;21(5):648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boire A, Zou Y, Shieh J, Macalinao DG, Pentsova E, Massagué J. Complement component 3 adapts the cerebrospinal fluid for leptomeningeal metastasis. Cell. 2017;168(6):1101–1113.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–791. [DOI] [PubMed] [Google Scholar]
- 18. Negin BP, Cohen SJ. Circulating tumor cells in colorectal cancer: past, present, and future challenges. Curr Treat Options Oncol. 2010;11(1-2):1–13. [DOI] [PubMed] [Google Scholar]
- 19. Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14(9):985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yan WT, Cui X, Chen Q, et al. Circulating tumor cell status monitors the treatment responses in breast cancer patients: a meta-analysis. Sci Rep. 2017;7:43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14(19):6302–6309. [DOI] [PubMed] [Google Scholar]
- 22. Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213–3221. [DOI] [PubMed] [Google Scholar]
- 23. Hyun MH, Sung JS, Kang EJ, et al. Quantification of circulating cell-free DNA to predict patient survival in non-small-cell lung cancer. Oncotarget. 2017;8(55):94417–94430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Momtaz P, Pentsova E, Abdel-Wahab O, et al. Quantification of tumor-derived cell free DNA(cfDNA) by digital PCR (DigPCR) in cerebrospinal fluid of patients with BRAFV600 mutated malignancies. Oncotarget. 2016;7(51):85430–85436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malani R, Fleisher M, Lin X, et al. Cerebrospinal fluid circulating tumor cells (CSF CTC) for real-time patient monitoring and response to treatment [abstract]. J Clin Oncol. 2016; 35:11549. [Google Scholar]
- 26. Hyun JW, Jeong IH, Joung A, Cho HJ, Kim SH, Kim HJ. Leptomeningeal metastasis: clinical experience of 519 cases. Eur J Cancer. 2016;56:107–114. [DOI] [PubMed] [Google Scholar]
- 27. Niwińska A, Pogoda K, Michalski W, Kunkiel M, Jagiełło-Gruszfeld A. Determinants of prolonged survival for breast cancer patient groups with leptomeningeal metastasis (LM). J Neurooncol. 2018;138(1):191–198. [DOI] [PubMed] [Google Scholar]
- 28. Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. [DOI] [PubMed] [Google Scholar]
- 29. Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. [DOI] [PubMed] [Google Scholar]
- 30. Soria JC, Ohe Y, Vansteenkiste J, et al. ; FLAURA Investigators Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. [DOI] [PubMed] [Google Scholar]
- 31. Maemondo M, Inoue A, Kobayashi K, et al. ; North-East Japan Study Group Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. [DOI] [PubMed] [Google Scholar]
- 32. Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol. 2016;11(11):1962–1969. [DOI] [PubMed] [Google Scholar]
- 33. Boire A, Brandsma D, Brastianos PK, et al. Liquid biopsy in central nervous system metastases: a RANO review and proposals for clinical applications. Neuro Oncol. 2019;21(5):571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.