Abstract
Building on an initiative to enhance clinical trial participation involving the Society for Neuro-Oncology, the Response Assessment in Neuro-Oncology Working Group, patient advocacy groups, clinical trial cooperative groups, and other partners, we evaluate the impact of eligibility criteria and trial conduct on neuro-oncology clinical trial participation. Clinical trials often carry forward eligibility criteria from prior studies that may be overly restrictive and unnecessary and needlessly limit patient accrual. Inclusion and exclusion criteria should be evaluated based on the goals and design of the study and whether they impact patient safety and/or treatment efficacy. In addition, we evaluate clinical trial conduct as a barrier to accrual and discuss strategies to minimize such barriers for neuro-oncology trials.
Keywords: clinical trials, primary brain tumor, eligibility, inclusion criteria, exclusion criteria
It is estimated that only 21% of patients with primary brain tumors and only 8–11% of newly diagnosed glioblastoma (GBM) patients participate in clinical trials,1,2 even though there is limited therapeutic benefit associated with available standard therapies and there are several promising investigational approaches under evaluation in clinical trials. Although the etiology of poor accrual is likely multifactorial,3 failure to incorporate optimal eligibility criteria is a contributing factor. Despite the increasing sophistication of clinical trial designs, eligibility criteria for these trials are often overly burdensome and restrictive. The intent of eligibility criteria is to protect patients from harm and to identify a well-defined population to effectively address the key questions of a given trial. Often eligibility criteria are included “out of habit” or copied from prior protocols without reevaluating the continuing value of each individual inclusion or exclusion criterion relative to the key questions of the study. Consequently, patients may be deemed ineligible for reasons that do not directly impact safety or efficacy.
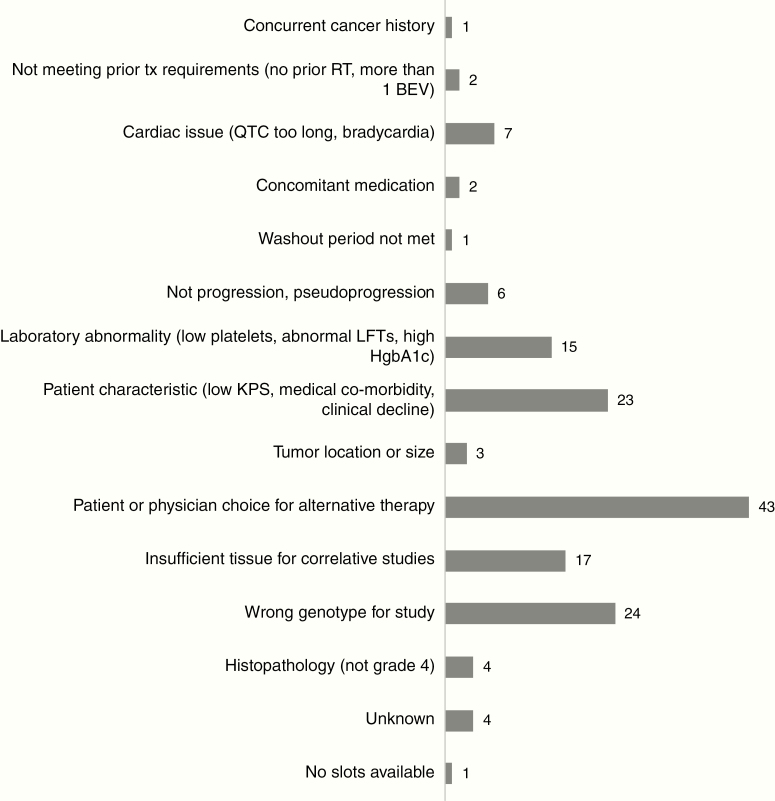
When brain tumor patients and caregivers were asked about barriers to clinical trial participation in a recent National Brain Tumor Society survey, “I did not qualify” was the second most common reason, after “my doctor did not recommend participating in a clinical trial.” 1 Clearly physicians caring for brain tumor patients need to prioritize presentation of clinical trial options, but failure to qualify underscores the critical need to carefully optimize eligibility criteria on a trial-by-trial basis in order to ensure that as many patients as possible can participate. The reasons for ineligibility vary depending on the clinical trial and are difficult to capture. We attempted to examine the reasons for ineligibility at an academic institution with high referral basis for clinical trials. However, our analysis was limited to those patients who signed consent but ultimately did not enroll on study (ie, screen failures). As some clinical trial providers “pre-screen” potential participants for trial eligibility before offering those trials, some patients who did not meet criteria never signed consent for a trial and their reasons for exclusion were not documented. Based on patients with newly diagnosed or recurrent GBM who signed consent and were screened for one of 6 principal investigator–initiated clinical trials at Dana-Farber Cancer Institute between March 2009 and February 2019 but ultimately did not enroll on the study, ineligibility was due to a variety of reasons, including incorrect histopathology (ie, not World Health Organization [WHO] grade IV), insufficient tissue for correlative studies, and laboratory abnormalities (Figure 1). Although further prospective studies are needed to more clearly document the reasons for ineligibility in brain tumor clinical trials, the neuro-oncologic academic community can and should move toward more deliberate examination of eligibility criteria.
Fig. 1.
Reasons for ineligibility at screening for 153 patients with newly diagnosed or recurrent glioblastoma who signed consent for one of 6 principal investigator–initiated clinical trials at Dana-Farber Cancer Institute between March 2009 and February 2019.
The American Society for Clinical Oncology (ASCO) and the Friends of Cancer Research (Friends) recently led an effort to optimize oncology trial eligibility in 5 specific areas: brain metastases,4 minimum age,5 HIV infection,6 organ dysfunction, and prior and concurrent malignancies.7 They recognized that overly restrictive eligibility criteria slow trial accrual, restrict patient access to investigational drugs, reduce the chances of knowing how the drug will work in the real world (ie, limit generalizability), and result in duplicative efforts with respect to drug development.4 Based on this ASCO/Friends initiative, the US FDA8–11 issued new draft guidance documents for inclusion and exclusion criteria with respect to these 5 specific areas, and the National Cancer Institute (NCI)12 amended protocol templates to reflect some of these changes. Building on this work, an effort has been made to join forces to increase clinical trial accrual to neuro-oncology trials by the Society for Neuro-Oncology (SNO), the Response Assessment in Neuro-Oncology Working Group, patient advocacy groups, clinical trial cooperative groups including the Adult Brain Tumor Consortium (ABTC), the Brain Tumor Committee of NRG Oncology, the Neuro-Oncology Committee for the Alliance for Clinical Trials in Oncology, the European Organisation for Research and Treatment of Cancer Brain Tumor Group, and other partners.3 Here, we evaluate the impact of eligibility criteria and trial conduct on neuro-oncology clinical trial participation (Table 1). The recommendations that follow represent consensus guidelines based on evidence (when available) and expert opinion. They are meant to provide a framework for critically evaluating eligibility criteria and conduct in current-day neuro-oncology trials. As our understanding of brain tumors evolve, trial design, including eligibility criteria, will similarly need to evolve beyond what is discussed here. We also note that the desire to increase clinical trial participation must be balanced with the ability to answer a scientific question, which may sometimes warrant restricting eligibility, such as limiting participation to the appropriate molecular subgroup for targeted therapy. Finally, critical evaluation of eligibility criteria and clinical trial conduct will be for naught if we do not increase the number of high-quality, thoughtful clinical trials (a topic outside the scope of this paper).
Table 1.
Summary of recommendations for neuro-oncology clinical trial eligibility
| Criterion | Types of Trials | Recommendation |
|---|---|---|
| Age | Primary brain tumor | • Allow children (age ≥12) to participate in adult trials when disease biology and clinical course is similar in children and adults • Allow older patients (age ≥65) to participate on trials, particularly in diseases such as GBM where older patients represent a significant portion of the patient population |
| Functional status | Solid tumor phase I trials | • Performance score requirement can be of ECOG ≤2 or equivalent KPS of ≥60 for selected phase 1 clinical trial based on mechanism of action and expected toxicity profile. |
| Comorbid medical conditions | Primary brain tumor | • Allow participation of patients with a prior or concurrent history of malignancy whose natural history or treatment does not have the potential to interfere with the safety or efficacy assessment of the investigational regimen, rather than specifying a specific time frame since completion of treatment |
| Immunotherapy | • Allow patients with select, well-controlled autoimmune diseases to enroll on immune checkpoint inhibitor trials, eg, thyroiditis | |
| Concomitant medications | Immunotherapy | • Allow corticosteroids at baseline but consider limiting maximum total daily doses of 2 mg dexamethasone and/or stratification according to dexamethasone dose in randomized trials |
| Long washout | Primary brain tumor | • Use 5 half-lives rather than a 4-week washout from prior therapy with short half-life. A general statement that the patient must have recovered from the effects of prior treatment would allow for even broader participation, particularly when prior therapy has long half-life. |
| Archival tissue requirements | Primary brain tumor | • The amount of tissue required for study enrollment needs a strong rationale and should be limited to what is necessary |
| Molecular subtypes | Primary brain tumor | • For molecular targets that are not stable throughout the disease course, a repeat biopsy should be considered to confirm target expression |
| Laboratory values | Primary brain tumor | • Only the relevant laboratory tests based on the safety profile of the study agent should be used as the basis for eligibility criteria • For those laboratory tests included as eligibility criteria, allow for a safe range above normal parameters |
| Immunotherapy | • Depending on the trial design and primary outcome, baseline ALC > 1000 cells/µL is ideal, but ≥500 cells/µL may be reasonable | |
| Pathology | GBM | • Patients with tumors meeting criteria for “diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV” should be allowed to participate on GBM clinical trials • Patients with IDH-mutant GBM can be included in phase 0/I GBM studies where efficacy is not primary endpoint or patients can be stratified by IDH status in randomized studies |
| Solid tumor phase I trials | • Patients with primary brain tumors including lower-grade gliomas and other rare CNS tumors should be included in dose escalation phases of solid phase I clinical trials • Exploratory expansion cohorts of specific brain tumor histopathology should be included if there is a biologic rationale for efficacy | |
| Prior therapy | Phase I | • Allow inclusion regardless of prior therapy unless a particular study question makes the prior therapy relevant • Allow prior exposure to bevacizumab |
| Phase II/III recurrent GBM | • When efficacy is an important endpoint and there is a high likelihood that outcomes may be influenced by prior therapies, strategies to allow broader enrollment include specifying separate analyses for patients who have or have not received the particular treatment (eg, bevacizumab refractory vs bevacizumab naïve), enrolling separate arms for these patient populations, or stratifying randomization based on prior exposure. | |
| Number of relapses | Recurrent GBM and phase I | • Allow any number of prior relapses, especially in phase 0/I trials and especially in bevacizumab- naïve patients |
| Recurrent GBM and phase II | • Allow at least 2 prior relapses in bevacizumab-naïve patients |
General Eligibility Criteria
Patient Factors
Patient factors that form the basis for eligibility criteria across neuro-oncology trials include age, functional status, past medical history, and prior therapies. Adult trials typically restrict enrollment to age 18 and older. ASCO/Friends5 and the FDA8 provide guidance on when to allow children as young as age 12 to participate in adult cancer trials. This is particularly relevant in tumor subtypes where the disease biology and clinical course are similar in children and adults, such as H3K27M mutant diffuse midline glioma,13 or when an adult disease rarely presents in adolescents.14 At the other end of the age spectrum, older patients (age ≥65) are not well represented in clinical trials.15,16 Indeed, the phase III trial which established radiation and temozolomide as standard of care for newly diagnosed GBM excluded patients above the age of 70.17 Since the median age at GBM diagnosis in the US is 65 years,18 excluding older patients leads to a lack of data for an important portion of the GBM population. Even when not explicitly excluded by age, patients can be excluded by comorbidities or concomitant medications. The FDA provides a guidance document to promote inclusion of elderly patients on trials when the drugs are likely to be used in the elderly.16
Historically, patients with a prior malignancy have been excluded from clinical trials with a few exceptions. When the risk of the prior malignancy interfering with the trials endpoints or safety is deemed to be low, participation should be allowed. We agree with the ASCO/Friends recommendation to allow participation of patients with a prior or concurrent history of malignancy whose natural history or treatment does not have the potential to interfere with the safety or efficacy assessment of the investigational regimen.19
Requirements for overly long washout periods of previous drug treatments may also interfere with study enrollment. If the goal is to eliminate the carryover effects from the prior treatment to avoid overlapping toxicities, then the often applied 4-week washout period from prior investigational agents is often excessive. Instead, a washout period of 5 times the half-life of the prior treatment may suffice for cytotoxic chemotherapies or targeted therapies. Even so, this may be unnecessarily long for treatments with long half-lives such as bevacizumab and checkpoint inhibitors. A general statement that the patient must have recovered from the anticipated effects of prior treatment would allow for even broader participation.
Archival Tissue Requirements
Some clinical trials mandate central review of the tumor as well as molecular testing (eg, promotor methylation status of O6-methylguanine-DNA methyltransferase [MGMT], genotyping), which may be redundant when similar data from laboratories certified by Clinical Laboratory Improvement Amendments or the equivalent are available. In these situations, the demand for large amount of tissues, and more specifically frozen tissue, will severely hamper accrual. The amount and type of tissue required for study enrollment needs a strong rationale and should be limited to only what is absolutely necessary to assess the study’s key questions. Of note, advances in tissue-based testing are resulting in large reductions in the amount of tumor required for even advanced molecular analyses, which can usually be performed on formalin-fixed material.
Molecular Subtypes for Targeted Therapy Trials
Genomic data for trial eligibility is often derived from the initial sample. While this is reasonable for genomic alterations that are generally stable across recurrences (such as isocitrate dehydrogenase [IDH] mutational status), gliomas can harbor new molecular changes at recurrences.20 For example, epidermal growth factor receptor (EGFR) variant III mutations are lost at tumor recurrence in 37–59% of patients.20,21 Therefore, depending on the target, a repeat biopsy should be considered to confirm target expression prior to participation on a molecularly driven trial.
Laboratory Values and Organ Dysfunction
Specifying parameters for laboratory values and organ function is critical for patient safety and to ensure that the study can be completed without excessive treatment modification or discontinuations based on poor tolerance and toxicity. Overly stringent requirements without an allowance for a safe range above normal parameters can limit enrollment, particularly for patients with advanced disease. We agree with recommendations from Friends/ASCO regarding increased inclusiveness of patients with organ dysfunction, including renal, hepatic, and cardiac dysfunction when appropriate.19 Only the relevant laboratory tests and cardiac status based on the safety profile of the study agent should be used as the basis for eligibility criteria (Table 2).
Table 2.
Summary of recommendations from Friends/ASCO,20 NCI,13 and FDA10 regarding inclusion of patients with organ dysfunction and abnormal laboratory values
| Organ | Summary Recommendation |
|---|---|
| Renal function | • Eligibility criteria should include assessment of GFR rather than serum creatinine concentrations. |
| • Inclusion of patients with renal dysfunction when available nonclinical and clinical data indicate that inclusion is safe. | |
| • For ETCTN and phase I trials: GFR ≥60 mL/min/1.73 m2 unless data exist supporting safe use at lower kidney function values, no lower than 30 mL/min/1.73m.2 • For non-network (mostly large phase II and III) trials: GFR ≥50 mL/min/1.73 m2 unless data exist supporting safe use at lower kidney function values, no lower than 30 mL/min/1.73 m2. | |
| Cardiac function | • Patients with known history or current symptoms of cardiac disease, or history of treatment with cardiotoxic agents, should have a clinical risk assessment of cardiac function using the New York Heart Association Functional Classification. To be eligible for this trial, patients should be class 2B or better. |
| • If QTc prolongation is not identified as a concern in first-in-human studies, QTc interval eligibility criteria in phase IB and later trials should be reevaluated, and ongoing ECG monitoring may not be required. | |
| Hepatic function | • Inclusion of patients with mild to moderate hepatic impairment (ie, NCI CTCAE grade 1) when nonclinical and clinical data indicate risk is not unreasonable |
| • Inclusion of patients with AST/ALT elevations ≤3 × institutional ULN. | |
| • Eligibility criteria for patients with asymptomatic elevations in unconjugated bilirubin (eg, Gilbert syndrome, hereditary spherocytosis, sickle cell disease, thalassemia intermedia) should be defined in the protocol. |
Abbreviations: ALT, alanine transaminase; ASCO, American Society of Clinical Oncology; AST, aspartate transaminase; CTCAE, Common Terminology Criteria for Adverse Events; ETCTN, Experimental Therapeutics Clinical Trial Network; GFR, glomerular filtration rate; NCI, National Cancer Institute; ULN, upper limit of normal.
Immunotherapy Trials
Although effective immunotherapy remains elusive for the majority of brain tumor patients,22,23 recent scientific and translational advances have ignited a plethora of immunotherapy trials, particularly in GBM and brain metastases. To maximize enrollment to these studies and produce generalizable results,24 several key eligibility criteria for immunotherapy trials must be carefully considered.
First, a key consideration is the use of corticosteroids. Consistent with their immunosuppressive effect, recent data from preclinical GBM models25 as well as immunotherapy trials in GBM26 have suggested that corticosteroid use is associated with quantitative and qualitative T-cell dysfunction and poorer outcomes. When possible, corticosteroids should therefore be avoided. However, despite their significant drawbacks, corticosteroids continue to play an essential role in the management of peritumoral edema and resultant symptoms in brain tumor patients.27 Although bevacizumab represents a potential alternative, it is expensive, associated with its own toxicity and risks, some of which can impact the timing of surgery, and may confound response assessment when administered with other antineoplastic therapies.28 Thus, some degree of corticosteroid use may be unavoidable in the majority of patients with aggressive brain tumors. Routine exclusion of patients requiring dexamethasone would severely limit eligibility for immunotherapy trials and lead to selection bias and less generalizable results. What the lowest dose of corticosteroids allowable for participation on an immunotherapy trial should be depends on several factors, including the patient population and the goals of the study. In patients with non-small-cell lung cancer (NSCLC) treated with programmed death 1 (PD-1) or programmed death ligand 1 (PD-L1) blockade, baseline corticosteroid use of ≥10 mg/day of prednisone equivalent (ie, 1.5 mg/day dexamethasone) was associated with poorer outcome.29 For trials where efficacy is an important endpoint, a reasonable compromise would be to stratify patients to those who do not require corticosteroids, those who require modest dosing such as maximum total daily doses of 2 mg dexamethasone and, if deemed appropriate, those requiring higher doses. For early phase trials where toxicity is a primary endpoint, consensus opinion is to limit baseline dexamethasone dose to 2 mg/day or less as high doses of dexamethasone could mask toxicity from immunotherapy. Future studies of the dose relationship of corticosteroids to immune reactivity may help refine these guidelines and provide a better understanding of the duration of corticosteroid effects after successful cessation of treatment.
Once a study participant has initiated immunotherapy treatment, steroid dosing could be liberalized to manage symptoms related to toxicity and/or cerebral edema. Data from the use of immunotherapies in systemic cancers suggest that implementation of short-term corticosteroids to manage immune-related adverse events (irAEs) does not seem to significantly alter efficacy.30,31 Laboratory-based studies in GBM models suggest that once antitumor immunity has been initiated, the negative impact of corticosteroids on immune function is markedly reduced.25 Pragmatically, for symptom management, patients able to start immunotherapy without corticosteroids may be able to use corticosteroids after immunotherapy has been implemented. The impact of this intervention also warrants careful prospective study.
Second, given the key role of lymphocytes in mounting an antitumor immune response, cutoffs for minimum absolute lymphocyte counts (ALCs) have been implemented in numerous immunotherapy trials. In GBM patients, however, lymphopenia is common, both at baseline due to sequestration of T cells in the bone marrow32 as well as due to corticosteroids and chemoradiation.33 Although it is not yet known whether baseline ALC predicts immunotherapy outcomes in GBM, data from other cancers have yielded mixed results. Some studies have demonstrated an association between baseline lymphopenia (ALC < 1000 cells/µL) and poor antitumor immune response and reduced immunologic toxicities34,35; others have failed to show such a relationship.36 What baseline ALC values should be chosen as study entry criteria for immunotherapy trials is unclear and depends on the patient population and trial goals. In an ideal setting, when efficacy is a primary endpoint, baseline ALC > 1000 cells/µL may be a reasonable cutoff. We recognize that this will make the study results less generalizable to the overall GBM population and could significantly limit study accrual. It can also be argued that, given the lack of clear data as well as a lack of proven efficacy of immunotherapy in primary brain tumors to date, it may be prudent to use a more liberal ALC cutoff, such as a minimum ALC of 500 cells/µL (ie, NCI Common Terminology Criteria for Adverse Events grade ≤3), until data from ongoing immunotherapy trials in brain tumors can determine if baseline ALC predicts response to immunotherapy.
Finally, the presence of preexisting autoimmune disease is a common exclusion criterion that may limit enrollment and generalizability in immune checkpoint inhibitor trials. Given that flares and irAEs in patients with preexisting autoimmune disease receiving immune checkpoint inhibitors for NSCLC and melanoma are often manageable without discontinuing therapy,37 as well as the dismal prognosis of GBM,18 it is reasonable to allow patients with select, well-controlled autoimmune diseases to enroll on GBM immune checkpoint inhibitor trials.
Glioblastoma Eligibility Criteria
Molecular Classification
Advances in molecular diagnostics over the past decade have led to changes in the classification and grading of CNS tumors.38 A working group of the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy (cIMPACT-NOW) made recommendations for a new integrated diagnosis for a subset of IDH-wildtype astrocytomas that exhibit an aggressive clinical course similar to GBM but do not meet histopathologic criteria for GBM.39,40 Based on expert opinion and an extensive literature review, cIMPACT-NOW established that histologic IDH-wildtype astrocytomas of WHO grade II or III can be considered “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV” if any one of the following is present41–45: high-level amplification of EGFR, whole chromosome 7 gain and whole chromosome 10 loss (+7/−10), or telomerase reverse transcriptase promoter mutation. Patients with tumors meeting these criteria should be considered eligible to participate in clinical trials for newly diagnosed or recurrent GBM.
Another important change to glioma classification based on molecular profiling is the evolving definition of the term “secondary GBM.” Traditionally, secondary GBM has referred to a GBM arising from a known grade II or III astrocytoma, proven by pathology.46 However, secondary GBMs may also now refer to a tumor that is histologically grade IV at initial diagnosis but harbors an IDH1 or IDH2 mutation, even without a known history of a lower-grade astrocytoma.47 For IDH-mutant GBM, it may be reasonable to include these patients along with IDH-wildtype patients in phase 0/I studies, where efficacy is not the primary endpoint. For phase II/III trials with survival endpoints, however, stratification or exclusion based on IDH mutational status may be considered, as IDH-mutant tumors have a distinct biology and may have a more indolent natural history.48,49
Tumor-Treating Fields in Newly Diagnosed GBM Trials
Tumor-treating fields (TTFields) are approved by the US FDA for use in combination with maintenance temozolomide in adults with newly diagnosed, supratentorial GBM following maximal debulking surgery and completion of radiation therapy. Discussions on approval and reimbursement are ongoing in many other countries worldwide. US approval, as well as its Category I recommendation status in the National Comprehensive Cancer Center (NCCN) guidelines for CNS Tumors, is based on the randomized, phase III EF-14 trial, which demonstrated survival benefit from the addition of TTFields to maintenance temozolomide chemotherapy.50 Despite this, a substantial proportion of patients with newly diagnosed GBM in the US do not use TTFields,51 the reasons for which are not fully understood but may include the encumbrances related to carrying and maintaining the device. In other countries, there is large variation in access and request for this treatment.
The question of whether to allow use of TTFields in upfront GBM clinical trials needs to be addressed for each clinical trial. It is difficult to mandate the use of TTFields, especially for trials in countries where access and reimbursement is limited and especially when a substantial proportion of patients in the US chose not to use TTFields. On the other hand, routine exclusion of TTFields from front-line GBM trials in the US may impact enrollment from health care providers and patients motivated to use TTFields. More recently, the US NCI Brain Malignancies Steering Committee has advised against routine exclusion of TTFields, unless its use is harmful for participants on study.
When and how TTFields should be incorporated into upfront GBM trials was heavily debated within the group. In general, the group did not favor mandating TTFields in trials. We discussed a variety of strategies for incorporating TTFields based on the clinical trial design and primary outcomes. In phase I studies, as long as TTFields is not considered harmful50,52 to combine with the experimental therapy, its use could be allowed with careful consideration of toxicity attribution to TTFields versus the experimental therapy. For single arm studies with efficacy endpoints, inconsistent use of TTFields across the study population may skew results and thereby limit the ability to isolate the treatment effect of the experimental therapy. For randomized trials in the newly diagnosed GBM setting, patients could theoretically be stratified by use of TTFields. Study teams would need to be aware of the additional logistical and statistical challenges with stratification by “intent to use TTFields” as most patients do not know at the time of randomization (for upfront studies, this could be at the time of initial surgery or before starting radiation) whether they would want to add TTFields to their post-radiation treatment regimen. Lessons can be learned from the experience of “intent to use temozolomide” in a randomized phase III trial of standard of care with or without sitimagene ceradenovec in high-grade glioma.53,54 During study proceedings, data emerged supporting the addition of temozolomide to radiotherapy and the protocol was amended to stratify according to intent to use temozolomide. However, non-adherence confounded the results; 24% of patients in both arms who had intended to use temozolomide did not use temozolomide on study.54
Prior Treatments and Number of Relapses in Recurrent GBM Trials
Studies of hypofractionated radiation therapy in elderly adults with GBM55–57 and our increased understanding of the predictive value of MGMT promoter methylation on chemosensitivity58,59 have resulted in heterogeneous treatment approaches at diagnosis. Some patients may have received upfront hypofractionated radiation (instead of standard 6-week radiation) with or without temozolomide or temozolomide alone without radiation, while others may have participated on an upfront clinical trial with experimental agents and/or radiation techniques. Limiting enrollment only to patients who received standard of care as upfront therapy excludes patients who may otherwise be reasonable trial candidates. In phase 0/I studies, exclusion of patients for alternative front-line therapy is generally not warranted. In general, recurrent GBM trials should not exclude based on prior treatment unless a particular study question or treatment-related toxicity makes the prior treatment relevant. In phase II/III studies, where efficacy is an important endpoint, investigators should carefully consider (i) whether prior receipt of temozolomide or short-course radiation would realistically be expected to impact the efficacy of the investigational treatment (we would argue that in most cases, it would not) and (ii) whether stratification rather than exclusion based on prior therapy received is warranted.
A second major issue that limits trial eligibility in recurrent GBM is the exclusion of patients who are beyond first or second relapse. While the number of relapses may be relevant when considering efficacy endpoints,60,61 this is much less important in phase 0/I studies (discussed further below). We recommend including all patients with recurrent GBM, irrespective of number of relapses, in early phase studies provided that the patient is otherwise an appropriate trial candidate in terms of performance status, expected survival, and comorbidities.
Overall survival (OS) is potentially influenced by whether a patient is in first versus second relapse. It is less clear whether progression-free survival (PFS) or radiographic response (RR) would be influenced by first versus second relapse but could be influenced by prior therapies. For example, patients who experience disease progression while receiving bevacizumab rarely respond to further salvage therapy.62 Thus, for single arm phase II studies with a non-OS endpoint (ie, PFS or RR), we recommend inclusion of patients in first or second relapse. Instead of discriminating based on the number of relapses, studies may consider discriminating based on relevant prior therapies which would predict failure on trial. Randomized studies, including those with an OS endpoint, could stratify based on first versus second relapse. When efficacy is an important endpoint and there is a high likelihood that outcomes may be influenced by prior therapies, strategies to allow broader enrollment include specifying separate analyses for patients who have or have not received the particular treatment (eg, bevacizumab refractory versus bevacizumab naïve), enrolling separate arms for these patient populations, or stratifying randomization based on prior exposure.
Eligibility Criteria for Phase I Studies
Patients with primary CNS tumors are often excluded from first-in-human solid tumor clinical trials. Perceived poor prognosis and fear of excessive CNS toxicities are the major reasons for limiting access to early phase clinical trials,63 but there is evidence to refute this perception. A pooled analysis of patients with recurrent WHO grades III and IV gliomas enrolled onto ABTC phase I trials compared findings with the published outcomes of patients with solid tumors enrolled onto phase I oncology trials of the same treatments. Patients with WHO grades III and IV gliomas who fulfilled the standard phase I eligibility criteria and were enrolled onto trials of appropriately chosen single-agent drugs successfully met phase I endpoints (namely, safety, toxicity, and efficacy). The serious toxicities observed in these patients were within the acceptable toxicity rates seen in other solid tumor phase I trials. The maximum tolerated dose was identical or marginally higher in WHO grades III and IV glioma patients who were not receiving enzyme-inducing anti-epileptic drugs (EIAEDs) compared with non-glioma patients or with glioma patients on EIAEDs.64 Therefore, one can argue that all phase I clinical trials for solid tumors should allow enrollment of patients with WHO grade III or IV glioma who otherwise meet the standard eligibility criteria, provided the investigational agent has adequate penetration across the blood–brain barrier or the mechanism of action does not require it to do so (eg, certain immunotherapies). We also recommend addition of a specific expansion cohort for these tumors when there is a sound biologic rationale and favorable pharmacokinetic properties, including evidence of blood–brain barrier penetration as observed in preclinical models. Alternatively, expansion cohorts can be designed in surgical patients who would receive experimental drug before tumor resection for clinical indications, and pharmacokinetic and pharmacodynamic data can be obtained on resected tumor tissue. These cohorts would help with making go/no-go decisions based on early outcomes data.
In addition to patients with WHO grade III or IV glioma, phase I trials may offer a way for patients with rare primary CNS tumors (eg, diffuse midline gliomas, ependymomas, medulloblastomas) to obtain access to novel agents. These cancers have been less studied in the clinical research setting, mainly due to their low incidence and slow accrual to clinical trials, as well as lack of incentive for pharmaceutical companies due to a small potential market. As with glioma patients, patients with rare CNS tumors can provide valuable data on safety and dose-finding in early-phase clinical trials. In addition, one could potentially gain valuable efficacy signals that could then be evaluated further in expansion cohorts or in follow-up efficacy trials. This approach does offer opportunities for patients with rare diseases to participate but also underscores the need to mandate that the phase I efficacy data not be routinely used as a go/no-go decision for further investigation of the treatment agent or regimen.
Restrictive eligibility criteria are also a significant barrier for patient accrual to clinical trials that are specifically designed for patients with primary brain tumors. The majority of early-phase clinical trials in neuro-oncology are open only to patients with GBM. Other therapy restrictions such as the prior use of anti-angiogenic drugs (typically bevacizumab) are also common, but not germane to the goals of phase I clinical trials. In addition, patients may be ineligible if their initial diagnosis was WHO grade II or grade III, even though they have pathologically proven GBM at the time of progression. Neither the number of prior recurrences nor lines of prior therapy nor pathologic grade (provided they meet criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”) should exclude patients from phase I GBM trials so long as they meet other eligibility criteria required for enhancing the safety of clinical trials and completing the dose-limiting toxicity period of observation.
Finally, many phase I clinical trials limit eligibility to patients with good Eastern Cooperative Oncology Group (ECOG) performance score of ≤1 or equivalent Karnofsky performance score (KPS) of ≥80. The expected toxicity profile and mechanism of action of the investigational therapeutics should drive eligibility criteria in phase I trials, not a required functional status score. The primary brain tumor population specifically may experience neurologic deficits that do not directly affect their ability to tolerate treatments but limit their ability to self-care and thus lower their ECOG score to 2 or equivalent KPS to 60 (such as hemiparesis). Therefore, in selected phase I trials, primary brain tumor patients with lower performance scores (KPS ≥60) due to fixed deficits can be accrued without affecting the integrity of the study.
Optimizing Patient-Related Factors
Patient accrual and retention are two critical obstacles to the successful completion of clinical trials. We currently live in an era of unprecedented connectivity, access to information, and reliance on mobile technologies; these conditions may be exploited to improve the conduct of clinical trials for patients with brain tumors.
Lack of awareness of available clinical trials is arguably the first barrier to improving patient accrual. A report from the Center for Information and Study on Clinical Research Participation found that most people (51%) would prefer to receive information on trials from their primary care physician or from study staff (44%).65 However, younger patients may rely on online resources or social media for information on trial options, and 84% of the adult population in the US use the internet.66 CenterWatch, a resource for information on clinical trials, reported that 38% of patients find available studies through major search engines on the internet.67 Clearly, the potential for increasing dissemination of trial information using outreach and social media exists; the Collaborative Ependymoma Research Network Foundation applied these strategies to form community-academic partnerships to successfully accrue patients to ependymoma studies. Modern-day technologies and mobile applications designed to aid clinicians and patients to browse available clinical trials may boost awareness of appropriate studies and could also support platforms to recruit underrepresented populations.
Geographic access to clinical trials is another hurdle to both accrual and retention of patients. As Janet Woodcock, Director of the FDA’s Center for Drug Evaluation and Research, expressed, “sites for clinical trials are frequently selected on the basis of where the investigators are located, as opposed to where the patients are, creating difficulties in patient recruitment.” 68 A recent retrospective analysis of 1600 patients with cancer at a single center indicated that the overall median unidirectional distance traveled from home to study site was 25.8 miles, with patients enrolled on phase I studies having the longest travel (median of 41.2 miles).69 To offset the burden of travel, especially for patients with brain tumors who may have limited mobility, many routine clinical trial assessments such as blood work could be completed locally, with results provided to the study centers per a specified protocol. In addition, “remote” or “virtual” visits may be able to replace some clinical assessments. This telemedicine approach has successfully been implemented in other disease areas and has been shown to reduce costs and improve care for patients with neuro-degenerative conditions that impair mobility and travel to clinical centers.70 Moreover, the use of wearable devices to gather patient-specific information could facilitate data collection of functional outcomes. Two recent large, randomized studies (ClinicalTrials.gov identifiers: NCT02511405 and NCT02152982) conducted in patients with GBM completed accrual much sooner than expected; it is possible that these successes were as a result of allowing administration of backbone therapies (radiation therapy, bevacizumab) to be delivered locally in the community. Finally, use of a central institutional review board (IRB) for multicenter trials could circumvent the need for a cumbersome local IRB approval requirement that can hinder patient enrollment. All these novel approaches require changes in how institutions and methods for data collection are sanctioned as acceptable for use in clinical trials.
Another impediment to clinical trial accrual and retention may be the lack of patient-focused approaches. Study designers do not generally consider the patient experience in writing clinical trials, as most efforts are focused on evaluating the efficacy and safety of an intervention and often maximizing the amount of data collected. However, patients are the key “customers” for clinical trials and hence their perspective is crucial to capturing and maintaining their participation. A recent survey indicated that patient involvement in trial design early on, including selection of outcomes and measurement tools, is recommended to improve the completion rate of trials for rare diseases.71 This is in keeping with the recent emphasis on “patient-focused drug development” that takes into consideration patients’ priorities. To gather stakeholder input, Leiter et al instituted an internet crowdsourcing platform to collect feedback from clinicians, patients, and advocates that led to significant modifications to an oncology trial. They found that crowdsourcing participation in clinical trial design was not only feasible, but worthwhile.72
Conclusions
Building on work by Friends/ASCO, FDA, and NCI in optimizing eligibility criteria for oncology trials, we provide additional recommendations regarding eligibility criteria and the conduct of neuro-oncology trials involving primary brain tumors. It is also important to consider the trial design or phase of development. As long as known safety data about a study agent is taken into account, eligibility criteria for phase I trials should be more permissive, particularly with respect to histology and grade. For randomized, phase III trials aimed at assessing definitive therapeutic benefit, patients can be stratified according to key characteristics such as number of prior relapses or IDH mutation status to allow greater inclusion.
This discussion also provides an introduction to some of the strategies that may transform clinical trial conduct for patients with brain tumors. There are multiple opportunities to exploit existing technologies and information networks to improve access to clinical trials for both patients and providers. However, as with any proposal to include novel approaches, introducing these changes will come with challenges. For example, the practical and regulatory frameworks for many of these applications are unclear. Who will pay for local testing and telemedicine visits? Will community physicians be responsible for following up with clinical trial laboratory results? Where do referring physicians and community oncologists fit in the clinical trial structure? How do we prioritize fostering awareness, outreach, and education for all stakeholders? Despite these uncertainties, it is imperative that we as a community move forward to address these issues, endeavor to overcome resistance to change, and work toward optimizing the conduct of clinical trials for our patients.
Funding
This work was supported by the Adult Brain Tumor Consortium (ABTC) NIH/NCI UM1 CA137443.
Conflicts of Interest (in alphabetical order)
Manmeet Ahluwalia: Receipt of grants/research supports: Astrazeneca, Abbvie, BMS, Bayer, Incyte, Pharmacyclics, Novocure, Merck. Receipt of honoraria or consultation fees: Elsevier, Wiley, Astrazeneca, Abvvie, VBI Vaccines, Flatiron, Varian Medical Systems, Prime Education, Bayer, karyopharm, Tocagen, Forma therapeutics. Stock shareholder: Doctible, Mimivax.
Stephen J. Bagley: Research support from Eli Lilly, Incyte, Novocure, and Tesaro. Participated on advisory boards for Bayer, Northwest Biotherapeutics, and Novocure.
Jian L. Campian: Consultant for Alexion, Abbvie, Arbor Pharmaceuticals, Dova Pharmaceuticals, Inovio Pharmaceuticals, Incyte, Novocure. Research support from Merck and NeoImmuneTech.
Evanthia Galanis: General Consulting from MedImmune, Inc; F. Hoffman La Roche, Ltd (compensation to Mayo), Tactical Therapeutics, Inc; Oncorus (personal compensation). Advisory board on Vyriad (compensation to Mayo), Celgene Corporation; KIYATEC (personal compensation). Clinical Trial Funding from MedImmune, Inc.; Denovo Biopharma; Tracon; Genentech; Bristol-Myers Squibb (Mayo).
Matthias Holdhoff: Consulting relationships/advisory boards with Celgene, Abbvie, BTG International Ltd, NewLink Genetics, DPClinical.
Eudocia Q. Lee: Consulting for Eli Lilly. Royalties from Up to Date, Inc. Honoraria from Prime Oncology.
Glenn Lesser: Research support (paid to institution) from Novartis, Vascular Biogenics, Pfizer, Incyte, New Link Genetics; Consulting for Monteris Medical, Cancer Expert Now; DSMB membership for Stemline Therapeutics, Agios
Minesh Mehta: Consulting relationships with honoraria with Varian, Agenus, Insys, Remedy, IBA, Astra-Zeneca, Celgene, Tocagen, Abbvie; Board of Directors position with stock options with Oncoceutics; and DSMB for Monteris.
L. Burt Nabors: Scientific advisory board for Karyopharm, Blue Earth Diagnostics, and BTG Pharmaceuticals
David Reardon: Research support (paid to institution): Acerta Phamaceuticals; Agenus; Celldex; EMD Serono; Incyte; Inovio; Midatech; Omniox; Tragara. Advisory/consultation (paid to self): Abbvie; Advantagene; Agenus; Bristol-Myers Squibb; Celldex; EMD Serono; Genentech/Roche; Inovio; Merck; Merck KGaA; Monteris; Novocure; Oncorus; Oxigene; Regeneron; Stemline; Taiho Oncology, Inc. Honoraria (paid to self): Abbvie; Advantagene; Agenus; Bristol-Myers Squibb; Celldex; EMD Serono; Genentech/Roche; Inovio; Merck; Merck KGaA; Monteris; Novocure; Oncorus; Oxigene; Regeneron; Stemline; Taiho Oncology, Inc.
Solmaz Sahebjam: Research support (paid to institution) from BMS and Merck.
Roy E. Strowd: Research support (paid to institution) from JAZZ Pharmaceuticals, Conquer Cancer Foundation of the American Society of Clinical Oncology, Southeastern Brain Tumor Foundation, Vaximm Pharmaceuticals. Consulting for Novocure, Peloton Therapeutics, Monteris Medical. Editorial Stipend: American Academy of Neurology.
Martin van den Bent: Consultanting for Cellgene, BMS, Agios, Boehringer, Abbvie, Bayer, Carthera.
Michael Vogelbaum: Indirect equity and patient royalty interests from Infuseon Therapeutics. Honoraria from Celgene, Tocagen, and Blue Earth Diagnostics.
Tobias Walbert: Advisory board of Orbus Therapeutics, Tocagen.
Michael Weller: Research grants from Abbvie, Bayer, Merck, Sharp & Dohme (MSD), Dracen, Merck (EMD), Novocure, OGD2, Piqur, Roche and Adastra. Honoraria for lectures or advisory board participation or consulting from Abbvie, Basilea, Bristol Meyer Squibb, Celgene, Merck, Sharp & Dohme (MSD), Merck (EMD), Novocure, Orbus, Roche, Teva, Tocagen.
Patrick Wen: Research Support from Agios, Astra Zeneca, Beigene, Celgene, Eli Lily, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, Oncoceutics, Sanofi-Aventis, Vascular Biogenics, VBI Vaccines. Advisory Board for Agios, Astra Zeneca, Bayer, Blue Earth Diagnostics, Immunomic Therapeutics, Karyopharm, Kiyatec, Puma, Taiho, Vascular Biogenics, Deciphera, VBI Vaccines, Tocagen. Speaker for Merck, Prime Oncology.
No conflicts reported for Susan M. Chang, Mark R. Gilbert, Stuart Grossman, Frank S. Lieberman, Marta Penas-Prado, Karisa Schreck, and Joohee Sul.
No part of this manuscript has been previously published or submitted concurrently to any other journal.
All coauthors have read and approved of its submission to this journal.
References
- 1. Bates AJ, Couillard SA, Arons DF, et al. hout-15. brain tumor patient and caregiver survey on clinical trials: identifying attitudes and barriers to patient participation. Neuro Oncol. 2017;19(suppl_6):vi109. [Google Scholar]
- 2. Vanderbeek AM, Rahman R, Fell G, et al. The clinical trials landscape for glioblastoma: is it adequate to develop new treatments? Neuro Oncol. 2018;20(8):1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee EQ, Chukwueke UN, Hervey-Jumper SL, et al. Barriers to accrual and enrollment in brain tumor trials. Neuro Oncol. 2019;pii:5513026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin NU, Prowell T, Tan AR, et al. Modernizing clinical trial eligibility criteria: recommendations of the American Society of Clinical Oncology–Friends of Cancer Research Brain Metastases Working Group. J Clin Oncol. 2017;35(33):3760–3773. [DOI] [PubMed] [Google Scholar]
- 5. Gore L, Ivy SP, Balis FM, et al. Modernizing clinical trial eligibility: recommendations of the American Society of Clinical Oncology–Friends of Cancer Research Minimum Age Working Group. J Clin Oncol. 2017;35(33):3781–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uldrick TS, Ison G, Rudek MA, et al. Modernizing clinical trial eligibility criteria: recommendations of American Society of Clinical Oncology–Friends of Cancer Research HIV Working Group. J Clin Oncol. 2017;35(33):3774–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lichtman SM, Harvey RD, Smit M-AD, et al. Modernizing clinical trial eligibility criteria: recommendations of the American Society of Clinical Oncology–Friends of Cancer Research Organ Dysfunction, Prior or Concurrent Malignancy, and Comorbidities Working Group. J Clin Oncol. 2017;35(33):3753–3759. [DOI] [PubMed] [Google Scholar]
- 8. Food and Drug Administration. Cancer Clinical Trial Eligibility Criteria: Minimum Age for Pediatric Patients 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cancer-clinical-trial-eligibility-criteria-minimum-age-pediatric-patients. Accessed July 20, 2019.
- 9. Food and Drug Administration. Cancer Clinical Trial Eligibility Criteria: Patients with Organ Dysfunction or Prior or Concurrent Malignancies 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cancer-clinical-trial-eligibility-criteria-minimum-age-pediatric-patients. Accessed July 20, 2019.
- 10. Food and Drug Administration. Cancer Clinical Trial Eligibility Criteria: Brain Metastases 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cancer-clinical-trial-eligibility-criteria-minimum-age-pediatric-patients. Accessed July 20, 2019.
- 11. Food and Drug Administration. Cancer Clinical Trial Eligibility Criteria: Patients with HIV, Hepatitis B Virus, or Hepatitis C Virus Infections 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cancer-clinical-trial-eligibility-criteria-minimum-age-pediatric-patients. Accessed July 20, 2019.
- 12. Cancer Therapy Evaluation Program. Inclusion/Exclusion Criteria for National Cancer Institute (NCI) Sponsored Clinical Trials 2018. https://ctep.cancer.gov/protocolDevelopment/docs/NCI_ASCO_Friends_Eligibility_Criteria.pdf. Accessed April 12, 2019.
- 13. Kleinschmidt-DeMasters BK, Mulcahy Levy JM. H3 K27M-mutant gliomas in adults vs. children share similar histological features and adverse prognosis. Clin Neuropathol. 2018;37(2):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gaspar N, Marshall LV, Binner D, et al. Joint adolescent–adult early phase clinical trials to improve access to new drugs for adolescents with cancer: proposals from the multi-stakeholder platform—ACCELERATE. Ann Oncol. 2018;29(3):766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Food and Drug Administration. Workshop Report: Evaluating Inclusion and Exclusion Criteria in Clinical Trials 2018. https://www.fdanews.com/ext/resources/files/2018/08-22-18-FDAsummary.pdf?1534970149. Accessed July 20, 2019.
- 16. Food and Drug Administration. Study of Drugs Likely to Be used in the Elderly 1997. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM072048.pdf. Accessed July 20, 2019.
- 17. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 18. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the united states in 2011–2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lichtman SM, Harvey RD, Damiette Smit MA, et al. Modernizing clinical trial eligibility criteria: recommendations of the American Society of Clinical Oncology–Friends of Cancer Research Organ Dysfunction, Prior or Concurrent Malignancy, and Comorbidities Working Group. J Clin Oncol. 2017;35(33):3753–3759. [DOI] [PubMed] [Google Scholar]
- 20. Draaisma K, Chatzipli A, Taphoorn M, et al. Molecular evolution of IDH wild-type glioblastomas treated with standard of care affects survival and design of precision medicine trials: a report from the EORTC 1542 study. J Clin Oncol. 2020;38(1):81–99. [DOI] [PubMed] [Google Scholar]
- 21. Weller M, Butowski N, Tran DD, et al. ; ACT IV trial investigators Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. [DOI] [PubMed] [Google Scholar]
- 22. Reardon DA, Omuro A, Brandes AA, et al. OS10.3 randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: CheckMate 143. Neuro Oncol. 2017;19(suppl_3):iii21–iii21. [Google Scholar]
- 23. Lim M, Xia Y, Bettegowda C, Weller M. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol. 2018;15(7):422–442. [DOI] [PubMed] [Google Scholar]
- 24. Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncol. 2017;35(33):3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giles AJ, Hutchinson MND, Sonnemann HM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keskin DB, Anandappa AJ, Sun J, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee EQ, Wen PY. Corticosteroids for peritumoral edema: time to overcome our addiction? Neuro Oncol. 2016;18(9):1191–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics. 2017;14(2):307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36(28):2872–2878. [DOI] [PubMed] [Google Scholar]
- 30. Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35(7):785–792. [DOI] [PubMed] [Google Scholar]
- 31. Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chongsathidkiet P, Jackson C, Koyama S, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24(9):1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31(2):140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Diehl A, Yarchoan M, Hopkins A, Jaffee E, Grossman SA. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget. 2017;8(69):114268–114280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ho WJ, Yarchoan M, Hopkins A, Mehra R, Grossman S, Kang H. Association between pretreatment lymphocyte count and response to PD1 inhibitors in head and neck squamous cell carcinomas. J Immunother Cancer. 2018;6(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun R, Champiat S, Dercle L, et al. Baseline lymphopenia should not be used as exclusion criteria in early clinical trials investigating immune checkpoint blockers (PD-1/PD-L1 inhibitors). Eur J Cancer. 2017;84:202–211. [DOI] [PubMed] [Google Scholar]
- 37. Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann Intern Med. 2018;168(2):121–130. [DOI] [PubMed] [Google Scholar]
- 38. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 39. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018;136(5):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aibaidula A, Chan AK, Shi Z, et al. Adult IDH wild-type lower-grade gliomas should be further stratified. Neuro Oncol. 2017;19(10):1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aoki K, Nakamura H, Suzuki H, et al. Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro Oncol. 2018;20(1):66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stichel D, Ebrahimi A, Reuss D, et al. Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt astrocytoma to glioblastoma. Acta Neuropathol. 2018;136(5):793–803. [DOI] [PubMed] [Google Scholar]
- 44. Weller M, Weber RG, Willscher E, et al. Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol. 2015;129(5):679–693. [DOI] [PubMed] [Google Scholar]
- 45. Wijnenga MMJ, Dubbink HJ, French PJ, et al. Molecular and clinical heterogeneity of adult diffuse low-grade IDH wild-type gliomas: assessment of TERT promoter mutation and chromosome 7 and 10 copy number status allows superior prognostic stratification. Acta Neuropathol. 2017;134(6):957–959. [DOI] [PubMed] [Google Scholar]
- 46. Hamisch C, Ruge M, Kellermann S, et al. Impact of treatment on survival of patients with secondary glioblastoma. J Neurooncol. 2017;133(2):309–313. [DOI] [PubMed] [Google Scholar]
- 47. Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19(4):764–772. [DOI] [PubMed] [Google Scholar]
- 48. Nobusawa S, Watanabe T, Kleihues P, Ohgaki H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15(19):6002–6007. [DOI] [PubMed] [Google Scholar]
- 49. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Burri SH, Gondi V, Brown PD, Mehta MP. The evolving role of tumor treating fields in managing glioblastoma: guide for oncologists. Am J Clin Oncol. 2018;41(2):191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48(14):2192–2202. [DOI] [PubMed] [Google Scholar]
- 53. Westphal M, Ylä-Herttuala S, Martin J, et al. ; ASPECT Study Group Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(9):823–833. [DOI] [PubMed] [Google Scholar]
- 54. European Medicines Agency. Withdrawal Assessment Report of Cerepro 2011; https://www.ema.europa.eu/en/documents/withdrawal- report/withdrawal-assessment-report-cerepro_en-0.pdf. Accessed October 7, 2019.
- 55. Perry JR, Laperriere N, O’Callaghan CJ, et al. ; Trial Investigators Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 56. Malmström A, Grønberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 57. Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–1588. [DOI] [PubMed] [Google Scholar]
- 58. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 59. Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Majewska P, Ioannidis S, Raza MH, Tanna N, Bulbeck H, Williams M. Postprogression survival in patients with glioblastoma treated with concurrent chemoradiotherapy: a routine care cohort study. CNS Oncol. 2017;6(4):307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wann A, Tully PA, Barnes EH, et al. Outcomes after second surgery for recurrent glioblastoma: a retrospective case-control study. J Neurooncol. 2018;137(2):409–415. [DOI] [PubMed] [Google Scholar]
- 62. Quant EC, Norden AD, Drappatz J, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol. 2009;11(5):550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wen PY, Schiff D, Cloughesy TF, et al. It is time to include patients with brain tumors in phase I trials in oncology. J Clin Oncol. 2011;29(24):3211–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gounder MM, Nayak L, Sahebjam S, et al. Evaluation of the safety and benefit of phase I oncology trials for patients with primary CNS tumors. J Clin Oncol. 2015;33(28):3186–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Center for Information and Study on Clinical Research Participation (CISCRP). 2015 Perceptions & Insights Study 2015; http://0393122.netsolhost.com/programs-events/research-and-studies/perceptions-and-insights/. Accessed November 1, 2019.
- 66. United States Census Bureau. Computer and Internet Use in the United States 2011; https://www.census.gov/data/tables/2011/demo/computer-internet/p20-569.html. Accessed November 1, 2019.
- 67. CenterWatch. How Do Patients Find Clinical Trials?2015; https://www.centerwatch.com/images/infographics/how-patients-find-clinical-trials.pdf. Accessed November 1, 2019.
- 68. Institute of Medicine (US) Forum on Drug Discovery, Development, and Translation. Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary. Washington (DC): National Academies Press (US); 2010. https://www.ncbi.nlm.nih.gov/books/NBK50892/ doi: 10.17226/12900. Accessed November 1, 2019.
- 69. Borno HT, Zhang L, Siegel A, Chang E, Ryan CJ. At what cost to clinical trial enrollment? A retrospective study of patient travel burden in cancer clinical trials. Oncologist. 2018;23(10):1242–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Beck CA, Beran DB, Biglan KM, et al. ; Connect.Parkinson Investigators National randomized controlled trial of virtual house calls for Parkinson disease. Neurology. 2017;89(11):1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gaasterland CMW, van der Weide MCJ, du Prie-Olthof MJ, et al. The patient’s view on rare disease trial design—a qualitative study. Orphanet J Rare Dis. 2019;14(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Leiter A, Sablinski T, Diefenbach M, et al. Use of crowdsourcing for cancer clinical trial development. J Natl Cancer Inst. 2014; 106(10):dju258. [DOI] [PubMed] [Google Scholar]